2014 Volume 62 Issue 7 Pages 700-708
2014 Volume 62 Issue 7 Pages 700-708
Nuclear transcription factor nuclear factor-kappa B (NF-κB) has diverse pathophysiological functions, and NF-κB inhibitors are considered to be candidates for multiple therapeutic applications. We previously reported a novel triazine-based NF-κB inhibitor, 2-anilino-4,6-dichloro-1,3,5-triazine (NI241), that directly inhibits DNA binding of NF-κB. Here, we report synthesis of a series of triazine derivatives and evaluation of their structure–activity relationships for NF-κB inhibition. We found that 2-amino-4,6-dichloro-1,3,5-triazine substructure is essential for the inhibitory activity of the lead compound NI241, and modification of NI241 by introduction of an m-methoxy substituent on the phenyl ring afforded the more potent derivative 28. The structure–activity relationships identified in this study suggested a possible mechanism of irreversible NF-κB inhibition by NI241, and should be helpful in the design of other NF-κB inhibitors.
Nuclear factor-kappa B (NF-κB) is a transcriptional factor that regulates many crucial physiological and pathological processes, such as immune response, inflammation, infection, cell differentiation, and cancer development.1–5) NF-κB is activated by various factors, such as cytokines, toxins, and other cellular stimuli, through multiple pathways including the canonical pathway and non-canonical pathway.6–11) Inhibitors of NF-κB are expected to be candidates for multiple therapeutic applications, and various compounds that inhibit NF-κB signaling pathways have been developed in recent decades.12–14) Though activation of NF-κB can be blocked at a variety of steps, compounds that block upstream and midstream steps of the NF-κB pathway might affect multiple targets and pathways. On the other hand, compounds that directly inhibit DNA binding of NF-κB might be very effective in regulating the expression of target genes, because NF-κB signaling pathways converge at the level of DNA binding. On the basis of these considerations, we previously screened NF-κB DNA-binding inhibitors and identified the 2,4-dichloro-1,3,5-triazine derivative NI241.15) Structurally, NI241 has a quite simple scaffold, and in the present work we planned to investigate its structure–activity relationship (SAR) with the aim of developing more potent compounds.
NI241 consists of three parts: dichlorotriazine, a phenyl ring, and the linking secondary amino group. C-Chlorinated triazine is chemically reactive toward nucleophiles. Here, we focused on pyrimidine and triazine derivatives bearing dichloro groups or other functional group(s) such as alkoxy or amino groups (Fig. 1). Since our previous studies on docking simulation of NI241 to NF-κB suggested the importance of both the molecular shape of NI241 and a secondary amino group that interacts with amino acid residues of NF-κB, we also examined various N-substituents on 2-amino-4,6-dichloro-1,3,5-triazine.
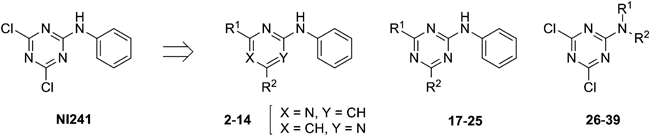
Synthesis of pyrimidine derivatives is illustrated in Chart 1. Aromatic nucleophilic substitution reactions using aniline and 2,4,6-trichloropyrimidine gave anilinopyrimidines 2 and 3. Further substitution of 2 or 3 using alkoxides afforded compounds 4–8. The use of excess alkoxide afforded dialkoxylated compounds 5 and 8. Amination of 2 or 3 using 4-methoxybenzylamine gave compounds 9 or 11, respectively. Cleavage of the 4-methoxybenzyl group of 9 and 11 under acidic conditions gave compounds 10 and 12, respectively. Methylpyrimidine derivative 14 was prepared from 4,6-dichloro-2-methylpyrimidine (13) and aniline (Chart 1).
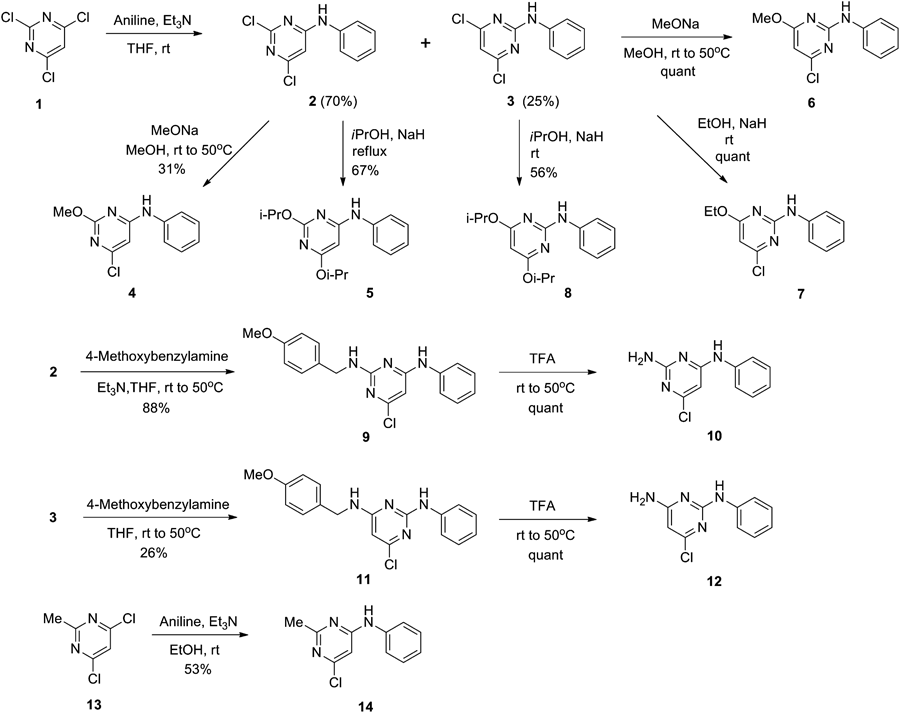
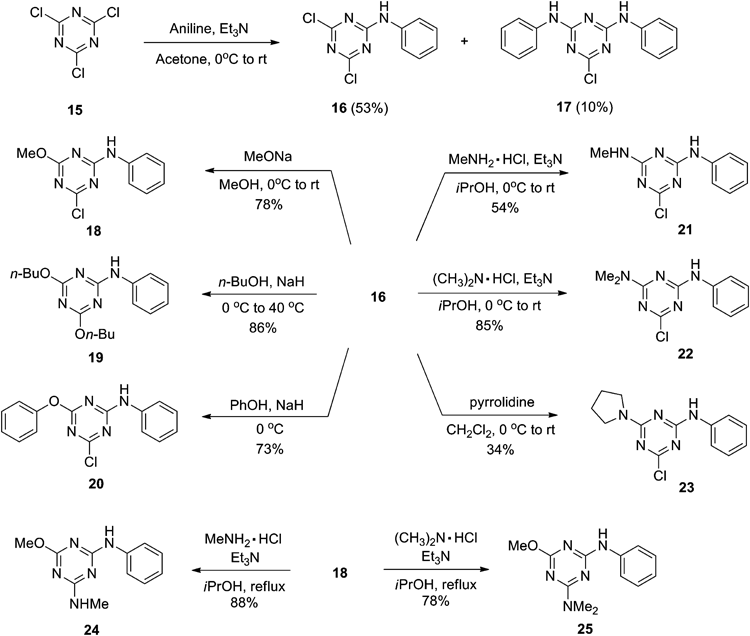
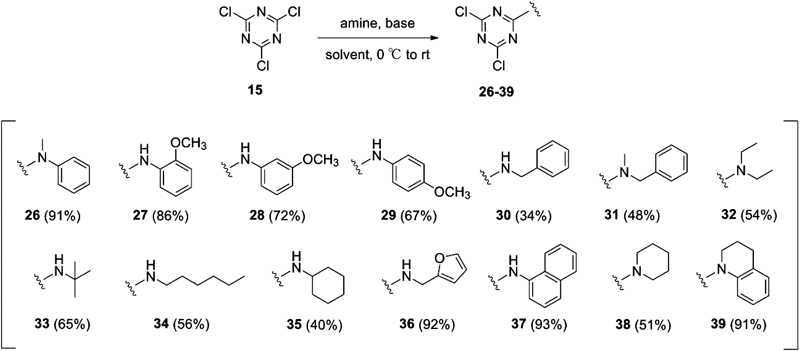
Synthesis of 1,3,5-triazine derivatives is illustrated in Charts 2 and 3. Nucleophilic substitution reaction of 2,4,6-trichloro-1,3,5-triazine (15) by aniline gave mono-substituted compound 16 (NI241) and disubstituted compound 17. Further substitution of 16 with various nucleophiles afforded mono-chlorotriazine derivatives 18–23. Compounds 24 and 25 were prepared from compound 18 by amination using methylammonium chloride or dimethylammonium chloride, respectively (Chart 2). Various 2,4-dichloro-1,3,5-triazine derivatives were synthesized in one step by substitution of 15 using corresponding amines (Chart 3).
