2016 Volume 64 Issue 12 Pages 1755-1762
2016 Volume 64 Issue 12 Pages 1755-1762
Acetylcholinesterase (AChE) is a key enzyme which present in the central nervous system of living organisms. Organophosphorus pesticides (OPs) that serve as insecticides are AChE inhibitors which have been used widely in agriculture. A series of novel OPs containing pyrazole moiety have been designed and synthesized. The biological evaluation indicated compound 4e appeared 81% larvicidal activity against Plutella xylostella at the concentration of 0.1 mg/L and the inhibition of AChE by compound 4e was distinctly enhanced with the increasing doses. Molecular docking of compound 4e into the three dimensional X-ray structure of the Drosophila melanogaster AChE (DmAChE, PDB code: 1QO9) was carried out utilizating the Discovery Studio (DS), the binding model revealed that the title structure was tightly embedded in the binding sites of DmAChE. Therefore, we suggest that compound 4e may serve as a novel AChE inhibitor that can be utilized as a new insecticidal drug.
The synthesis of organophosphorus compound began in early 1930s.1) Since then, organophosphorus insecticides represent one of the major classical insecticides that have been used over the past several decades around the world. However, organophosphorus insecticides have been excessively used over the past decades, and insects have became difficult to control due to their resistance to the traditional organophosphorus insecticides that cause enormous losses of crop production. The undifferentiated acts of organophosphorus on acetylcholinesterase (AChE) of organism are threatening to human health.2) Thus, the discovery of new organophosphorus insecticides that have low toxicity and can inhibit the inhibition of AChE is particularly important.
AChE is a key enzyme present in the central nervous system of living organisms. The crucial function of AChE is to terminate impulse transmission at cholinergic synapses through rapid hydrolysis of acetylcholine (ACh), thus to keep the normal transmission of nerve impulses,3–5) while the catalytic mechanism of ACh hydrolysis is a successive two-step process, namely, acylation and deacylation.6) Inhibition of AChE cannot lead to the hydrolysis of neurotransmitter ACh or accumulation of ACh in the synaptic cleft that results in that impeded neurotransmission is the primary mechanism of AChE inhibitors.7–10) Especially, many organophosphorus pesticides (OPs) that serve as insecticides are AChE (EC 3.1.1.7) inhibitors that are widely used in agriculture11–13) (Fig. 1). Those organophosphorus compounds prevent ACh hydrolysis, which results in overstimulation of the cholinergic synapses and leads to neuromuscular paralysis. As a consequence, this will contribute to the death of the insect.14–16) The electrophilic phosphorous atom of OPs can react with the hydroxyl group of the serine in the AChE through nucleophilic attack, which leads to the enzymes’ phosphorylation and deactivation, so these organophosphorus compounds are highly toxic.

If we introduce some heterocyclic (e.g., pyridine, furan, pyrimidine, thiazole) into the organophosphorus scaffold, the resulting compounds may have an interesting structure to reduce the toxicity to human health while maintaining its insecticidal activity, and the introduction of further structurally diversity to OPs is also conducive to delay the onset of resistance. The constitution of pyrazole derivatives is very important in pesticide field due to their broad range of biological activities, high efficiency and low toxicity,17–21) we thus choose pyrazole ring introduced into the α-aminophosphonate skeleton to synthesize a series of title compounds (Fig. 2), and some single-crystal structures were obtained. All compounds have been evaluated for their insecticidal activities against Plutella xylostella and were performed molecular docking, and the binding model revealed that the title structure was tightly embeded in the binding sites of DmAChE (PDB code: 1QO9). The structure–activity relationship for the inhibitory effects of the title structure on AChE was discussed.

All the title compounds 4a–t were synthesized by using the modified Thirumurugan’s method22) and the general synthetic route was depicted in Chart 1.

Reagents and conditions: (a) sodium acetate, ethanol, r.t.; (b) DMF, POCl3, 80–85°C, 5 h; (c) BF3·Et2O, acetonitrile, 3 h, reflux.
