2016 Volume 64 Issue 11 Pages 1616-1621
2016 Volume 64 Issue 11 Pages 1616-1621
A menthol–diphenhydramine cream is prepared in hospital pharmacies and then prescribed to patients for the treatment of pruritus associated with chronic kidney disease. The purpose of this study is to design a stable formulation without any concern about phase separation during its clinical use on patients. As a preventive measure against phase separation, various surfactants and thickeners were incorporated into the creams. The test creams were stored at 40°C, and then their phase separation behaviors were monitored. The key technology was magnetic resonance imaging T2 mapping. From the T2 maps, some surfactants showed a certain stabilizing effect. In addition, the data analysis using Kohonen’s self-organizing map revealed that hydrophilic–lipophilic balance of the surfactant is an important factor for the stabilizing effects of the surfactants. However, as a whole, the effect of surfactant was not sufficient to improve completely the low stability. By contrast, the creams were significantly stabilized by addition of thickeners. In particular, the stabilizing effect of carbomer Hiviswako105® (H105) was very high; no phase separation was observed from the cream containing H105 even after 30 d storage at 40°C. This study also verified the combination effect of surfactants and thickeners on the improvement of the emulsion stability. In conclusion, we successfully established a stable formulation of menthol–diphenhydramine cream.
Chronic kidney disease (CKD)-associated pruritus (also known as uremic pruritus) is a significant problem for many patients receiving long-term hemodialysis.1–3) Pruritus is an unpleasant symptom that negatively impacts a patient’s quality of life; for example, moderate/severe pruritus seriously affects sleeping and appetite regulation of patients.4) In hospitals, a cream that contains menthol and diphenhydramine is prescribed to patients for the treatment of the symptoms. This cream is made in hospital pharmacies by mixing a menthol-containing ethanol solution with a commercial diphenhydramine cream. It can give a pleasant cooling sensation to patients because of the menthol, as well as the antihistaminic action of diphenhydramine; therefore, it has received favorable responses from patients. However, this cream has a serious problem in terms of stability.
Various methods have been proposed to evaluate the stability of pharmaceutical emulsions. These include droplet-size analysis,5,6) light scattering,5,7,8) and turbidity measurements.9) From a series of our studies, we have recently verified that magnetic resonance imaging (MRI) is a promising tool to investigate emulsion stability.10–13) MRI is a popular molecular imaging method using the principle of NMR.14) As well as nondestructive monitoring of a sample, it enables visualization of the molecular mobility of a sample using magnetic resonance (MR) parameters. Based on the MR techniques, we previously investigated the stability of menthol–diphenhydramine creams in detail.10) They were stored at different temperatures (25, 30, 40°C), and then time–course monitoring with MRI was performed. According to a T2 mapping, we successfully characterized the phase-separation behaviors of the samples. Although the sample stored at 25°C remained stable until the end of the experimental period of two weeks, the samples stored at 30 and 40°C showed obvious phase separation into two distinct layers. In the previous study,10) we also conducted a component analysis of each phase-separated layer using MR spectroscopy and chemical shift selective images, and it was verified that the upper layer consisted of packed oil droplets (creaming layer), whereas the lower layer was an aqueous phase. The development of phase separation became more obvious with increasing temperature. From these findings, we concluded that the current formulation has a significant risk of phase separation occurring during practical clinical use.
The purpose of this study is to improve the low stability of menthol–diphenhydramine cream. To achieve this purpose, various surfactants and thickeners were incorporated into the creams. The initial phase of this study was dedicated to screening surfactants to find the most suitable candidate to improve the stability problem. In this study, 13 kinds of surfactants were tested. A detailed analysis of the observed imaging data was performed using Kohonen’s self-organizing map (SOM). Furthermore, this study also investigated the stabilizing effects of thickeners. As a consequence, we successfully established a stable formulation of the cream for use in practical clinical practice.
