2016 Volume 64 Issue 10 Pages 1445-1449
2016 Volume 64 Issue 10 Pages 1445-1449
The aims of this study were to improve in vitro dissolution property of poorly water-soluble everolimus (EVR) for enhanced bioavailability without using organic solvents and characterize the effects of microfluidization and freeze-drying on physicochemical properties of EVR nanosuspension and nanoparticle, respectively. EVR nanosuspension was prepared using microfluidization with various types and concentrations of stabilizers. After that, it was solidified into nanoparticle using freeze-drying with various concentrations of xylitol, a cryoprotectant. The particle size, zeta potential, physical stability, and chemical stability of EVR nanosuspension and nanoparticle were measured. In vitro release of EVR nanoparticle was also measured and compared with that of physical mixture. Zero point five percent (w/w) poloxamer 407 (P407) was chosen as the stabilizer considering particle size, zeta potential, and yield of EVR nanosuspension. Freeze-drying with 1% (w/w) xylitol improved both physical and chemical stability of EVR nanoparticle. In vitro release test showed improved dissolution property compared to that of physical mixture, implying enhanced bioavailability.
Everolimus (EVR), the 40-O-(2-hydroxyethyl) derivatives of rapamycin is a mammalian target of rapamycin (mTOR) inhibitor which reduces cell proliferation, angiogenesis, and glucose uptake.1) It has been currently used as an immunosuppressant to prevent rejection after organ transplantation2) and approved for the treatment of the patients with advanced renal cell carcinoma (RCC) after failure of treatment with sunitinib or sorafenib.3,4) Also, it was used for the treatment of other cancers including leukemia, other hematological malignancies, subependymal giant cell astrocytoma (SEGA), and tuberous sclerosis complex (TSC).3,4) EVR is available under the tradenames Zortress (U.S.A.) and Certican (Europe and other countries) as an immunosuppressant, and Afinitor (U.S.A.) and Votubia (EU) as an anticancer. Because of the expansion of its indication and the number of patients, sales of EVR has been increased.5)
In spite of its clinical significance as an anticancer agent and immunosuppressant, EVR therapy has limitation on its low oral bioavailability (mice: 5–12%, rats: 14–26%, monkeys: 6%).6–8) Low aqueous solubility (below 1 mg/mL) of EVR9) is attributable to its low oral bioavailability. Commercial products were made using bottom-up method to improve the solubility and dissolution rate of EVR.10) It showed improved dissolution rate compared to drug and physical mixture. However, environmental issues have arisen concerning residual solvent used in the dissolving step.
Microfluidization can be an alternative to overcome this unwanted environmental issues. It is one way to manufacture the nanoparticle. Particles dispersed in the suspension pass through the interaction chambers at high pressure, causing the jet streams. Under the jet streams, particles collide each other and become smaller.11) Compared to bottom-up method which needs organic solvent to dissolve the poorly water soluble drug, this top-down method has no need to use organic solvent. However, this colloidal system has both physical (e.g., Ostwald ripening) and chemical (e.g., hydrolysis) stability issues.12) For this reason, solidification techniques (e.g., spray-drying and freeze-drying) were employed to further stabilize the system.13–15) In case of thermolabile drugs like EVR, freeze-drying was preferred to spray-drying because the latter introduced high temperature in the process.16)
The aims of this study were to improve the in vitro dissolution properties of poorly water-soluble EVR for enhanced bioavailability without using organic solvents. EVR nanoparticles were prepared using microfluidization and freeze-drying. The effects of microfluidization and freeze-drying on physicochemical properties of EVR nanosuspension and nanoparticle were characterized.
The following materials were used as received without any further purification: EVR (Hangzhou Pharma & Chem Co., Ltd., Korea), poloxamer 188 (Kolliphor® P188, Average molecular weight: 7680–9510), poloxamer 407 (Koliphor® P407, Average molecular weight: 9840–14600, BASF Co., Germany), polyoxyethylene sorbitan monostearate (Tween® 60, molecular weight: 1312), polyoxyethylene sorbitan monooleate (Tween® 80, molecular weight: 1310), potassium phosphate monobasic (Samchun Pure Chemical Co., Korea), sodium hydroxide (Daejung Chemical & Metals Co., Korea), sodium lauryl sulfate (SLS) (molecular weight: 288, Sigma-Aldrich Co., U.S.A.), and xylitol (Roquette Co., France). Acetonitrile, ethanol, and methanol were of HPLC grade (J.T. Baker Chemical Co., U.S.A.).