The crystal data and refinement parameter for part of the title compounds are listed in Table 1, the crystal structure is shown in Fig. 3. The structure was solved by direct methods and refined on F2 by full-matrix least-squares methods using SHELXTL.23) The unit cell dimensions and intensity data were recorded at 293 K. The program SAINT/XPREP was used for data reduction and APEX2/SAINT for cell refinement.24) All non-hydrogen atoms were refined with anisotropic thermal parameters. All hydrogen atoms with the exception of those on nitrogen atoms were geometrically fixed and refined using a riding model. Molecular graphics employed were MERCURY and PLATON.25) Crystallographic data (excluding structure factors) for the structure were deposited with the Cambridge Crystallographic Data Center as supplementary publication No. CCDC-1414578 (4e), CCDC-1414579 (4b), CCDC-1414580 (4i), CCDC-1414581 (4j), CCDC-1414582 (4k), CCDC-1414583 (4n), CCDC-1414584 (4r).
| Compound | 4b | 4e | 4i | 4j | 4k | 4n | 4r |
|---|---|---|---|---|---|---|---|
| Empirical formula | C27H30N3O4P | C28H32N3O3P | C27H29ClN3O3P | C27H29ClN3O4P | C26H26ClFN3O3P | C28H32N3O5P | C27H29FN3O4P |
| Molecular weight | 491.51 g/mol | 489.54 g/mol | 509.95 g/mol | 525.95 g/mol | 513.92 g/mol | 521.54 g/mol | 509.50 g/mol |
| Temperature (K) | 273(2) | 273(2) | 273(2) | 273(2) | 273(2) | 273(2) | 273(2) |
| Radiation | MoKα (0.71073 Å) | MoKα (0.71073 Å) | MoKα (0.71073 Å) | MoKα (0.71073 Å) | MoKα (0.71073 Å) | MoKα (0.71073 Å) | MoKα (0.71073 Å) |
| Crystal system | Orthorhombic | Monoclinic | Monoclinic | Monoclinic | Monoclinic | Monoclinic | Triclinic |
| Space group | Pbca | P2(1)/n | P2(1)/c | P2(1)/c | P2(1)/c | P2(1)/c | P-1 |
| a (Å) | 19.970(5) | 14.570(5) | 14.889(5) | 14.729(5) | 14.977(5) | 8.247(5) | 9.234(5) |
| b (Å) | 11.280(5) | 11.953(5) | 12.064(5) | 12.243(5) | 11.766(5) | 13.643(5) | 11.744(5) |
| c (Å) | 23.426(5) | 17.058(5) | 17.420(5) | 17.468(5) | 17.486(5) | 24.492(5) | 13.031(5) |
| α (°) | 90.000(5) | 90.000(5) | 90.000(5) | 90.000(5) | 90.000(5) | 90.000(5) | 81.400(5) |
| β (°) | 90.000(5) | 112.905(5) | 109.169(5) | 112.688(5) | 108.706(5) | 93.122(5) | 72.404(5) |
| γ (°) | 90.000(5) | 90.000(5) | 90.000(5) | 90.000(5) | 90.000(5) | 90.000(5) | 79.395(5) |
| V (Å3) | 5276.9(3) | 2736.3 (8) | 2955.4(6) | 2906.1(5) | 2918.5(2) | 2752(2) | 1317.3(10) |
| Z | 8 | 4 | 4 | 4 | 4 | 4 | 2 |
| Dc (g cm−3) | 1.237 | 1.188 | 1.146 | 1.202 | 1.170 | 1.259 | 1.284 |
| μ (mm−1) absort. coeff. | 0.141 | 0.133 | 0.213 | 0.221 | 0.221 | 0.141 | 0.149 |
| F (000) | 2080 | 1040 | 1072 | 1104 | 1072 | 1104 | 536 |
| θ rang (deg) | 2.25–25.20 | 2.35–25.20 | 2.30–25.20 | 2.27–25.20 | 2.25–25.20 | 2.24–25.20 | 2.30–30.75 |
| Reflections collected | 49287 (Rint=0.0302) | 13899 (Rint=0.0564) | 15930 (Rint=0.0453) | 28098 (Rint=0.0325) | 28353 (Rint=0.0266) | 26506 (Rint=0.0248) | 13623 (Rint=0.0154) |
| Indep. reflns | 4721 | 4737 | 5122 | 5219 | 5243 | 4929 | 4671 |
| Refns obs. [I>2σ(I)] | 3863 | 3131 | 3210 | 3677 | 4006 | 3908 | 4050 |
| Data/restr./paras | 4721/0/316 | 4737/0/317 | 5122/0/316 | 5219/0/334 | 5243/0/316 | 4929/0/334 | 4671/0/343 |
| Goodness-of-fit on F2 | 10.23 | 1.031 | 1.014 | 10.018 | 1.023 | 1.008 | 1.054 |
| R1, wR2 (all data) | 0.0714/0.1819 | 0.1590/0.3186 | 0.1323/0.2422 | 0.0851/0.1854 | 0.0652/0.1588 | 0.0730/0.1928 | 0.0666/0.1929 |
| R1, wR2 [I>2σ(I)] | 0.0590/0.1686 | 0.1092/0.2820 | 0.0889/0.2242 | 0.0569/0.1584 | 0.0496/0.1476 | 0.0585/0.1777 | 0.0600/0.1847 |
| Larg.peak/hole (e. Å) | 0.558/−0.403 | 0.580/−0.356 | 0.302/−0.264 | 0.334/−0.258 | 0.288/−0.282 | 1.259/−0.245 | 0.671/−0.463 |

All the compounds 4a–t were evaluated for their insecticidal activities against P. xylostella using procedures reported previously.26) Standard insecticidal agent chlorpyrifos were also screened under identical conditions for comparison. The results revealed that some of the synthetic compounds exhibited significant insecticidal activities in Table 2.
