2015 Volume 63 Issue 7 Pages 485-488
2015 Volume 63 Issue 7 Pages 485-488
A new type of O-alkylation of 2-hydroxy-1,4-naphthoquinone with alkoxymethyl chlorides is described. The reaction course can be controlled by the choice of base and yields O-alkylated or O-alkoxymethylated products in high yield with high selectivity.
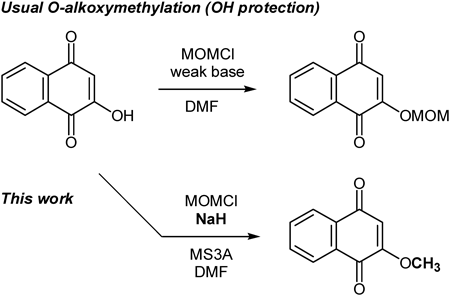
1,4-Naphthoquinone is one of the common skeletons found in a various biologically active compounds.1–7) Recently, our research interests have focused on the total synthesis of naturally occurring naphthoquinones, such as rinacanthin A,8) dehydroiso-β-lapachone9) and lantalucratins10) having biological activities including anti-tumor activity, in order to confirm the chemical structure and determine the absolute configuration. In the course of our synthetic study, a key intermediate (I) was prepared from 1,4-dimethoxynaphthalene, which is relatively expensive as a commercial source, by the introduction of a hydroxyl group using directed ortho lithiation-substitution reaction11) and the following hydroxyl protection by methoxymethyl group (Chart 1).

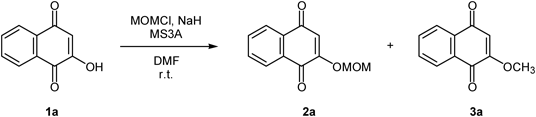
In order to utilize intermediate (I) for the syntheses of naphthoquinones containing various natural products at a practical level, a more efficient synthetic method had to be developed and established. This led us to try to develop a concise synthesis of the intermediate (I). Initial entry was started from ordinary hydroxyl protection of 2-hydroxy-1,4-naphthoquinone (1a) using chloromethyl methyl ether (MOMCl) in basic condition. As a result, an unusual side reaction was observed, namely O-methylation proceeded along with the normal methoxymethylation of the hydroxyl group (Chart 2). This result prompted us to study the generality and limitation of this unusual alkylation reaction. Here we describe our findings related to the changes in the ratio and yield of the product due to differences in the reaction conditions, and briefly consider the reaction pathway.
In the initial approach directed toward understanding the unusual alkylation, the reaction conditions were examined as summarized in Table 1.12) First, 1a was treated with sodium hydride (NaH) and MOMCl in N,N-dimethylformamide (DMF) at r.t., which is a conventional O-methoxymethylation method, and 3a was obtained in moderate yield (Table 1, entry 1). Unfortunately, the data obtained under this condition are not available for the discussion of the mechanism because of the poor reproducibility of the reaction. Various reaction conditions were examined to solve this problem, we found that the amount of water strongly affected the ratio of the product. Namely, reproducibility was dramatically improved by moisture control of the reaction system using molecular sieves 3A.13)
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Base (eq) | MOMCl (eq) | DMF (mol/L) | Time (h) | Product | |
| 2a (%) | 3a (%) | |||||
| 1b) | NaH (2.0) | 3.0 | 0.2 | 0.25 | 34 | 59 |
| 2 | NaH (2.0) | 3.0 | 0.2 | 1 | 29 | 61 |
| 3 | NaH (1.0) | 1.0 | 0.2 | 1 | 85 | 0 |
| 4 | NaH (2.0) | 2.0 | 0.2 | 1 | 14 | 83 |
| 5 | NaH (3.0) | 3.0 | 0.2 | 1 | 0 | 47 |
| 6 | NaH (3.0) | 2.0 | 0.2 | 1 | 0 | 31 |
| 7 | NaH (2.0) | 2.0 | 1.0 | 1 | 5 | 73 |
| 8 | NaH (2.0) | 2.0 | 0.05 | 1 | 13 | 69 |
| 9 | NaH (2.0) | 2.0 | 1.0 | 5 | Trace | 63 |
| 10c) | NaH (2.0) | 3.0 | 0.2 | 0.25 | 76 | 10 |
| 11 | LiH (2.0) | 2.0 | 1.0 | 1 | 82 | 0 |
| 12 | KH (2.0) | 2.0 | 1.0 | 1 | 20 | 40 |
| 13 | K2CO3 (2.0) | 2.0 | 1.0 | 1 | 75 | 0 |
| 14 | iPr2NEt (2.0) | 2.0 | 1.0 | 1 | 90 | 0 |
| 15 | KOtBu (2.0) | 2.0 | 1.0 | 1 | 50 | 10 |
| 16 | NaOMe (2.0) | 2.0 | 1.0 | 2 | 64 | 0 |
| 17d) | NaOH (2.0) | 2.0 | 1.0 | 1 | Trace | 0 |
a) The reaction was carried out using flame-dried round bottom flask and filled with argon gas atmosphere. 1a (2 mmol) and activated MS3A (500 mg) was used, and stirred at room temperature. b) The reaction was conducted without using MS3A. c) The reaction was carried out with addition of water (1 drop). d) 1a was almost recovered.