The inhibitory activity of synthesized compounds toward DNA binding of NF-κB was evaluated by means of electrophoretic mobility shift assays (EMSA) using Alexa680-labeled NF-κB probe and His-tagged p50 recombinants (Tables 1, 2). All 4-phenylaminopyrimidine derivatives exhibited only weak activities, significantly lower than that of NI241 (Table 1). As for the 2-phenylaminopyrimidines, 4-ethoxy derivative 7 exhibited moderate activity, and p-methoxybenzylamino derivative 11 exhibited weak activity. However, the activity of these compounds was lower than that of NI241. These results suggested that the 1,3,5-triazine substructure is essential for the inhibitory activity of NI241. Therefore, we investigated structural development based on 1,3,5-triazine structure.
| Compound | R1 | R2 | Inhibition (%)a) | |
|---|---|---|---|---|
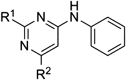 | NI241 | — | — | 40 |
| 2 | Cl | Cl | <5 | |
| 4 | MeO | Cl | <5 | |
| 5 | i-PrO | Oi-Pr | 11 | |
| 9 | NH–CH2C6H4–p-OMe | Cl | 10 | |
| 10 | NH2 | Cl | 7 | |
| 14 | Me | Cl | 12 |
a) Inhibition ratio was determined from the decrease of Alexa680-labeled NF-κB probe at the concentration of 100 µM bound to His-tagged p50 recombinants.
| Compound | R1 | R2 | Inhibition (%)a) | |
|---|---|---|---|---|
 | NI241 | — | — | 40 |
| 3 | Cl | Cl | <5 | |
| 6 | MeO | Cl | <5 | |
| 7 | EtO | Cl | 31 | |
| 8 | i-PrO | Oi-Pr | <5 | |
| 11 | NH–CH2C6H4–p-OMe | Cl | 22 | |
| 12 | NH2 | Cl | 14 |
a) Inhibition ratio was determined from the decrease of Alexa680-labeled NF-κB probe at the concentration of 100 µM bound to His-tagged p50 recombinants.
Table 3 summarizes the inhibitory activity of 2-phenylamino-1,3,5-triazine derivatives. Compounds bearing a bulky substituent, such as n-butoxy (19), phenoxy (20) or pyrrolidinyl (23), exhibited quite low activity. Though methoxy derivative 18 exhibited moderate activity, the lead compound 16 (NI241) exhibited the most potent inhibitory activity among the synthesized compounds. These results suggest that 2,4-dichloro-1,3,5-triazine substructure is the essential pharmacophore for NF-κB inhibitory activity of NI241, and aromatic nucleophilic substitution of NI241 decreased the activity.
| Compound | R1 | R2 | Inhibition (%)a) | |
|---|---|---|---|---|
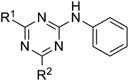 | 16 (NI241) | Cl | Cl | 51 |
| 17 | NHPh | Cl | 15 | |
| 18 | MeO | Cl | 31 | |
| 19 | n-BuO | On-Bu | 5 | |
| 20 | PhO | Cl | 9 | |
| 21 | MeNH | Cl | 14 | |
| 22 | Me2N | Cl | 18 | |
| 23 | Pyrrolidin-1-yl | Oi-Pr | 11 | |
| 24 | MeO | NHMe | <5 | |
| 25 | MeO | NMe2 | 22 |
a) Inhibition ratio was determined from the decrease of Alexa680-labeled NF-κB probe at the concentration of 100 µM bound to His-tagged p50 recombinants.
Thus, we next investigated the inhibitory activity of various 2,4-dichloro-1,3,5-triazine derivatives toward DNA binding of NF-κB. Table 4 summarizes the results of EMSA evaluation. Most of the synthesized compounds exhibited inhibitory activity, supporting the hypothesis that the 2,4-dichloro-1,3,5-triazine substructure is the key pharmacophore for this activity. N-Phenyl derivatives including naphthyl derivative 37 and tetrahydroquinoline derivative 39 exhibited significant activity, whereas N-alkyl derivatives exhibited moderate activity. N-Methylation of secondary amines NI241 and 30, yielding 26 and 31, respectively, increased the inhibitory activity. The results suggested that aromatic and bulky amines are favorable for the inhibitory activity. Finally we examined the IC50 values of the most potent compounds. Table 5 summarized the IC50 values of the selected compounds. m-Methoxy derivative 28 exhibited the most potent inhibitory activity, showing an approximately 50% increase of potency over the lead compound NI241.
| Compound | R1 | R2 | Inhibition (%)a) | |
|---|---|---|---|---|
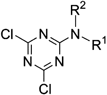 | 16 (NI241) | Ph | H | 51 |
| 26 | Ph | Me | 57 | |
| 27 | o-OMe–C6H4 | H | 52 | |
| 28 | m-OMe–C6H4 | H | 63 | |
| 29 | p-OMe–C6H4 | H | 51 | |
| 30 | Bn | H | 31 | |
| 31 | Bn | Me | 49 | |
| 32 | Et | Et | <5 | |
| 33 | t-Bu | H | 51 | |
| 34 | n-Hex | H | 29 | |
| 35 | c-Hex | H | 7 | |
| 36 | Furfuryl | H | 59 | |
| 37 | 1-Naphthyl | H | 62 | |
| 38 | –(CH2)5– | 47 | ||
| 39 | –C6H4–o-(CH2)3– | 55 | ||
a) Inhibition ratio was determined from the decrease of Alexa680-labeled NF-κB probe at the concentration of 100 µM bound to His-tagged p50 recombinants.
| Compound | 16 (NI241) | 26 | 28 | 29 | 37 |
|---|---|---|---|---|---|
| IC50 (µM) | 97 | 77 | 60 | 97 | 68 |
In our previous report, the binding mode of NI241 to NF-κB was simulated in silico and it was suggested that the N–H hydrogen of the aminophenyl group and two nitrogen atoms of triazine adjacent to the amino group form hydrogen bonds to NF-κB.15) However, the present structure–activity relationship data indicate that the N–H hydrogen is not important, and that the whole 2,4-dichloro-1,3,5-triazine substructure is required for inhibitory activity of NI241. Dichloro-1,3,5-triazine is an electrophilic species that could react with nucleophilic targets in proteins or water molecules in assay media. Thus, we speculate that NI241 and its derivatives function as irreversible inhibitors by reacting with NF-κB protein, or activated by conversion into hydrated form. These are also possible reasons of low biological activity of 2 and 3, the dichloropyrimidine derivatives with lower activity, corresponding to NI241. Further binding simulation studies based on the present results are in progress.