| Compound | R1 | R2 | Larvicidal activity (%) at a concentration of (mg/L) | ||||
|---|---|---|---|---|---|---|---|
| 10 | 5 | 1 | 0.5 | 0.1 | |||
| 4a | H | CH3 | 100 | 100 | 100 | 64 | 21 |
| 4b | H | OCH3 | 84 | 54 | 12 | 0 | 0 |
| 4c | H | F | 100 | 100 | 92 | 37 | 36 |
| 4d | H | Cl | 100 | 100 | 84 | 67 | 15 |
| 4e | CH3 | CH3 | 100 | 100 | 100 | 100 | 81 |
| 4f | CH3 | OCH3 | 100 | 100 | 85 | 54 | 16 |
| 4g | CH3 | F | 100 | 100 | 100 | 90 | 44 |
| 4h | CH3 | Cl | 100 | 100 | 100 | 85 | 34 |
| 4i | Cl | CH3 | 63 | 40 | 11 | 0 | 0 |
| 4j | Cl | OCH3 | 74 | 56 | 28 | 0 | 0 |
| 4k | Cl | F | 100 | 100 | 100 | 87 | 57 |
| 4l | Cl | Cl | 100 | 86 | 57 | 34 | 0 |
| 4m | OCH3 | CH3 | 100 | 95 | 72 | 46 | 21 |
| 4n | OCH3 | OCH3 | 100 | 94 | 67 | 27 | 15 |
| 4o | OCH3 | F | 100 | 100 | 100 | 84 | 55 |
| 4p | OCH3 | Cl | 79 | 51 | 18 | 0 | 0 |
| 4q | F | CH3 | 100 | 100 | 84 | 56 | 25 |
| 4r | F | OCH3 | 100 | 88 | 75 | 34 | 14 |
| 4s | F | F | 100 | 100 | 100 | 79 | 44 |
| 4t | F | Cl | 89 | 46 | 13 | 0 | 0 |
| Chlorpyrifos | 100 | 100 | 100 | 100 | 89 | ||
As Table 2 showed, some of the synthetic compounds exhibited the nearly equal inhibitory effects to chlorpyrifos at 10 or 5 mg/L, such as compounds 4g and e. Especially, compound 4e showed the most potent insecticidal activity against P. xylostella, which could be comparable with chlorpyrifos. In addition, introducing different substituent groups on benzene ring could lead to a remarkable change in bioactivity. For instance, when compound introduced electron-donating substituent groups on benzene ring, such compound insecticidal activity significantly increased, and when introduced electron-withdrawing substituent groups, compound insecticidal activity significantly decreases. In electron-withdrawing substituent groups, F showed the most potent insecticidal activity such as compounds 4c, g, k and o. However, not all compounds containing methyl are beneficial such as 4i and m. Similarly, not all compounds containing methyl are harmful such as 4g and h. Therefore, the activity of compounds is determined by R1 and R2. Further to say, as to the R1, R2 place, the contribution of electron-donating substituents is greater than electron-withdrawing substituents’, wherein CH3 has the greatest contribution. The results provided valuable information for the design of insecticidal agents.