The amount of reagent and concentration of solution affected the products. The product ratio of 3a increased in accordance with the use of a larger amount of base and MOMCl, although excess use of these reagents caused a decrease in the 3a yield (Table 1, entries 3–5). Under the slight basic conditions, namely, the reaction using excess amount of base over MOMCl resulted in a great decrease of 3a (Table 1, entry 6). Remarkably, the selectivity was completely regulated by using one or three equivalents of the reagents against 1a. These results strongly suggested that the rate of the formation of 2a was faster than that of 3a, and 3a was produced via the formation of 2a. Under higher concentration, good selectivity of 3a was observed (Table 1, entries 4, 7 and 8). Prolonged reaction time resulted in a slight decrease of 3a (Table 1, entries 7 vs. 9).
In order to clarify the moisture effect in this O-alkylation, the reaction was performed under wet condition (Table 1, entry 10). As expected, the product ratio of 2a/3a was dramatically changed, 2a was obtained as a major product. Various types of bases were examined, and the results indicated that the base must be of the appropriate basicity and molecular size to obtain the methyl ether 3a. In case of using alkoxide which can also act as nucleophile, 3a was not obtained at all (Table 1, entry 16). Using weaker base or hydride base, including a smaller size of countercation, afforded the methoxymethyl ether 2a in good to excellent yield with complete selectivity (Table 1, entries 11, 13, 14). In addition, it became clear that there were no significant impurities such as methanol in MOMCl confirmed by 1H-NMR analysis.
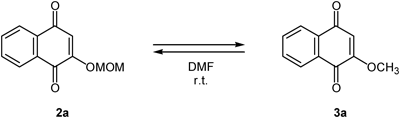
In order to certify that methyl ether 3a was actually produced via 2a, isolated 2a or 3a was treated with the NaH/MOMCl reagent system (Table 2). As expected, 3a was generated from 2a, and this molecular conversion required both NaH and MOMCl (Table 2, entries 1–4). The reverse reaction from 3a into 2a did not proceed under anhydrous condition (Table 2, entry 7). Interestingly, the transformation of 3a to 2a occurred in the presence of water, and this result indicated that the water in the reaction promoted the degradation of 3a (Table 2, entry 8). On the other hand, the alkoxides are considered as one of the degradation fragments of the methoxymethyl (MOM) group and might promote the O-methylation. However, contrary to our expectation, only a small amount of methyl ether 3a was obtained using sodium methoxide as a base (Table 2, entry 9). In addition, the O-methylation was not inhibited in the presence of dibutylhydroxytoluene (BHT) as a free radical scavenger (Table 2, entry 6).
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Substrate | Base (eq) | MOMCl (eq) | Time (h) | Product (Recovered) | |
| 2a (%) | 3a (%) | |||||
| 1 | 2a | NaH (1.0) | 1.0 | 1 | 39 | 21 |
| 2 | 2a | none | 1.0 | 1 | 91 | 0 |
| 3 | 2a | NaH (1.0) | none | 1 | <26b) | 0 |
| 4 | 2a | none | none | 24 | quant | 0 |
| 5c) | 2a | NaH (1.0) | 1.0 | 1 | 69 | 11 |
| 6d) | 2a | NaH (1.0) | 1.0 | 1 | 61 | 36 |
| 7 | 3a | NaH (1.0) | 1.0 | 1 | 0 | 88 |
| 8c) | 3a | NaH (1.0) | 1.0 | 1 | 36 | 33 |
| 9d) | 2a | NaOCH3 (1.0) | 1.0 | 2 | 90 | 4 |
a) The reaction was carried out using flame-dried round bottom flask and filled with argon gas atmosphere. 0.5 mmol of substrate (2a or 3a), anhydrous DMF (0.5 mL) and activated MS3A (125 mg) was used, and stirred at room temperature. b) Unidentified compounds were also obtained. c) One drop of water was added to the reaction solution. d) 0.6 mmol of BHT (dibutylhydroxytoluene) was added.
The O-alkylation also occurred using other alkoxyalkyl halides, such as benzyl chloromethyl ether (Chart 3). The reaction of 1a using benzyl chloromethyl ether with NaH proceeded smoothly; benzyl ether (3b)14) was obtained along with benzyloxymethyl ether (2b).15) It was noteworthy that methyl ether (3a) was not obtained at all in this case. This result indirectly indicated that the methyl group of 3a did not originate from the methylene moiety of 2a but from the terminal methyl moiety in case of the reaction of 1a with NaH and MOMCl.