The diphenhydramine cream (Restamin Kowa cream®) was purchased from Kowa Co., Ltd. (Tokyo, Japan). l-Menthol and thymol were purchased from Pfizer Japan Inc. (Tokyo, Japan). Mint oil was purchased from Yoshida Pharmaceutical Co., Ltd. (Tokyo, Japan). d-Camphor was purchased from Kozakai Pharmaceutical Co., Ltd. (Tokyo, Japan). Hydroxyethyl cellulose (HEC), xanthane gum, and crosslinked acrylic acids (carbomers) including several types of carboxyvinyl polymer (Hiviswako® 103, 104, and 105 (H103, H104, and H105)) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Nikkol® nonionic surfactants were kindly donated by Nikko Chem. Co., Ltd. (Tokyo, Japan). They include polyoxyethylene 9-lauryl ether (BL-9EX), polyoxyethylene stearyl ether (BS-4), poly(ethylene glycol) (PEG)-20-hydrogenated castor oil (HCO-20), PEG-40-hydrogenated castor oil (HCO-40), PEG-60-hydrogenated castor oil (HCO-60), PEG-40-monostearate (MYS-40MV), sorbitan laurate (SL-10), sorbitan oleate (SO-10V), sorbitan trioleate (SO-30V), polysorbate 20 (TL-10), polysorbate 80 (TO-10MV), and polysorbate 60 (TS-10MV). All other chemicals were of analytical grade.
Sample PreparationThe menthol–diphenhydramine creams tested were prepared according to our previous study.10) The standard formulation of the cream is shown in Table 1. In brief, after preparing the menthol-containing ethanol solution, it was mixed with diphenhydramine cream using a revolution/rotation-type hybrid mixer (Model HM-500; Keyence, Osaka, Japan). The revolution and rotation speeds of the mixer were 2000 and 800 rpm, respectively. Further details of the sample preparation were described in full previously.10) For improvement of the formulation, surfactants and thickeners were added to the standard cream. Additional surfactants were added by dissolving them in the menthol-containing ethanol solution, and the final concentration in the creams was adjusted to 2.0 wt%. Thickeners were added as an aqueous solution to the other components at a weight ratio of 1 : 9, and then they were mixed using the hybrid mixer. Regarding the carbomers’ aqueous solutions, the pH was adjusted to 7.0 by adding triethanol amine. The final concentration of the thickeners in the creams ranged from 0.02 to 0.30%. After sample preparation, they were packed in a tube (1.5 mL) and stored in an oven at 40°C. At designated intervals, they were removed from the oven, and then their phase separations were monitored by MRI and visible observation.
| Components | Weight (g) |
|---|---|
| Menthol solution | 14 |
| l-Menthol | 7 |
| Thymol | 0.4 |
| Mint oil | 0.9 |
| 30% Camphor in ethanol | 1.75 |
| Ethanol | ad 14 |
| Diphenhydramine cream | 86 |
The MRI experiments were performed at room temperature using a 9.4 T vertical MRI scanner (Varian, Palo Alto, CA, U.S.A.). T2-Weighted images were acquired using a spin–echo pulse sequence with TR=2000 ms, TEs=25 and 40 ms, field of view=35×35 mm2, matrix size=128×128, number of excitations=2, and slice thickness=1 mm. After the acquisition, a quantitative T2 map was constructed from the images. The decay of the echo amplitude is given by the equation:
 | (1) |
The viscosity of the samples was measured at 40.0±0.1°C with a cone-and-plate-type viscometer (TV-30; Toki Sangyo Co., Ltd., Tokyo, Japan). The samples were prepared immediately prior to the measurement. The shear rate was fixed at 38.3 s−1. After 2 min shearing at the constant shear rate, the viscosity value was recorded.
SOM ClusteringViscovery SOMine (Version 4.0; Eudaptics Software; Vienna, Austria) software was used for SOM clustering. The lower layer area to the total area (%) after storage for 24, 48, and 72 h at 40°C were used as tutorial data for the SOM clustering. The clustering was performed based on SOM-Ward method.