We investigated the SAR of the NF-κB DNA-binding inhibitor NI241. Its 2,4-dichloro-1,3,5-triazine substructure was identified as the essential pharmacophore for this activity. Based on the structure of NI241, we developed the more potent inhibitor 28. Since NF-κB has diverse pathophysiological functions, the SAR information obtained in this study is expected to contribute to the development of novel NF-κB inhibitors as candidates for multiple therapeutic applications.
All reagents were purchased from Sigma-Aldrich Chemicals Co., Tokyo Kasei Co., Wako Pure Chemical Industries, Ltd., and Kanto Chemical Co., Inc. Silica gel column chromatography was purchased from Kanto Chemical Co., Inc. 1H-NMR spectra were recorded at 400 MHz on a Brucker Avance 400 spectrometer or 500 MHz on a Brucker Avance 500 spectrometer. 13C-NMR spectra were recorded on at 125 MHz on a Brucker Avance 500 spectrometer. Chemical shifts are reported as parts per million (ppm) relative to chloroform (7.26 ppm for 1H-NMR and 77.16 ppm for 13C-NMR) or dimethylsulfoxide (DMSO) (2.50 ppm for 1H-NMR and 39.52 ppm for 13C-NMR). Data are reported as follows; chemical shift, multiplicity (s, singlet; d, doublet; t, triplet; q, quartet; quint, quintet; sex, sextet; sep, septet; br, broad; m, multiplet) coupling constants (Hz), integration. Melting points were taken on a Yanagimoto micro melting point apparatus. Mass spectral data were obtained with a JEOL AX-505 spectrometer. Elemental analyses were carried out on a Yanaco MT-6 CHN CORDER.
2,6-Dichloro-4-phenylaminopyrimidine (2) and 4,6-Dichloro-2-phenylaminopyrimidine (3)Aniline (1.0 mL, 10.90 mmol) was added dropwise to 2,4,6-trichloropyrimidine (2.00 g, 10.90 mmol) in tetrahydrofuran (30 mL). The mixture was stirred at room temperature for 3 h, then Et3N (2.27 mL, 16.35 mmol) and aniline (0.50 mL, 5.45 mmol) were further added in one portion. The mixture was stirred at room temperature for 18 h, then poured into water and extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulfate and concentrated. Purification of the residue by silica gel chromatography (eluent: hexane–ethyl acetate 10 : 1 to 4 : 1) gave 2 (70%) and 3 (25%). 2: colorless crystals (dichloromethane–hexane); mp 143.2–144.6°C; 1H-NMR (CDCl3, 400 MHz) δ: 7.45 (td, 2H, J=7.0, 0.6 Hz), 7.30 (tt, 1H, J=7.5, 1.0 Hz), 7.28 (dd, 2H, J=7.4, 1.2 Hz), 7.15 (br s, 1H), 6.57 (s, 1H); 13C-NMR (CDCl3, 125 MHz) δ: 163.3, 161.2, 160.1, 136.2, 129.9, 126.8, 123.8, 100.7. Anal. Calcd for C10H7Cl2N3: C, 50.03; H, 2.94; N, 17.50. Found: C, 50.00; H, 2.96; N, 17.39. 3: colorless needles (hexane); mp 115.1–116.9°C; 1H-NMR (CDCl3, 400 MHz) δ: 7.57 (dd, 2H, J=8.7, 1.1 Hz), 7.36 (td, 2H, J=7.1, 1.0 Hz), 7.23 (br s, 1 H), 7.11 (tt, 1H, J=7.4, 1.1 Hz), 6.79 (s, 1H); 13C-NMR (CDCl3, 125 MHz) δ: 161.8, 158.9, 137.7, 129.1, 124.0, 119.7, 111.2. Anal. Calcd for C10H7Cl2N3: C, 50.03; H, 2.94; N, 17.50. Found: C, 50.16; H, 3.08; N, 17.46.
4-Chloro-2-methoxy-6-phenylaminopyrimidine (4)Sodium methoxide (58.5 mg, 1.08 mmol) was added to 2 (200 mg, 0.83 mmol) in methanol (4.2 mL) at 0°C. The mixture was stirred at room temperature for 4 h. The mixture was stirred at 50°C for 2 h. The contents were poured into saturated aqueous ammonium chloride and extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulfate and concentrated. Purification of the residue by silica gel chromatography (eluent: hexane–ethyl acetate 5 : 1) gave 4 (32%) as colorless needles (dichloromethane–hexane); mp 174.2–176.8°C; 1H-NMR (CDCl3, 400 MHz) δ: 7.40 (td, 2H, J=7.4, 0.9 Hz), 7.32 (dd, 2H, J=8.6, 1.7 Hz), 7.22 (tt, 1H, J=7.3, 1.2 Hz), 6.97 (br s, 1H), 6.35 (s, 1H), 3.96 (s, 3H); 13C-NMR (CDCl3, 125 MHz) δ: 165.2, 163.6, 161.2, 137.4, 129.5, 125.6, 123.2, 96.8, 55.0. Anal. Calcd for C11H10ClN3O: C, 56.06; H, 4.28; N, 17.83. Found: C, 56.07; H, 4.34; N, 17.88.
2,4-Diisopropoxy-6-phenylaminopyrimidine (5)Sodium hydride (60% dispersion in mineral oil, 66.6 mg, 1.67 mmol) in 2-propanol (6 mL) was added to 2 (100 mg, 0.42 mmol) under Ar. The mixture was stirred at room temperature for 1 h and at 100°C for 24 h, then sodium hydride (60% dispersion in mineral oil, 16.66 mg) suspended in 2-propanol (3 mL) was further added in one portion. The resulting mixture was stirred at 100°C for 24 h, then poured into water and extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulfate and concentrated. Purification of the residue by silica gel flash chromatography (eluent: hexane–ethyl acetate 5 : 1) gave 5 (67%) as colorless needles (hexane); mp 86.1–88.8°C; 1H-NMR (CDCl3, 400 MHz) δ: 7.57 (dd, 2H, J=8.7, 1.1 Hz), 7.25 (dd, 2H, J=7.1, 0.8 Hz), 7.12 (tt, 1H, J=7.4, 1.2 Hz) 6.54 (br s, 1H), 5.32 (sep, 1H, J=6.2 Hz), 5.21 (sep, 1H, J=6.3 Hz), 1.38 (d, 6H, J=6.4 Hz), 1.30 (d, 6H, J=6.0 Hz); 13C-NMR (CDCl3, 125 MHz) δ: 171.7, 164.5, 163.6, 138.8, 129.3, 124.3, 122.4, 80.9, 69.5, 68.7, 22.1, 22.0. Anal. Calcd for C15H18N3O2: C, 66.88; H, 7.37; N, 14.62. Found: C, 66.82; H, 7.19; N, 14.72.