MethodsPreparation of EVR NanosuspensionMicrofluidization was used to prepare EVR nanosuspension. SLS, Kolliphor® P188 (P188), P407, Tween® 60 (T60), and Tween® 80 (T80) were reported to be used for the stabilization of nanosuspension.13) Therefore, 5 surfactants mentioned above were screened for the stabilizer. Each of them were dispersed in distilled water and used as the stabilizer solution. Twenty milligrams of EVR was dispersed in 20 mL of stabilizer solution using a magnetic stirrer (coarse suspension) and was homogenized at 10000 rpm for 30 min using an Ultra-Turrax T8 homogenizer (IKA Labortechnik, Germany) (macrosuspension). After that, the macrosuspension was processed by an M110S microfluidizer (Microfluidics, U.S.A.) applying 10 cycles at 200 and 500 bar, followed by additional cycles at 1000 bar.
Preparation of EVR NanoparticleEVR nanoparticle was created by freeze-drying of the EVR nanosuspension using a Bondiro freeze dryer (Ilshin Lab, Korea). Xylitol was used as a cryoprotectant and dispersed in 10 mL of EVR nanosuspension overnight using a magnetic stirrer. It was then transferred to a 50 mL disposable polypropylene tube sealed with parafilm which had small air holes made using a sterile needle and frozen at −40°C for 24 h. In brief the freeze drying conditions were as follows: drying temperature −85°C, sublimation time 48 h, fill depth was less than 2.7 cm and pressure applied was maintained below 100 mTorr.
Particle Size and Zeta PotentialThe particle size of the EVR nanosuspension and nanoparticle was determined by dynamic light scattering and its zeta potential was determined by laser Doppler micro-electrophoresis using Zetasizer 3000HS system (Malvern Instruments, U.K.). Samples were diluted with distilled water before measurement. All measurements were made in triplicate and the mean values and standard deviations were reported.
In Vitro DissolutionThe dissolution studies were conducted using a U.S. Pharmacopeia (USP) Apparatus 2 (Vision Elite 8, Hanson, U.S.A.). The stirring speed was 50 rpm and the temperature was maintained at 37±0.5°C. Each capsule, containing physical mixture and EVR nanoparticle (equivalent to 5 mg of EVR), was put into a sinker and placed in the 500 mL of pH 6.8 phosphate buffer (0.05 M). Physical mixture was prepared by lyophilization after simple mixing EVR, P407, and xylitol at the ratio of 1 : 5 : 10 (w/w) without microfluidization. In vitro dissolution medium was prepared according to the Korea Food and Drug Administration (KFDA) comparative dissolution guidelines by adjusting to about pH 6.8 and filling to 1000 mL with water after adding 118 mL of 0.2 M sodium hydroxide solution to 250 mL of 0.2 M potassium phosphate monobasic solution. Five milliliters of the dissolution medium was withdrawn at predetermined time intervals and filtered through a polytetrafluoroethylene (PTFE) syringe filter (25 mm i.d., 0.2 µm, Whatman, U.K.). At each sampling time, an equal volume of the medium was replaced. The drug concentrations of samples were determined using a slight modification of a validated HPLC-UV method.17) The HPLC system was Hitachi L-7000 series (Tokyo, Japan). Chromatographic separation was carried out on a Gemini-NX C18 (250×4.60 mm, i.d., 5 µm, Phenomenex, U.S.A.) using a binary isocratic mobile phase composed of acetonitrile and deionized water (3 : 2, v/v) at flow rate of 1.5 mL/min. The column temperature was maintained at 25°C and the injection volume was 50 µL. The detection wavelength was 278 nm. All measurements were made in triplicate and the mean values and standard deviations were reported.
StabilityThe stability studies were conducted in accordance with International Conference on Harmonization (ICH) tripartite guidelines.18) The EVR nanosuspension and nanoparticle were placed in stoppered glass vials sealed with parafilm and aluminum foil and stored in stability chamber maintained at 25±2°C and 60±5% relative humidity (RH) for long-term stability. Samples were taken at predetermined time intervals and their particle sizes and contents were analyzed. All measurements were made in triplicate and the mean values and standard deviations were reported.