To improve the emulsion stability, we first introduced additional surfactants to the menthol–diphenhydramine cream. Thus, the initial phase of this study was designed to screen the stabilizing effect of surfactants. To perform continuous nondestructive monitoring of the phase separation occurring in the creams, MRI T2 mapping was employed. From our previous studies, it has been confirmed that T2 maps are a powerful tool to detect phase separation in pharmaceutical emulsions.10,11,15) As far as oil-in-water (o/w)-type emulsion is concerned, the state of water is substantially changed depending on its surroundings.16) The mobility of water molecules adjacent to oil droplets is more restricted than that of bulk water; for one thing, water molecules interact with surfactants on the oil droplets. Because of this mechanism, T2 derived from water located in the region with close-packed droplets is assumed to be much shorter than that in the region with a lower density of droplets. By taking advantage of the difference in the state of water, these maps can detect the phase separation with high sensitivity. It is worth noting that the detection sensitivity of change in the emulsion state is much superior to that by visible observation.12,15)
The creams containing various surfactants were stored at 40°C, and their phase separations were monitored continuously. As shown in Fig. 1a, substantial phase separation was eventually observed from all creams at the end of the experimental period. According to the visible observation (Fig. 1b), the aspects of the phase-separated samples were the same as those shown in the previous study10); thus, the upper layer consisted of packed oil droplet layers, whereas the lower was an aqueous phase. We further note that the development of phase separation was different from sample to sample. To quantify the phase separation of each sample, the lower layer area to the total area was measured from the T2 maps, then the area under the curve (AUC) was calculated from the change in the lower layer areas (Table 2). Regarding the control without any additional surfactant, the lower layer areas were increased with increasing storage period as follows: 6.57±5.61, 16.34±3.02, and 46.37±0.33% at 24, 48, and 72 h, respectively (Table 2). Compared with the control, more substantial phase separations were observed from the creams containing SL-10 and SO-10V; for instance, the lower layer areas at 24 h were much higher than that of the control: 30.73±0.66 and 23.01±9.65% for the creams containing SL-10 and SO-10V, respectively. By contrast, the creams containing BL-9EX, MYS-40MV, TL-10, TO-10MV, and TS-10MV appeared to be more stable than the control; AUCs were 783±45, 667±86, 820±87, 760±102, and 801±73%·h for the creams containing BL-9EX, MYS-40MV, TL-10, TO-10MV, and TS-10MV, respectively; these values were much lower than that of the control (1131±162%·h).
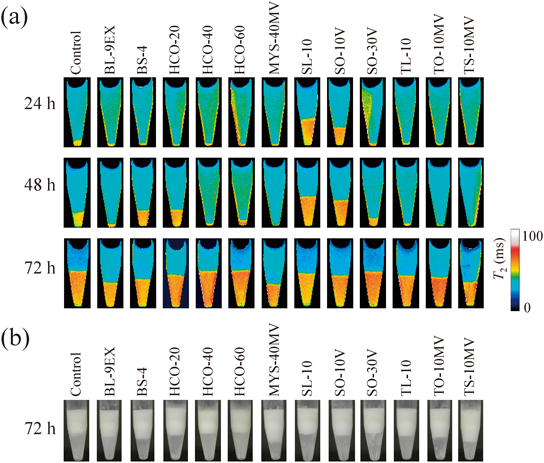
The concentration of the additional surfactants was fixed at 2.0%. The test creams were stored at 40°C for up to 72 h. Acquisition of each T2 map was triplicated. Representative images are shown in this figure.