4-Chloro-6-methoxy-2-phenylaminopyrimidine (6)Sodium methoxide (58.50 mg, 1.08 mmol) was added to 3 (200 mg, 0.83 mmol) in methanol (4 mL) at 0°C. The mixture was stirred at room temperature for 23 h, then poured into saturated aqueous ammonium chloride and extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulfate, and evaporated. Recrystallization of the residue gave 6 (quant.) as colorless plates (hexane); mp 105.0–105.9°C; 1H-NMR (CDCl3, 400 MHz) δ: 7.59 (dd, 2H, J=8.7, 1.1 Hz), 7.34 (td, 2H, J=7.1, 1.0 Hz), 7.22 (br s, 1 H), 7.07 (tt, 1H, J=7.4, 1.1 Hz), 6.22 (s, 1H), 3.97 (s, 3H); 13C-NMR (CDCl3, 125 MHz) δ: 171.2, 160.6, 159.0, 138.8, 128.9, 123.1, 119.4, 97.9, 54.3. Anal. Calcd for C11H10ClN3O: C, 56.06; H, 4.28; N, 17.83. Found: C, 56.26; H, 4.31; N, 17.82.
4-Chloro-6-ethoxy-2-phenylaminopyrimidine (7)Sodium hydride (60% dispersion in mineral oil) (33.6 mg, 0.84 mmol) in ethanol (6 mL) was added to 3 (70 mg, 0.29 mmol) at 0°C under Ar. The mixture was stirred at room temperature for 5 h, then poured into water and extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulfate and concentrated. Purification of the residue by silica gel chromatography (eluent: hexane–ethyl acetate 5 : 1) gave 7 (quant.) as pale-yellow needles (hexane); mp 109.1–111.2°C; 1H-NMR (CDCl3, 400 MHz) δ: 7.57 (dd, 2H, J=8.8, 1.2 Hz), 7.33 (td, 2H, J=7.2, 2.0 Hz), 7.06 (tt, 1H, J=7.6, 0.8 Hz), 7.08–7.04 (br s, 1H), 6.20 (s, 1H), 4.41 (q, 2H, J=7.2 Hz), 1.40 (t, 3H, J=7.2 Hz); 13C-NMR (CDCl3, 125 MHz) δ: 170.9, 159.6, 158.5, 138.5, 129.0, 123.2, 119.5, 98.2, 63.4, 14.3. Anal. Calcd for C12H12ClN3O: C, 57.72; H, 4.84; N, 16.83. Found: C, 57.65; H, 4.80; N, 16.93.
4,6-Diisopropoxy-2-phenylaminopyrimidine (8)Sodium hydride (60% dispersion in mineral oil, 45.5 mg, 1.14 mmol) in 2-propanol (3 mL) was added to 3 (136.4 mg, 0.57 mmol) at 0°C under Ar. The mixture was stirred at room temperature for 1 h and at 100°C for 24 h, then sodium hydride (60% dispersion in mineral oil, 68.17 mg, 1.70 mmol) suspended in 2-propanol (3 mL) was further added in one portion. The resulting mixture was stirred at 100°C for 24 h, then poured into water and extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulfate and concentrated. Purification of the residue by silica gel flash chromatography (eluent: hexane–ethyl acetate 20 : 1) gave 8 (56%) as a colorless oil; 1H-NMR (CDCl3, 400 MHz) δ: 7.59 (dd, 2H, J=8.8, 1.2 Hz), 7.30 (td, 2H, J=8.0, 1.2 Hz), 7.00 (tt, 1H, J=7.4, 1.2 Hz), 6.83 (br s, 1H), 5.48 (s, 1H), 5.23 (sep, 2H, J=7.4 Hz), 1.35 (d, 12 H J=6.4 Hz); 13C-NMR (CDCl3, 125 MHz) δ: 171.1, 158.8, 139.8, 128.7, 122.0, 118.8, 82.4, 69.0, 22.0.
4-Chloro-2-(4-methoxybenzyl)amino-6-phenylaminopyrimidine (9)4-Methoxybenzylamine (68.6 mg, 0.50 mmol) and Et3N (63.3 mg, 0.63 mmol) were added to 2 (100 mg, 0.42 mmol) in THF (4 mL) at 0°C. The mixture was stirred at room temperature for 18 h and at 50°C for 4 h, then 4-methoxybenzylamine (28.6 mg, 0.21 mmol) and Et3N (21.1 mg, 0.21 mmol) were further added in one portion. The resulting mixture was stirred at 50°C for 3 h, then poured into water and extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulfate and then concentrated. Purification of the residue by silica gel chromatography (eluent: hexane–ethyl acetate 5 : 1) gave 9 (88%). 9: colorless crystals (hexane); mp 143.8–146.3°C; 1H-NMR (CDCl3, 400 MHz) δ: 7.35 (td, 2H, J=7.0, 2.3 Hz), 7.30 (dd, 2H, J=8.6, 1.5 Hz), 7.26 (dd, 2H, J=6.1, 2.0 Hz), 7.15 (tt, 1H, J=7.0, 1.5 Hz), 6.87 (dd, 2H, J=6.6, 2.1 Hz), 6.57 (br s, 1 H), 6.04 (s, 1H), 5.27 (s, 1H), 4.53 (d, 2H, J=5.8 Hz), 3.80 (s, 3H); 13C-NMR (CDCl3, 125 MHz) δ: 162.4, 161.8, 160.4, 158.8, 138.0, 130.9, 129.3, 128.7, 124.7, 122.4, 114.0 93.1, 55.3, 44.9. Anal. Calcd for C18H17ClN4O: C, 63.44; H, 5.03; N, 16.44. Found: C, 63.28; H, 5.03; N, 16.45.
2-Amino-4-chloro-6-phenylaminopyrimidine (10)Trifluoroacetic acid (1.0 mL) was added to a solution of 9 (34.6 mg, 0.10 mmol). The mixture was stirred at room temperature for 25 h, then poured into saturated aqueous sodium hydrogen carbonate and extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulfate and concentrated. Purification of the residue by silica gel chromatography (eluent: hexane–ethyl acetate 2 : 1) gave 10 (quant.) as colorless powder (ethyl acetate–hexane); mp 209.6–210.4°C; 1H-NMR (CDCl3, 400 MHz) δ: 7.38 (td, 2H, J=8.0, 0.8 Hz), 7.30 (dd, 2H, J=8.8, 1.6 Hz), 7.19 (tt, 1H, J=7.4, 1.2 Hz), 6.59 (br s, 1 H), 6.11 (s, 1 H), 4.90 (br s, 2H); 13C-NMR (CDCl3, 125 MHz) δ: 162.8, 162.4, 160.7, 137.6, 129.5, 125.2, 122.9, 93.8. Anal. Calcd for C10H9ClN4: C, 54.43; H, 4.11; N, 25.39. Found: C, 54.38; H, 4.22; N, 25.15.