The particle size of EVR nanosuspension containing 0.1% (w/w) stabilizers with various microfluidization cycle numbers was shown in Fig. 1. The microfluidization cycle number tested at 1000 bar were 10, 20, 30, and 60. The particle sizes of EVR nanosuspension with all other stabilizers (except SLS) had a tendency to become smaller with increasing microfluidization cycle number. Similar results have been reported in other studies.6,19,20) The 60 cycles at 1000 bar was employed in microfluidization process.
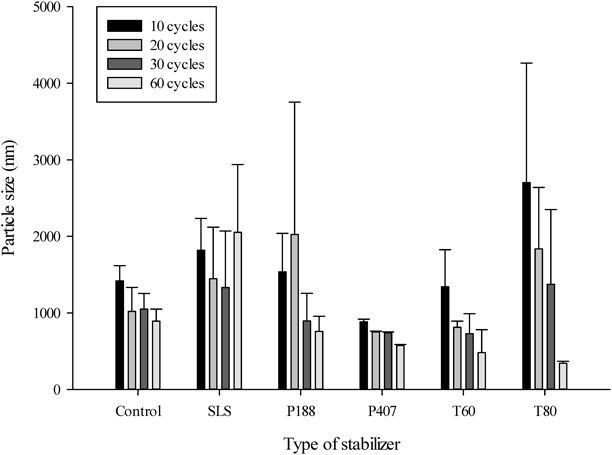
Nanosuspension was known for its susceptibility to aggregation and necessity of stabilizer to prevent this unwanted result.19,21) For this reason, 5 stabilizers mentioned above were used to stabilize EVR nanosuspension and their effects on particle size and zeta potential of EVR nanosuspension were studied. Compared to control, all other stabilizers (except SLS) were effective in particle size reduction of EVR nanosuspension at the concentration of 0.1% (w/w) (Fig. 1). There are two types of stabilizers used to stabilize a colloidal system, steric stabilizer and electrostatic stabilizer.21) Among the stabilizers used in this study, only SLS is an electrostatic stabilizer which has a low molecular weight (288) and negative charge. An ionic surfactant has self-repulsion which inhibits the surface coverage on the drug particles.21) Furthermore, small molecule is more prone to Ostwald ripening and particle growth.22) This is why SLS increases the particle size. Polyoxyethylene sorbitan fatty acid esters (T60, T80) and poloxamers (P188, P407) were used as steric stabilizers. In terms of molecular weight, poloxamer and polyoxyethylene sorbitan fatty acid esters are categorized as polymer and surfactant, respectively. As a polymer with a high molecular weight is more physically adsorbing,13) P407 which has the highest molecular weight (9840–14600) represented narrow standard deviation of particle size indicating stabilizing effect on EVR nanosuspension regardless of microfluidization cycle number. The hydrophobic (polyoxypropylene) segment of P407 interacted with EVR surface and P407 adsorbed onto EVR. While hydrophilic (polyoxyethylene) segment of it interacted with water and stabilized EVR nanosuspension. Zeta potential of EVR nanosuspension containing 0.1% (w/w) stabilizers was represented in Fig. 2. Zeta potential change of EVR nanosuspension with stabilizers can be explained by the functional group of stabilizer used. In case of an anionic stabilizer, SLS, imparted higher absolute zeta potential than that of the control to EVR nanosuspension. It is attributable to the negative charge of SLS.23) On the other hand, nonionic stabilizers masked the negative charge of EVR by surrounding it and caused the reduction of the absolute zeta potential. Zeta potential is used as an index of physical stability in colloidal system and its absolute value above 30 is regarded as physically stable.24) However, in case of steric stabilizers, zeta potential is used as an indicator of surface coverage.19) Therefore, the reduction of absolute zeta potential via surface coverage of drug particle meant physically stable state rather than unstable state. There were no relationship observed between the particle size and zeta potential for steric stabilizers. Yield of the EVR nanosuspension was significantly high when using P407 compared to all other stabilizers at the concentration of 0.1% (w/w) (Fig. 3). As microfluidization process is prolonged, particle agglomeration was formed and resulted in the loss of the drug, which reduced the efficiency of the process.25,26) As discussed earlier, stabilizing effect of P407 on EVR nanosuspension regardless of microfluidization cycle number minimized aggregation and improved the yield of the process. Considering particle size, zeta potential, and yield of EVR nanosuspension, P407 was chosen as the stabilizer used in this study. Further studies were conducted to investigate the effect of P407 concentration on particle size of EVR nanosuspension.