| Surfactant | HLB | MW | Number of EG unit | Area of the lower layer (% of whole sample)a) | AUCa,b) | |||
|---|---|---|---|---|---|---|---|---|
| 6 h | 24 h | 48 h | 72 h | |||||
| Control | — | — | — | 3.70±0.77 | 6.57±5.61 | 16.34±3.02 | 46.37±0.33 | 1131±162 |
| BL-9EX | 14.5 | 583 | 9 | 6.09±1.50 | 6.69±2.97 | 6.91±2.54 | 33.58±5.64 | 783±45 |
| BS-4 | 9.0 | 446 | 4 | 4.06±1.81 | 10.26±4.63 | 22.38±4.12 | 34.40±2.37 | 1214±107 |
| HCO-20 | 10.5 | 1829 | 20 | 9.37±7.30 | 9.70±0.85 | 13.22±5.28 | 40.73±1.83 | 1122±151 |
| HCO-40 | 12.5 | 2709 | 40 | 6.91±3.93 | 8.56±3.15 | 13.95±6.15 | 46.68±1.53 | 1158±91 |
| HCO-60 | 14.0 | 3589 | 60 | 8.11±5.40 | 11.83±6.18 | 14.33±2.21 | 46.62±2.24 | 1249±199 |
| MYS-40MV | 17.5 | 2030 | 40 | 6.30±4.46 | 7.59±2.31 | 5.39±1.88 | 25.22±3.58 | 667±86 |
| SL-10 | 8.6 | 346 | 0 | 7.41±4.41 | 30.73±0.66 | 36.30±0.44 | 41.51±1.07 | 2104±44 |
| SO-10V | 4.3 | 429 | 0 | 9.09±5.07 | 23.01±9.65 | 30.36±0.55 | 38.66±0.49 | 1785±244 |
| SO-30V | 1.7 | 957 | 0 | 4.23±3.23 | 14.48±7.83 | 12.96±3.03 | 45.15±0.47 | 1208±214 |
| TL-10 | 16.9 | 1228 | 20 | 5.00±3.48 | 6.15±2.29 | 7.36±0.96 | 37.82±4.05 | 820±87 |
| TO-10MV | 15.0 | 1310 | 20 | 4.82±2.61 | 7.28±1.71 | 6.69±4.59 | 32.39±3.18 | 760±102 |
| TS-10MV | 14.9 | 1312 | 20 | 7.58±2.11 | 6.77±1.95 | 8.76±0.09 | 29.79±5.76 | 801±73 |
a) Each value represents the mean±S.D. (n=3). b) The value was calculated from the change behavior of the lower layer areas at individual sampling points.
To investigate the stabilizing effects of the surfactants in more detail, SOM analysis was performed. SOM is a feedforward-type neural network model.17) It enables the expression of relationships of multidimensional data as a two-dimensional surface. The typical structure of the SOM comprises one input layer and one output layer. The output layer has an array of nodes, and each node has the same number of parametric reference vectors as the input vector. The nodes in the output layer associate with the adjacent ones; thus, the distance between the nodes expresses the degree of similarity and neighboring nodes have similar properties. In this study, the lower layer areas after storage for 24, 48, and 72 h (Table 1) were used as a tutorial data set for SOM clustering. As shown in Fig. 2a, the surfactants tested were classified into three distinct clusters. Figure 2b shows SOM feature maps; red or blue regions in the feature maps indicate higher or lower values of the responses, respectively. From the SOM feature maps of the lower layer areas and AUC, it was clarified that clusters 1 and 3 were represented as the clusters having low and high emulsion stability, respectively. We further examined the distribution of tutorial data in SOM to characterize the stabilizing effects of the surfactants. Surfactants whose data sets were distributed in the same cluster can be regarded as having similar stabilizing effects. As anticipated, SL-10 and SO-10V were classified into the surfactants having the lowest stabilizing effect, because their data sets were concentrated in cluster 1 (unstable cluster); all data sets concerning SL-10 and one-in-three data set concerning SO-10V were contained in the cluster. By contrast, BL-9EX, MYS-40MV, TL-10, TO-10MV, and TS-10MV can be regarded as surfactants with a strong stabilizing effect because all of their data sets were distributed in cluster 3 (stable cluster).

The SOM clustering was performed using the lower layer areas of the test creams after storage for 24, 48, and 72 h at 40°C (n=3) as tutorial data sets. The number in the brackets attached to the SOM (a) represents the number of the tutorial data sets contained in each cluster. Regarding the SOM feature maps (b), each variable is shown in color: red and blue regions represent higher and lower values of the factors, respectively.
We also prepared SOM feature maps of the intrinsic physical properties of the surfactants (Fig. 2b), and then compared the patterns of these feature maps. They include hydrophilic–lipophilic balance (HLB), molecular weight (MW), and number of ethylene glycol (EG) units. After a closer look at the SOM feature maps, it occurred to us that, in cluster 3, HLB values of surfactants were obviously higher than those in the other clusters. This suggests that HLB of surfactants plays an important role in their stabilizing effects. In general, HLB is well known to be an important element for stabilization of emulsion systems, and optimization of the HLB value is a crucial issue to investigate in designing pharmaceutical emulsions.6,18) The optimal HLB value depends on the formulation of the emulsion.19) As far as the cream is concerned, it was clarified that surfactants with higher HLB were preferable for improvement of the stability. As for the other physical properties of the surfactants, they appeared not to affect the stability because their feature maps’ patterns were quite different from those concerning stability (Fig. 2b).