4-Chloro-6-(4-methoxybenzylamino)-2-phenylaminopyrimidine (11)4-Methoxybenzylamine (57.1 mg, 0.42 mmol) and Et3N (31.6 mg, 0.31 mmol) were added to 3 (50 mg, 0.20 mmol) in THF (2 mL). The mixture was stirred at room temperature for 1 h and at 50°C for 14 h, then poured into water and extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulfate and concentrated. Purification of the residue by silica gel chromatography (eluent: hexane–ethyl acetate 2 : 1) gave 11 (81%) as a colorless oil; 1H-NMR (CDCl3, 400 MHz) δ: 7.53 (dd, 2H, J=8.4, 0.8 Hz), 7.27 (td, 2H, J=7.2, 2.0 Hz), 7.24 (dd, 2H, J=6.8, 2.0 Hz), 7.00 (tt, 1H, J=8.0, 1.2 Hz), 7.02–6.98 (br s, 1H), 6.89 (dd, 2H, J=6.8, 2.0 Hz), 5.88 (s, 1H), 5.18 (br s, 1H), 4.46 (br s, 2H), 3.80 (s, 3 H); 13C-NMR (CDCl3, 125 MHz) δ: 163.7, 159.2, 139.2, 128.8, 112.5, 119.3, 114.2, 55.3, 53.4, 45.2, 45.1.
4-Amino-6-chloro-2-phenylaminopyrimidine (12)Trifluoroacetic acid (2.0 mL) was added to a solution of 11 (92.0 mg, 0.42 mmol). The mixture was stirred at room temperature for 17 h, then poured into saturated aqueous sodium hydrogen carbonate and extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulfate and concentrated. Purification of the residue by silica gel chromatography (eluent: hexane–ethyl acetate 1 : 1) gave 2–13 (quant.) as colorless plates (dichloromethane–hexane); mp 139.2–141.0°C; 1H-NMR (CDCl3, 400 MHz) δ: 7.55 (dd, 2H, J=8.8, 1.2 Hz), 7.31 (td, 2H, J=7.6, 2.0 Hz), 7.04 (tt, 1H, J=7.2, 1.2 Hz), 6.91 (br s, 1H), 5.97 (s, 1H), 4.81 (br s, 2H); 13C-NMR (CDCl3, 125 MHz) δ: 164.3, 160.0, 159.6, 139.0, 128.8, 122.8, 119.6, 95.0. Anal. Calcd for C10H9ClN4: C, 54.43; H, 4.11; N, 25.39. Found: C, 54.29; H, 4.26; N, 25.52.
4-Chloro-2-methyl-6-phenylaminopyrimidine (14)Aniline (560 µL, 6.13 mmol) was added dropwise to 4,6-dichloro-2-methylpyrimidine (1.0 g, 6.13 mmol) in ethanol (30 mL) at 0°C. The mixture was stirred at room temperature for 19 h, then poured into water and extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulfate and concentrated. Purification of the residue by silica gel flash chromatography (eluent: hexane–ethyl acetate 5 : 1) gave 14 (53%) as colorless needles (hexane); mp 109.1–111.2°C; 1H-NMR (CDCl3, 400 MHz) δ: 7.42 (td, 2H, J=7.6, 0.8 Hz), 7.24 (dd, 2H, J=8.8, 1.2 Hz), 7.24 (tt, 1H, J=7.6, 0.8 Hz), 6.87 (br s, 1H), 6.54 (s, 1H), 2.54 (s, 3H); 13C-NMR (CDCl3, 125 MHz) δ: 167.43, 161.1, 160.9, 136.5, 129.9, 129.6, 129.2, 126.3, 123.4, 99.9, 25.2. Anal. Calcd for C11H10ClN3: C, 60.14; H, 4.59; N, 19.13. Found: C, 60.36; H, 4.79; N, 19.15.
2,4-Dichloro-6-phenylamino-1,3,5-triazine (16) and 2-Chloro-4,6-bis(phenylamino)-1,3,5-triazine (17)Aniline (0.98 µL, 10.85 mmol) was added dropwise to cyanuric chloride (2.0 g, 10.85 mmol) in acetone (60 mL) at 0°C. Et3N (1.5 mL, 10.85 mmol) was added in one portion. The mixture was stirred at room temperature for 7 h, then poured into water and extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulfate and concentrated. Purification of the residue by silica gel chromatography (eluent: hexane–ethyl acetate 8 : 1 to 6 : 1) gave 16 (53%) and 17 (10%). 16: colorless crystals (ethyl acetate–hexane); mp 134.1–140.6°C; 1H-NMR (CDCl3, 400 MHz) δ: 7.54 (dd, 2H, J=8.8, 1.2 Hz), 7.49 (br s, 1H), 7.42 (td, 2H, J=7.2, 5.6 Hz), 7.23 (tt, 1H, J=7.6, 1.2 Hz). 13C-NMR (CDCl3, 125 MHz) δ: 171.4, 170.2, 164.1, 135.7, 129.3, 125.9, 121.2. Anal. Calcd for C9H6Cl2N4: C, 44.84; H, 3.51; N, 23.24. Found: C, 45.13; H, 2.69; N, 23.43. 17: colorless crystals (ethyl acetate–hexane); mp 201.8–204.0°C; 1H-NMR (CDCl3, 400 MHz) δ: 7.54 (d, 4H, J=8.0 Hz), 7.36 (t, 4H, J=7.6 Hz), 7.29 (br s, 2H), 7.16 (t, 2H, J=7.4 Hz); 13C-NMR (CDCl3, 125 MHz) δ: 168.6, 164.0, 137.0, 129.1, 121.4, 121.4. Anal. Calcd for C15H12ClN5: C, 60.51; H, 4.06; N, 23.52. Found: C, 60.75; H, 4.18; N, 23.65.
2-Chloro-4-methoxy-6-phenylamino-1,3,5-triazine (18)Sodium methoxide (22.1 mg, 0.41 mmol) was added to 16 (100 mg, 0.41 mmol) in methanol (2.0 mL) at 0°C. The mixture was stirred at room temperature for 18 h. Sodium methoxide (11.1 mg, 0.21 mmol) was further added in one portion and stirring was continued at room temperature for 3 h, then the mixture was poured into water and extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulfate and concentrated. Purification of the residue by silica gel chromatography (eluent: hexane–ethyl acetate 7 : 1) gave 18 (78%) as colorless needles (ethyl acetate–hexane); mp 174.2–176.8°C; 1H-NMR (CDCl3, 400 MHz) δ: 7.56 (dd, 2H, J=8.8, 1.2 Hz), 7.38 (td, 2H, J=7.6, 2.0 Hz), 7.41–7.36 (br s, 1H), 7.17 (tt, 1H, J=7.6, 0.8 Hz), 4.04 (s, 3H); 13C-NMR (CDCl3, 125 MHz) δ: 171.6, 170.7, 165.2, 136.7, 129.2, 124.9, 120.8, 55.8. Anal. Calcd for C10H9ClN4O: C, 50.75; H, 3.83; N, 23.67. Found: C, 50.74; H, 3.94; N, 23.88.