The effect of P407 concentration, ranging from 0.02 to 0.5% (w/w), on particle size of EVR nanosuspension was investigated. Among the tested concentrations, the particle size became smaller with increasing the concentration of P407, and the smallest particle size was observed in 0.5% (w/w) P407 (Fig. 4). However, EVR nanosuspension containing 0.5% (w/w) P407 was chemically unstable and the drug content of EVR nanosuspension stored at long term condition for 1 month was significantly different from that of initial (Fig. 5). Moreover, the drug content stored at long term condition for 1 month of EVR nanosuspension (88.4±2.2%) was lower than that of EVR itself (93.5±1.1%) reported in the other study.27) EVR is known for its susceptibility to oxidative degradation.8,28) Moisture contained in EVR nanosuspension seemed to accelerate this reaction. To overcome this problem, freeze-drying was used to improve the chemical stability of EVR by removing water from EVR nanosuspension and minimizing the chances of oxidation.


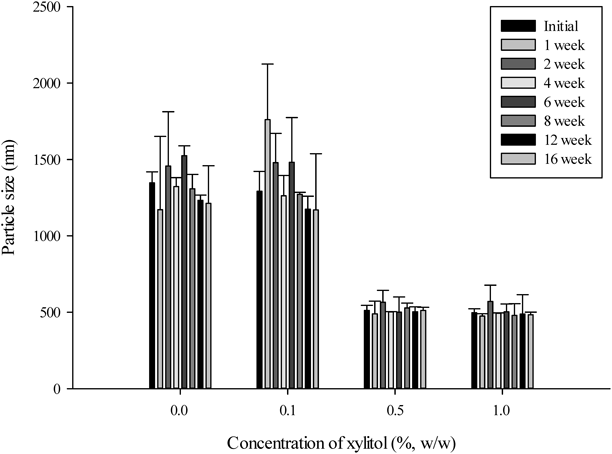
The chemical stability of EVR has been improved via freeze-drying (Fig. 5). The drug content of EVR nanoparticle stored for 1 month in long term condition (96.5±2.4%) was comparable to that of EVR itself.27) However, the physical stability of nanoparticle without xylitol was damaged and aggregation occurred (Fig. 6). To prevent the particle aggregation and enhance the physical stability of nanoparticle, xylitol was used as a cryoprotectant. Like other sugar alcohols, it acts as a matrix former based on its cryoprotective action. Furthermore, it was known for an antioxidative action29) and used to improve the chemical stability of EVR. The effects of xylitol concentration, ranging from 0.1 to 1.0% (w/w), on particle size and physical stability of nanoparticle have been investigated. Freeze dried EVR nanoparticles containing not less than 0.5% (w/w) xylitol seem to be stable without any collapse of the dried cake. The particle sizes of EVR nanoparticle containing not less than 0.5% (w/w) xylitol were comparable to that of EVR nanosuspension (Fig. 6) and physically stable for 4 months (Fig. 7).

Finally, in vitro dissolution of EVR nanoparticle was compared with physical mixture and the effects of xylitol concentration on in vitro dissolution has been studied. The cumulative drug release in the presence of xylitol reached plateau after approximately 60 to 90 min (Fig. 8). With increasing xylitol concentration, the amount of drug released from EVR nanoparticles after 2 h had a tendency to increase and EVR nanoparticle containing 1.0% (w/w) xylitol showed the significant difference with others (Fig. 8). It seemed that the aggregate in the sample affected the dissolution rate. The aggregate was included in the samples for in vitro dissolution study. As the amount of aggregate formed increased with decreasing xylitol concentration, the cumulative drug release decreased at low concentrations of xylitol. The dissolved amount of EVR nanoparticle containing 1.0% (w/w) xylitol (96.8±3.1%) was as 50 times high as that of physical mixture (1.9±0.2%) indicating its improved in vitro dissolution via microfluidization.

In this study, EVR nanoparticles were prepared using microfluidization and freeze-drying. In the microfluidization process, particle size become smaller with increasing microfluidization cycle number. Zero point five percent (w/w) P407 was used as the stabilizer considering particle size, zeta potential, and yield of EVR nanosuspension. However, EVR was unstable in the colloidal system, freeze-drying was employed to improve the chemical stability of EVR. One percent (w/w) of xylitol was used as the cryoprotectant considering particle size, physical stability, and in vitro dissolution of EVR nanoparticle. Improved in vitro dissolution property of EVR nanoparticle compared to physical mixture implied the possibility of bioavailability enhancement.
This study was supported by a Grant of the Korean Health Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A092018).
The authors declare no conflict of interest