Inhibition Assay against AChEIn order to determine the relationship between compounds and insecticidal effect, AChE inhibitory activity assays were performed. The results were given in Table 3. Chlorpyrifos showed the best inhibitory ability against AChE enzymes with IC50 values of 2.83 mg/L, and compound 4e showed slightly weak activity with IC50 values of 3.16 mg/L. Furthermore, these compounds show different inhibitory abilities with IC50 values from 3 to 10 mg/L exhibited the same tendency as the data acquired by using the insecticidal activity assay in vitro. AChE inhibitory activity assays fully proved that the target compounds displayed as good inhibitory effects as other successfully developed α-aminophosphonate insecticides. Therefore, we assumed that compound 4e could affect the function of AChE then lead to insect death.
| Compound | IC50 (mg/L)a) |
|---|---|
| 4a | 9.04±0.2 |
| 4e | 3.16±0.1 |
| 4g | 4.56±0.3 |
| 4h | 6.47±0.2 |
| 4o | 5.89±0.4 |
| 4s | 8.94±0.3 |
| Chlorpyrifos | 2.83±0.2 |
a) Values are the mean±standard deviation (S.D.) of three replicates.
The purpose was to ascertain at the molecular level that title compounds can provide a molecular explanation on the surprisingly good activity. Molecular docking of compound 4e into the three dimensional X-ray structures of the DmAChE (PDB code: 1QO9) was carried out using the Discovery Studio (DS) (version 3.5, NeoTrident Corporation, Beijing, China) software as implemented through the graphical user interface DS-CDOCKER protocol (Fig. 4). The three-dimensional (3D) structures of the aforementioned compounds were constructed using Chem. 3D ultra 12.0 software (Chemical Structure Drawing Standard, CambridgeSoft Corporation, Cambridge, MA, U.S.A.), then they were energetically minimized by using MMFF94 with 5000 iterations and minimum RMS gradient of 0.10. The crystal structures of protein complex were retrieved from the RCSB Protein Data Bank (http://www.rcsb.org/pdb/home/home.do) and prepared by DS 3.5 with all bound waters and ligands eliminated from the protein and the polar hydrogen added to the protein. The molecular docking procedure was performed by using CDOCKER protocol for receptor-ligand interactions section of DS 3.5.
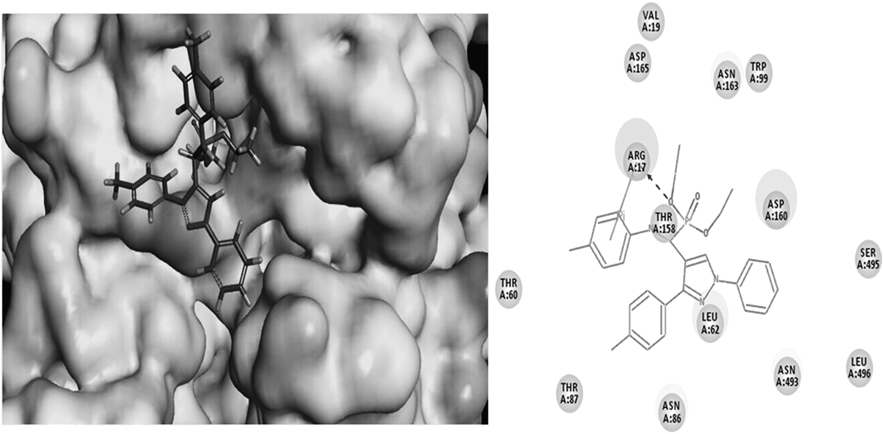
It is nicely bound to the DmAChE via one H-bonds (ARG 17-O: 2.32 Å, 103.31°) and a π–cation interaction (4.5 Å).
All of the synthesized compounds were chemically characterized by TLC, 1H-NMR and elemental microanalyses (CHN). 1H- and 13C-NMR spectra were measured on a Bruker AV-300 or a Agilent DD2 600 Hz spectrometer AV-600 spectrometer at 25°C and referenced to Me4Si. Chemical shifts were reported in ppm (δ) using the residual solvent line as internal standard. Splitting patterns were designated as s, singlet; d, doublet; t, triplet; m, multiplet. Electrospray ionization (ESI)-MS spectra were recorded on a Mariner System 5304 Mass spectrometer. Elemental analyses were performed on a CHN-O-Rapid instrument and were within ±0.4% of the theoretical values. Melting points (mp) were measured on a XT4 MP apparatus (Taike Corp, Beijing, China). Analytic TLC was performed on the glass backed silica gel sheets (silica gel 60A GF254). All compounds were detected using UV light (254 or 365 nm).