2,4-Di-n-butoxy-6-phenylamino-1,3,5-triazine (19)Sodium hydride (60% dispersion in mineral oil, 18.0 mg, 0.45 mmol) in 1-butanol (2 mL) was added to 16 (101 mg, 0.42 mmol) at 0°C under Ar. The mixture was stirred at room temperature for 1 h and at 40°C for 1 h, then sodium hydride (60% dispersion in mineral oil, 33.4 mg, 0.84 mmol) suspended in 1-butanol (2 mL) was further added in one portion. The resulting mixture was stirred at 40°C for 4 h, then poured into water and extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulfate and concentrated. Purification of the residue by silica gel chromatography (eluent: hexane–ethyl acetate 10 : 1) gave 19 (86%) as colorless plates (hexane); mp 58.4–59.4°C; 1H-NMR (CDCl3, 400 MHz) δ: 7.58 (dd, 2H, J=8.8, 1.2 Hz), 7.34 (td, 2H, J=7.2, 1.2 Hz), 7.10 (tt, 1H, J=7.4, 1.6 Hz), 7.12–7.08 (br s, 1H), 4.38 (t, 4H), 1.78 (quint, 4H, J=7.2 Hz), 1.48 (sex, 4H, J=7.4 Hz), 0.96 (t, 4H, J=7.4 Hz); 13C-NMR (CDCl3, 125 MHz) δ: 172.0, 166.32, 137.9, 128.9, 123.8, 120.4, 67.7, 30.7, 19.1, 13.8. Anal. Calcd for C17H24N4O2: C, 64.53; H, 7.65; N, 17.71. Found: C, 64.45; H, 7.37; N, 17.80.
2-Chloro-4-phenylamino-6-phenoxy-1,3,5-triazine (20)Sodium hydride (60% dispersion in mineral oil, 20.1 mg, 0.49 mmol) suspended in N,N-dimethylformamide (DMF) (1 mL) was added to phenol (67.3 mg, 0.72 mmol) and 16 (101 mg, 0.42 mmol) in DMF at 0°C under Ar. The mixture was stirred at 0°C for 2 h, then poured into water and extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulfate and concentrated. Purification of the residue by silica gel chromatography (eluent: hexane–ethyl acetate 2 : 1) gave 20 (73%) as colorless crystals (hexane); mp 137.1–138.8°C; 1H-NMR (DMSO-d6, 373 K, 400 MHz) δ: 10.36 (br s, 1H) 7.50–7.45 (m, 4H), 7.32 (tt, 1H, J=7.4, 1.2 Hz), 7.28 (dd, 2H, J=9.2, 1.6 Hz), 7.23 (t, 1H, J=7.6 Hz), 7.07 (t, 2H, J=7.4 Hz); Anal. Calcd for C15H11ClN4O: C, 60.31; H, 3.71; N, 18.76. Found: C, 60.53; H, 3.85; N, 18.87.
2-Chloro-4-methylamino-6-phenylamino-1,3,5-triazine (21)Methylammonium chloride (70 mg, 0.49 mmol) and Et3N (70 µL, 0.50 mmol) were added to 16 (101 mg, 0.42 mmol) in 2-propanol (2 mL) at 0°C. The mixture was stirred at room temperature for 4 h, and then methylammonium chloride (4 mg, 0.06 mmol) was further added in one portion. The resulting mixture was stirred at room temperature for 1 h, then poured into water and extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulfate and concentrated. Purification of the residue by silica gel chromatography (eluent: hexane–ethyl acetate 2 : 1) gave 21 (53%) as a colorless powder (ethyl acetate); mp 243.9–245.3°C; 1H-NMR (DMSO-d6, 373 K, 400 MHz) δ: 9.57 (br s, 1H), 7.69 (d, 2H, J=8.0 Hz), 7.60 (br s, 1H), 7.29 (t, 2H, J=8.0 Hz), 7.02 (t, 1H, J=7.2 Hz), 2.84 (s, 3H); 13C-NMR (CDCl3, 125 MHz) δ: 168.9, 168.1, 166.2, 164.1, 163.6, 139.4, 129.1, 123.3, 120.5, 17.9, 27.8. Anal. Calcd for C10H10ClN5: C, 50.96; H, 4.28; N, 29.72. Found: C, 51.17; H, 4.45; N, 29.74.
2-Chloro-4-dimethylamino-6-phenylamino-1,3,5-triazine (22)Dimethylammonium chloride (33.0 mg, 0.40 mmol) and Et3N (57 µL, 0.41 mmol) were added to 16 (100.2 mg, 0.41 mmol) in 2-propanol (2 mL) at 0°C. The mixture was stirred at room temperature for 4 h, then poured into water and extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulfate and concentrated. Purification of the residue by silica gel chromatography (eluent: hexane–ethyl acetate 10 : 1) gave 22 (85%) as colorless needles (ethyl acetate–hexane); mp 162.2–163.4°C; 1H-NMR (CDCl3, 400 MHz) δ: 7.57 (dd, 2H, J=8.8, 1.2 Hz), 7.34 (td, 2H, J=7.2, 2.0 Hz), 7.09 (tt, 1H, J=7.4, 1.2 Hz), 7.00 (br s, 1H), 3.20 (s, 6H); 13C-NMR (CDCl3, 125 MHz) δ: 169.0, 165.2, 163.4, 138.0, 128.9, 123.7, 120.1, 36.8, 36.7. Anal. Calcd for C11H12ClN5: C, 52.91; H, 4.82; N, 28.05. Found: C, 52.80; H, 4.99; N, 28.10.
2-Chloro-4-phenylamino-6-(pyrrolidin-1-yl)-1,3,5-triazine (23)Pyrrolidine (45 µL, 0.54 mmol) was added to a solution of 16 (101 mg, 0.42 mmol) in dichloromethane (2 mL) at 0°C. The mixture was stirred at room temperature for 15 min, then poured into water and extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulfate and concentrated. Purification of the residue by silica gel chromatography (eluent: hexane–ethyl acetate 7 : 1) gave 23 (34%) as colorless needles (ethyl acetate–hexane); mp 191.0–192.0°C; 1H-NMR (CDCl3, 400 MHz) δ: 7.40 (td, 2H, J=7.4, 0.9 Hz), 7.33 (dd, 2H, J=7.2, 2.0 Hz), 7.08 (tt, 1H, J=7.6, 1.2 Hz), 7.00 (br s, 1H), 3.63 (t, 4H, J=6.8 Hz), 1.99 (tt, 4H, J=3.4, 3.2 Hz); 13C-NMR (CDCl3, 125 MHz) δ: 168.7, 163.2, 1629, 138.2, 128.9, 123.5, 119.8, 46.8, 46.6, 25.2, 25.0. Anal. Calcd for C11H12ClN5: C, 56.63; H, 5.12; N, 25.40. Found: C, 56.62; H, 5.17; N, 25.43.
2-Methylamino-4-methoxy-6-phenylamino-1,3,5-triazine (24)Methylammonium chloride (36.1 mg, 0.42 mmol) and Et3N (70 µL, 0.50 mmol) were added to 18 (99.9 mg, 0.42 mmol) in methanol (2 mL) at 0°C. The mixture was stirred at room temperature for 2 h and at 40°C for 4 h, then methylammonium chloride (34.5 mg, 0.51 mmol) and Et3N (70 µL, 0.50 mmol) were further added. The resulting mixture was refluxed for 1 h, then poured into water and extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulfate and concentrated. Purification of the residue by silica gel chromatography (eluent: hexane–ethyl acetate 5 : 1) gave 24 (88%) as colorless crystals (ethyl acetate–hexane); mp 163.1–164.5°C; 1H-NMR (DMSO-d6, 373 K, 400 MHz) δ: 9.06 (br s, 1H), 7.73 (d, 2H, J=8.0 Hz), 7.25 (t, 2H, J=7.8 Hz), 7.01 (br s, 1H), 6.95 (t, 1H, J=7.4 Hz), 3.83 (s, 3H), 2.84 (d, 3 H J=4.8 Hz); Anal. Calcd for C11H13N5O: C, 57.13; H, 5.67; N, 30.28. Found: C, 57.31; H, 5.65; N, 30.28.