Effects of Thickeners on the Emulsion Stability of the CreamOwing to the SOM analysis, we could gain a comprehensive understanding of the stabilizing effect of surfactants on the stability. However, we have failed to find sufficient stabilizing effects to improve completely the lower stability of the cream with the test surfactants. In the next phase of this study, we investigated the stabilizing effect of various thickeners. As well as surfactants, a hydrophilic polymer as a thickener is a popular stabilizing agent of emulsions.20–22) The thickeners tested in this study include HEC, xanthan gum, and carbomers. At the beginning of this experiment, the concentration of the thickeners was fixed at 0.1%. The test creams were stored at 40°C, and then their phase separation behavior was monitored in the same way as described above. As a result, the stability was substantially improved by the addition of carbomers; no phase separation was observed from the test creams after 7 d at 40°C (Fig. 3). In particular, the cream containing H105 was quite stable; a homogeneous emulsion state was maintained even after 30 d at 40°C. The lower layer areas after 30 d storage were 36.6±1.2, 22.5±0.9, and 0.0±0.0% for the creams containing H103, H104, and H105, respectively. We believe that the cream containing H105 is stable enough for practical clinical practice with patients. By contrast, obvious improvement of the stability was not observed from the creams containing HEC and xanthan gum because substantial phase separations occurred in both test creams within 3 d storage.

H103, H104, and H105 are different grades of carbomers. The test creams were stored for 30 d at 40°C. Acquisition of each T2 map was triplicated. Representative images are shown in this figure.
The viscosity values of the test creams are shown in Fig. 4. The concentrations of thickeners and surfactants were 0.1 and 2.0%, respectively; these samples were the same ones as used in Figs. 3 and 1. The rank order of the viscosity of the creams containing carbomers was completely consistent with that of stability; the viscosity of the creams containing H103, H104, and H105 were 370.7±1.5, 523.3±4.9, and 924.7±13.3 mPa·s, respectively, at 38.3 s−1 at 40°C. Carbomers are polymers mainly composed of acrylic acid, and each grade is different in terms of the MW and polymer structure.23) The viscosity of the aqueous solutions is substantially increased by the ionization of carboxyl groups of carbomers, and, gelation usually occurs above 0.5% polymer concentration.23) H103 is rich in side chains or cross-links, while H105 is poor in side chains. The resultant carbomer gels show unique viscoelastic properties; under the ionized condition, the order of magnitude of viscosity is H103>H104>H105, while, under non-ionized condition, the order changes to the opposite one. In this study, the carbomer aqueous solutions used for mixing with the cream were adjusted to pH 7.0 (ionic condition). In addition, a preliminary experiment showed that the creams containing carbomers were kept at pH values above 7.0. According to these issues, we had speculated that the cream containing H103 showed the highest viscosity prior to the experiment. However, the highest viscosity was observed from the cream containing H105. From these findings, we considered that carbomers incorporated in the cream mostly existed as the non-ionized form. Although the exact reason is still unclear, the diphenhydramine cream contains a considerable amount of electrolytes (e.g., sodium lauryl sulfate); therefore, one possible explanation of this result is that the ionized carbomers were converted into non-ionized form caused by the salting-out effect of the electrolytes. As for the creams containing HEC and xanthan gum, the viscosity values were very low. These low viscosity well agreed with low stability. In particular, the viscosity of the cream containing HEC (107.0±4.6 mPa·s) was lower than the control level (196.3±7.5 mPa·s), indicating that the cream was just diluted with water contained in the aqueous solution.

The measurement of the viscosity was triplicated at 40°C at the shear rate of 38.3 s−1. The concentrations of thickeners and surfactants were fixed at 0.1 and 2.0%, respectively.