Preparation of the Intermediates 3-(4-Substituented Phenyl)-1-phenyl-1H-pyrazole-4-carbaldehyde Derivatives (3a–e)The intermediates 3-(4-substituented phenyl)-1-phenyl-1H-pyrazole-4-carbaldehyde (3a–e) were synthesized as following: para-substituted acetophenone (1a–e) (20 mmol) interacted with phenylhydrazine hydrochloride (20 mmol) couple with sodium acetate (40 mmol) in anhydrous ethanol to form 1-phenyl-2-(1-phenylethylidene) hydrazine (2a–e), which was then dissolved in a cold mixed solution of DMF (20 mL) and POCl3 (16 mL), stirred at 80–85°C for 5 h. The resulting mixture was poured into ice-cold water, a saturated solution of sodium hydroxide was added to neutralize the mixture, and the solid precipitate was filtered, washed with water, dried and recrystallized from ethanol to give the compounds 3a–e.27,28)
Preparation of Diethyl ((1,3-Diphenyl-1H-pyrazol-4-yl)(p-tolylamino)methyl)phosphonate Derivatives (4a–t)The intermediates 3-(4-substituented phenyl)-1-phenyl-1H-pyrazole-4-carbaldehyde 3a–e (1 mmol), para-substituted aniline (1 mmol) and diethyl phosphite (1 mmol) were added to acetonitrile (8 mL). Catalytic amount of BF3·Et2O solution in was also added. The mixture was stirred for 3 h at reflux temperature then cooled to ambient temperature. The solvent was concentrated under reduced pressure, and the solid was obtained, then the crude product was purified preparative TLC with a mixture of petroleum ether–ethyl acetate (1 : 1, v/v) as the developing solvent to yield the title compounds 4a–t.
Diethyl ((1,3-Diphenyl-1H-pyrazol-4-yl)(p-tolylamino)methyl)phosphonate (4a)White crystal, yield 88%; mp 184–185°C; 1H-NMR (300 MHz, CDCl3, δ ppm): 1.08 (t, J=7.2 Hz, 3H), 1.25 (t, J=7.2 Hz, 3H), 2.23 (s, 3H), 3.80–4.20 (m, 4H), 4.86 (d, J=21 Hz, 1H), 6.35 (d, J=8.4 Hz, 2H), 6.80 (d, J=8.4 Hz, 2H), 7.18–7.68 (m, 11H), 8.23 (d, J=2.04 Hz, 1H). MS (ESI): 476.5 (C27H30N3O3P, [M+H]+). Anal. Calcd for C27H30N3O3P: C, 68.15; H, 6.46; N, 8.92. Found: C, 68.20; H, 6.36; N, 8.84%.
Diethyl ((1,3-Diphenyl-1H-pyrazol-4-yl)((4-methoxyphenyl)amino)methyl)phosphonate (4b)White crystal, yield 90%; mp 190–192°C; 1H-NMR (300 MHz, CDCl3, δ ppm): 1.09 (t, J=7.2 Hz, 3H), 1.26 (t, J=7.2 Hz, 3H), 3.68 (s, 3H), 3.80–4.18 (m, 4H), 4.80 (d, J=21 Hz, 1H), 6.38 (d, J=8.4 Hz, 2H), 6.57 (d, J=8.4 Hz, 2H), 7.18–7.68 (m, 11H), 8.23 (d, J=2.04 Hz,1H). MS (ESI): 492.7 (C27H30N3O4P, [M+H]+). Anal. Calcd for C27H30N3O4P: C, 66.14; H, 6.27; N, 8.46. Found: C, 65.98; H, 6.15; N, 8.55%.
Diethyl ((1,3-Diphenyl-1H-pyrazol-4-yl)((4-fluorophenyl)amino)methyl)phosphonate (4c)White crystal, yield 75%; mp 166–167°C; 1H-NMR (300 MHz, CDCl3, δ ppm): 1.08 (t, J=7.2 Hz, 3H), 1.19 (t, J=7.2 Hz, 3H), 3.82–4.17 (m, 4H), 4.80 (d, J=21 Hz, 1H), 6.38 (q, 2H), 6.69 (q, 2H), 7.19-7.68 (m, 11H), 8.24 (d, J=2.04 Hz,1H). MS (ESI): 480.8 (C26H27FN3O3P, [M+H]+). Anal. Calcd for C26H27FN3O3P: C, 65.25; H, 5.72; N, 8.68. Found: C, 65.13; H, 5.68; N, 8.76%.