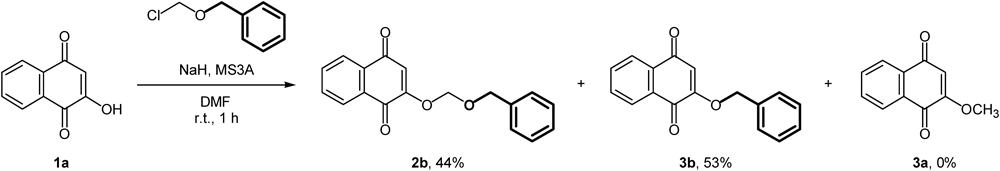
Reaction condition: 1a (2 mmol), NaH (2 eq), BOMCI (2 eq), MS3A (500 mg), anhydrous DMF (2 mL).
Based on the experimental findings, the proposed reaction pathway was illustrated in Chart 4. First, compound 2a was obtained by simple SN2 reaction from compound 1a under treatment with NaH and MOMCl. The following transformation can be explained by two pathways.16) In route A, deprotonation at the methylene carbon by a strong base such as NaH occurs to generate 6-membered chelate complex I17) which reacts with MOMCl to give intermediate II. The intramolecular Michael addition provides 5-membered spiro-ketal III, and compound 3a is finally obtained by cleavage of the methyl acetate group via formation of labile intermediate IV. On the other hand, in route B, 1,3-dioxetane intermediate V18) is generated directly by oxa-Michael addition of 2a, and subsequent removal of formaldehyde provides 3a. From the experimental results in Table 2, the transformation of 2a into 3a was surmised to require both NaH and MOMCl, and this result offered support for route A because those reagents are not involved in the transformation in route B. The radical reduction and/or radical chain reaction pathway perhaps could be eliminated (Table 2, entry 6). The reverse reaction from 3a into 2a is considered to occur by the following processes. A Michael addition between sodium hydroxide, which is generated from NaH and water, and 3a occurs first, and subsequent elimination provides 1a. The formation of 2a occurs in a same way as explained above. However, the detailed reaction pathway from 2a to 3a remains unclear.19)
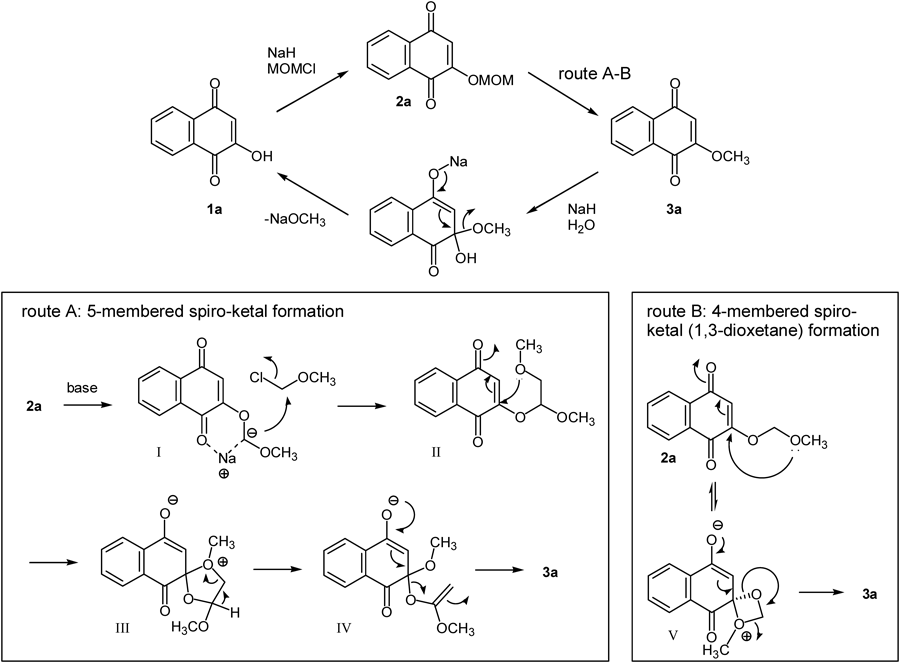
On the other hand, the reverse transformation (3a to 2a) can be clearly explained by the alkali hydrolysis pathway. Namely, 1a is reproduced by the alkaline hydrolysis of 3a, and following conventional methoxymethylation provide 2a.
In summary, we have developed a novel O-alkylation of 2-hydroxy-1,4-naphthoquinone with alkoxymethyl chlorides. Precise study of the reaction conditions was carried out, and which could be realized the separate formation of two type of product in high yield with high selectivity. Further study on the mechanism, especially for the transformation of 2a into 3a, substrate scope, limitation and applications are extensively in progress.
The authors declare no conflict of interest.