2-Dimethylamino-4-methoxy-6-phenylamino-1,3,5-triazine (25)Dimethylammonium chloride (69.0 mg, 0.85 mmol) and Et3N (120 µL, 0.80 mmol) were added to a solution of 18 (101 mg, 0.43 mmol) in methanol (2 mL) at 0°C. The mixture was stirred at room temperature for 2 h and at 50°C for 2 h, then methylammonium chloride (38.2 mg, 0.47 mmol) and Et3N (60.0 µL 0.43 mmol) were added in one portion. The resulting mixture was refluxed for 1 h, then poured into water and extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulfate and concentrated. Purification of the residue by silica gel chromatography (eluent: hexane–ethyl acetate 5 : 1) gave 25 (78%) as colorless needles (ethyl acetate–hexane); mp 163.1–164.5°C; 1H-NMR (CDCl3, 400 MHz) δ: 7.61 (dd, 2H, J=8.8, 1.2 Hz), 7.32 (td, 2H, J=7.2, 2.0 Hz), 7.04 (tt, 1H, J=7.2, 1.2 Hz), 6.85 (br s, 1H), 3.94 (s, 3H), 3.19 (s, 6H). 13C-NMR (CDCl3, 125 MHz) δ: 170.9, 166.6, 165.0, 139.0, 128.8, 122.8, 119.8, 54.0, 36.6, 36.3. Anal. Calcd for C12H15N5O: C, 58.76; H, 6.16; N, 28.55. Found: C, 58.90; H, 6.04; N, 28.66.
2,4-Dichloro-6-methyl(phenyl)amino-1,3,5-triazine (26)N-Methylaniline (58 µL, 0.54 mmol) and Et3N (75 µL, 0.54 mmol) were added dropwise to a solution of cyanuric chloride (101 mg, 0.55 mmol) in acetone (2 mL) at 0°C under Ar. The mixture was stirred at room temperature for 2 h, and then poured into water. The precipitate was collected by filtration, washed with water, dried, and recrystallized to gave 26 (91%) as colorless prisms (CH2Cl2–hexane); mp 134.0–132.7°C; 1H-NMR (CDCl3, 400 MHz) δ: 7.46 (td, 2H, J=7.8, 2.0 Hz), 7.36 (tt, 1H, J=7.4, 2.0 Hz), 7.24 (dd, 2H, J=7.2, 1.2 Hz), 3.55 (s, 3H); 13C-NMR (CDCl3, 125 MHz) δ: 170.3, 170.0, 165.1, 142.0, 129.5, 127.8, 126.1, 39.2. Anal. Calcd for C10H8Cl2N4: C, 47.12; H, 3.33; N, 21.85. Found: C, 47.08; H, 3.16; N, 21.96.
General Procedure for the Synthesis of Compounds 27–29Anisidine (1 eq) was added dropwise to a solution of cyanuric chloride (1 eq) in acetone (2 mL) at 0°C. The mixture was stirred at room temperature for 2 h. After the completion of the reaction, the mixture was evaporated. The crude product was purified by silica gel chromatography (eluent: hexane–ethyl acetate 5 : 1) and then recrystallized.
2,4-Dichloro-6-(2-methoxyphenyl)amino-1,3,5-triazine (27): Yield: 86%; pale-yellow plates (ethyl acetate–hexane); mp 179.4–181.2°C; 1H-NMR (CDCl3, 400 MHz) δ: 8.31 (dd, 1H, J=8.0, 1.6 Hz), 8.20 (br s, 1H), 7.15 (td, 1H, J=7.8, 1.2 Hz), 7.04 (td, 1H, J=7.8, 1.2 Hz), 6.94 (dd, 1H, J=8.0, 1.2 Hz), 3.91 (s, 3H); 13C-NMR (CDCl3, 125 MHz) δ: 171.4, 170.1, 148.9, 125.7, 125.5, 121.2, 120.9, 110.5, 56.0. Anal. Calcd for C10H8Cl2N4O: C, 44.30; H, 2.97; N, 20.67. Found: C, 44.24; H, 3.09; N, 20.71.
2,4-Dichloro-6-(3-methoxyphenyl)amino-1,3,5-triazine (28): Yield: 72%; colorless needles (dichloromethane–hexane); mp 116.7–117.8°C; 1H-NMR (CDCl3, 400 MHz) δ: 7.50 (br s, 1H), 7.30 (t, 1H, J=8.2 Hz), 7.26 (t, 1H, J=2.4 Hz), 7.05 (ddd, 1H, J=8.0, 2.0, 0.8 Hz), 6.77 (ddd, 1H, J=8.4, 2.4. 0.8 Hz), 3.84 (s, 3H); 13C-NMR (CDCl3, 125 MHz) δ: 171.5, 170.4, 164.1, 160.4, 137.0, 130.2, 113.4, 111.3, 107.4, 55.6. Anal. Calcd for C10H8Cl2N4O: C, 44.30; H, 2.97; N, 20.67. Found: C, 44.68; H, 3.25; N, 20.32.
2,4-Dichloro-6-(4-methoxyphenyl)amino-1,3,5-triazine (29): Yield: 67%; pale-yellow plates (ethyl acetate–hexane); mp 170.0–173.4°C; 1H-NMR (CDCl3, 400 MHz) δ: 7.43–7.40 (br s, 1H), 7.41 (dd, 2H, J=6.8, 2.4 Hz), 6.93 (dd, 2H, J=6.8, 2.4 Hz), 3.82 (s, 3H); 13C-NMR (CDCl3, 125 MHz) δ: 171.5, 170.2, 164.4, 128.5, 123.6, 114.6, 55.7. Anal. Calcd for C10H8Cl2N4O: C, 44.30; H, 2.97; N, 20.67. Found: C, 47.43; H, 3.26; N, 20.66.
General Procedure for the Synthesis of Compounds 30–35 and 38Amine (1 eq) was added dropwise to cyanuric chloride (1.2 eq) in acetone at 0°C. The mixture was stirred at room temperature for 1 h then poured into water and extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulfate and concentrated. The crude product was purified by silica gel chromatography (eluent: hexane–ethyl acetate) and then recrystallized.
2,4-Dichloro-6-benzylamino-1,3,5-triazine (30): Yield: 34%; colorless needles (dichloromethane–hexane); mp 120.1–121.3°C; 1H-NMR (CDCl3, 400 MHz) δ: 7.40 (m, 5H), 6.15 (br s, 1H), 4.68 (d, 2H, J=6.0 Hz); 13C-NMR (CDCl3, 125 MHz) δ: 171.3, 170.3, 166.0, 136.3, 129.2, 128.4, 127.9, 45.6. Anal. Calcd for C10H8Cl2N4: C, 47.08; H, 3.16; N, 21.96. Found: C, 47.05; H, 3.22; N, 21.99.
2,4-Dichloro-6-benzyl(methyl)amino-1,3,5-triazine (31): Yield: 48%; colorless needles (hexane); mp 99.4–101.6°C; 1H-NMR (CDCl3, 400 MHz) δ: 7.38–7.30 (m, 3H), 7.26–7.23 (m, 2H), 4.87 (s, 2H), 3.15 (s, 3H); 13C-NMR (CDCl3, 125 MHz) δ: 170.5, 170.2, 165.3, 135.5, 129.1, 128.3, 128.0, 52.8, 35.0. Anal. Calcd for C11H10Cl2N4: C, 49.09; H, 3.75; N, 20.82. Found: C, 49.19; H, 3.64; N, 20.92.