To identify the additional effects of HEC and xanthan gum on the stability, test creams with different amounts were prepared (Fig. 5). Regarding the creams containing xanthan gum, the stability was steadily improved with increasing the concentration. When its concentration reached 0.20%, a stable emulsion state persisted for more than 14 d. As for the creams containing HEC, no improvement of the stability was observed, even though the concentration was increased to 0.30%. In addition, we also prepared creams containing lower amounts of H105. As shown in Fig. 5, stable creams were prepared even if the concentration was decreased to 0.05%. The viscosity of the creams must be proportionally increased with increasing thickeners’ concentration. On the whole, the test cream with high viscosity showed high stability; therefore, the thickening effects of the thickeners are no doubt one of the essential elements for the stabilizing effects.

The test creams with different concentrations of thickeners were prepared and then stored at 40°C for 30 d. Acquisition of each T2 map was triplicated. Representative images are shown in this figure.
In the viscosity measurement experiment, we also tested the creams containing surfactants. As shown in Fig. 4, some creams showed relatively high viscosity; for instance, the viscosities of the creams containing BL-9EX and BS-4, 718.0±10.8 and 571.0±27.5, respectively, were higher than that of H104. However, such a high viscosity appeared not to associate with improvement of the emulsion stability because their emulsion stability was lower by far than that with H103. In addition, the pattern of the SOM feature map of the viscosity (see Supplementary Fig. S1) seemed to be quite different from those of the lower layer areas at individual points and AUC. Therefore, we concluded that increase in the viscosity is not a crucial factor for the stabilizing effect of the surfactant. One possible reason for the different contribution of viscosity to the stabilizing effects of surfactants and thickeners is that they are located in different regions in the cream. Surfactants are thought to be mostly located on the surface of the oil droplets, while thickeners, which are hydrophilic polymers by nature, were widely spread in the continuous phase.16)
Combination Effects of Thickeners and Surfactants on the Emulsion Stability of the CreamIn the final phase of this study, we investigated the combined effect of surfactant and thickener on the stability. Based on the result of the screening test of surfactants, BL-9EX and TL-10 were selected as test surfactants. As for carbomers, H105 and H103 were selected. As shown in Fig. 6, the emulsion stability of the creams containing H103 was obviously improved by addition of the surfactants. After 30 d storage at 40°C, the addition of TL-10 almost prevented phase separation, while the area of the lower layer separated was obviously decreased by addition of BL-9EX. The lower layer areas after 30 d storage were 2.5±0.6 and 21.2±1.1% for the creams containing TL-10 and BL-9EX, respectively. In addition, as anticipated, no phase separation was observed from the creams containing H105 and surfactants. From the results, the combination of carbomers and surfactants on the stability was proven. It could serve as an effective preventive measure against phase separation when there is a need to improve the stability of the creams further.

TL-10 and BL-9EX (2.0%) were incorporated into the creams containing 0.1% of H103 and H105. These creams were stored at 40°C for 30 d, and then were monitored by T2 maps and visible observation. Acquisition of each T2 map was triplicated. Representative images are shown in this figure. The T2 map of the cream without additional surfactants was the same one shown in Fig. 3.
In clinical practice using the menthol–diphenhydramine cream, its low stability has been a problem. This study screened the stabilizing effects of surfactants and thickeners on the stability in detail. In particular, the stabilizing effect of H105 was by far the strongest. We believe the cream containing H105 is stable enough for clinical practice. In the course of this study, we also gained a comprehensive understanding of the stabilizing effects of surfactants. The analysis using SOM clustering indicated the potential relationships between emulsion stability and HLB of the surfactant; higher HLB of surfactant plays an important role in the stabilizing effect. We believe that our findings offer profound insights into the design of externally applied pharmaceuticals.
This study was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science. We thank Mr. Koji Ito at Nikko Chem. Co., Ltd. for a generous gift of surfactants and technical support. We thank Mr. Daiki Higuchi and Mr. Shu Kozawa at Hoshi University for their technical assistance.
The Department of Pharmaceutical Technology is an endowed department, supported with an unrestricted grant from Nichi-Iko Pharmaceutical Co., Ltd. (Toyama, Japan).
The online version of this article contains supplementary materials.
Fig. S1. SOM Feature Map of Viscosity of the Creams Containing Various Surfactants
This feature map corresponds with those shown in Fig. 2.