2,4-Dichloro-6-diethylamino-1,3,5-triazine (32): Yield: 54%; colorless crystals (hexane); mp 78.0–78.8°C; 1H-NMR (CDCl3, 400 MHz) δ: 3.63 (q, 4H, J=7.2 Hz), 1.22 (t, 6H, J=7.2 Hz); 13C-NMR (CDCl3, 125 MHz) δ: 169.0, 163.0, 41.6, 11.7. Anal. Calcd for C7H10Cl2N4: C, 38.03; H, 4.56; N, 25.34. Found: C, 38.30; H, 4.56; N, 25.52.
2,4-Dichloro-6-t-butylamino-1,3,5-triazine (33): Yield: 65%; colorless plates (hexane); mp 129.4–132.0°C; 1H-NMR (CDCl3, 400 MHz) δ: 5.78 (br s, 1H), 1.46 (s, 9H).
2,4-Dichloro-6-n-hexylamino-1,3,5-triazine (34): Yield: 56%; colorless needles (hexane); mp 54.8–55.7°C; 1H-NMR (CDCl3, 400 MHz) δ: 3.47 (q, 2H, J=6.8 Hz), 1.60 (quint, 2H, J=7.2 Hz), 1.40–1.26 (m, 6H), 0.90 (t, 3H, J=6.8 Hz).
2,4-Dichloro-6-cyclohexylamino-1,3,5-triazine (35): Yield: 40%; colorless oil; 1H-NMR (CDCl3, 400 MHz) δ: 5.76 (br s, 1H), 3.93–3.88 (m, 1H), 2.01–1.96 (m, 2H), 1.77–1.70 (m, 2H), 1.67–1.61 (m, 1H), 1.47–1.36 (m, 2H), 1.29–1.20 (m, 3H); 13C-NMR (CDCl3, 125 MHz) δ: 171.1, 170.0, 165.1, 50.3, 32.6, 25.4, 24.5. Anal. Calcd for C9H12Cl2N4: C, 47.08; H, 3.16; N, 21.96. Found: C, 47.05; H, 3.22; N, 21.99.
2,4-Dichloro-6-(piperidin-1-yl)-1,3,5-triazine (38): Yield: 40%; colorless plates (hexane); mp 88.9–89.4°C; 1H-NMR (CDCl3, 400 MHz) δ: 3.81 (t, 4H, J=5.6 Hz), 1.75–1.69 (m, 2H), 1.67–1.61 (m, 4H); 13C-NMR (CDCl3, 125 MHz) δ: 170.3, 163.7, 45.5, 25.8, 24.4. Anal. Calcd for C8H10Cl2N4: C, 41.22; H, 4.32; N, 23.98. Found: C, 41.19; H, 4.68; N, 24.04.
General Procedure for the Synthesis of Compounds 36, 37 and 39Amine (1 eq) in tetrahydrofuran was added dropwise to cyanuric chloride (1.2 eq) and K2CO3 (1 eq) in tetrahydrofuran at 0°C. The mixture was stirred at room temperature for 3–4 h then poured into water and extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulfate and concentrated. The crude product was purified by silica gel chromatography (eluent: hexane–ethyl acetate) and then recrystallized.
2,4-Dichloro-6-(furfurylamino)-1,3,5-triazine (36): Yield: 92%; colorless needles (hexane–ethyl acetate); mp 104.7–105.4°C; 1H-NMR (CDCl3, 400 MHz) δ: 7.38 (dd, 1H, J=1.6, 0.4 Hz), 6.35 (dd, 1H, J=3.2, 2.0 Hz), 6.32 (dd, 1H, J=3.2, 0.8 Hz), 6.20 (br s, 1H), 4.67 (d, 2H, J=6.0 Hz); 13C-NMR (CDCl3, 125 MHz) δ: 171.2, 170.3, 165.8, 149.3, 143.0, 110.1, 108.7, 38.5. Anal. Calcd for C8H6Cl2N4O: C, 39.21; H, 2.47; N, 22.86. Found: C, 39.28; H, 2.49; N, 22.98.
2,4-Dichloro-6-(1-naphthylamino)-1,3,5-triazine (37): Yield: 93%; colorless needles (hexane–ethyl acetate); mp 156.7–157.8°C; 1H-NMR (CDCl3, 400 MHz) δ: 7.92–7.80 (m, 5H), 7.60–7.52 (m, 3H); 13C-NMR (CDCl3, 125 MHz) δ: 171.8, 170.7, 165.9, 134.4, 130.5, 129.0, 128.2, 128.0, 127.3, 126.7, 125.7, 122.9, 120.9. Anal. Calcd for C13H8Cl2N4: C, 53.63; H, 2.77; N, 19.24. Found: C, 53.58; H, 2.98; N, 19.26.
2,4-Dichloro-6-(1,2,3,4-tetrahydroquinolin-1-yl)-1,3,5-triazine (39): Yield: 93%; orange needles (hexane–ethyl acetate); mp 159.2–160.6°C; 1H-NMR (CDCl3, 400 MHz) δ: 7.72 (d, 1H, J=8.0 Hz), 7.27–7.16 (m, 3H),4.07 (t, 2H, J=6.4 Hz), 2.83 (t, 2H, J=6.8 Hz), 2.05 (quint, 2H, J=6.5 Hz); 13C-NMR (CDCl3, 125 MHz) δ: 170.5, 170.4, 164.1, 136.7, 132.4, 129.0, 126.2, 126.0, 125.1, 45.7, 26.8, 23.6. Anal. Calcd for C12H10Cl2N4: C, 51.27; H, 5.59; N, 19.93. Found: C, 51.38; H, 3.60; N, 20.08.
Electrophoretic Mobility Shift Assay (EMSA)Alexa680-labeled NF-κB probe was prepared by annealing with Alexa680-labeled single-stranded oligonucleotide and unlabeled complementary single-stranded oligonucleotide: 5′-Alexa680-AGTTGAGGGGACTTTCCCAGGC-3′ (sense) and 5′-GCCTGGGAAAGTCCCCTCAACT-3′ (antisense). The underlines show the sequence of the κB site. His-tagged NF-κB p50 recombinants were produced in E. coli and purified with HisTrap HP (GE Healthcare). Reaction mixtures containing binding buffer (15 mM Tris–HCl (pH 7.5), 75 mM NaCl, 1.5 mM ethylenediamine tetraacetic acid (EDTA), 1.5 mM dithiothreitol, 7.52% glycerol, and 0.3% Nonidet P-40), 0.5 µg of poly(dI-dC)·(dI-dC) and 62.5 ng of His-tagged p50 recombinants were left to stand on ice for 10 min, and then the candidate inhibitors were added and the mixtures were incubated at room temperature. After 30 min, 20 nM Alexa680 labeled NF-κB probe was added and incubation was continued at room temperature for 30 min. The samples in a volume of 10 µL were loaded on native 5% polyacrylamide gel prepared in 0.5×TBE and electrophoresed at 120 CV for 90 min. The gels were scanned and analyzed with an Odyssey Infrared Imaging System (LI-COR).