Diethyl (((4-Chlorophenyl)amino)(1,3-diphenyl-1H-pyrazol-4-yl)methyl)phosphonate (4d)White crystal, yield 83%; mp 188–190°C; 1H-NMR (300 MHz, CDCl3, δ ppm): 1.08 (t, J=7.2 Hz, 3H), 1.19 (t, J=7.2 Hz, 3H), 3.80–4.17 (m, 4H), 4.81 (d, J=21 Hz, 1H), 6.35 (d, J=8.79 Hz, 2H), 6.94 (d, J=6.78 Hz, 2H), 7.19–7.67 (m, 11H), 8.24 (d, J=2.04 Hz, 1H). MS (ESI): 497.3 (C26H27ClN3O3P, [M+H]+). Anal. Calcd for C26H27ClN3O3: C, 63.12; H, 5.47; N, 8.58. Found: C, 62.97; H, 5.49; N, 8.47%.
Diethyl ((1-Phenyl-3-(p-tolyl)-1H-pyrazol-4-yl)(p-tolylamino)methyl)phosphonate (4e)White crystal, yield 89%; mp 172–174°C; 1H-NMR (300 MHz, CDCl3, δ ppm): 1.16 (t, J=7.2 Hz, 3H), 1.25 (t, J=7.2 Hz, 3H), 2.19 (s, 3H), 2.43 (s, 3H), 3.70–4.28 (m, 4H), 4.91 (d, J=21 Hz, 1H), 6.42 (d, J=8.4 Hz, 2H), 6.88(d, J=8.4 Hz, 2H), 7.24–7.75 (m, 10H), 8.28 (d, J=2.04 Hz, 1H). 13C-NMR (151 MHz, CDCl3) δ: 152.66, 152.60, 139.80, 138.09, 129.81, 129.61, 129.38, 129.30, 128.47, 127.58, 127.56, 126.40, 118.89, 116.64, 114.41, 63.49 63.34, 47.32 (d, J=159.8 Hz, C-P), 21.34, 20.37, 16.46, 16.26. MS (ESI): 490.5 (C28H32N3O3P, [M+H]+). Anal. Calcd for C28H32N3O3P: C, 68.78; H, 6.67; N, 8.49; Found: C, 68.70; H, 6.59; N, 8.58%.
Diethyl (((4-Methoxyphenyl)amino)(1-phenyl-3-(p-tolyl)-1H-pyrazol-4-yl)methyl)phosphonate (4f)White crystal, yield 69%; mp 156–158°C; 1H-NMR (300 MHz, CDCl3, δ ppm): 1.08 (t, J=7.2 Hz, 3H), 1.23 (t, J=7.2 Hz, 3H), 2.35 (s, 3H), 3.62 (s, 3H), 3.80–4.18 (m, 4H), 4.79 (d, J=21 Hz, 1H), 6.39 (d, J=8.4 Hz, 2H), 6.65(d, J=8.4 Hz, 2H), 7.19–7.67 (m, 10H), 8.21 (d, J=2.04 Hz, 1H). MS (ESI): 506.6 (C28H32N3O4P, [M+H]+). Anal. Calcd for C28H32N3O4P: C, 66.54; H, 6.27; N, 8.21. Found: C, 66.52; H, 6.38; N, 8.31%.
Diethyl (((4-Fluorophenyl)amino)(1-phenyl-3-(p-tolyl)-1H-pyrazol-4-yl)methyl)phosphonate (4g)White crystal, yield 87%; mp 176–178°C; 1H-NMR (300 MHz, DMSO-d6, δ ppm): 1.18 (t, J=7.2 Hz, 3H), 1.28 (t, J=7.2 Hz, 3H), 2.43 (s, 3H), 3.80–4.18 (m, 4H), 4.85 (d, J=21 Hz, 1H), 6.45 (d, J=8.4 Hz, 2H), 6.78(d, J=8.4 Hz, 2H), 7.00–7.75 (m, 10H), 8.28 (d, J=2.04 Hz, 1H). MS (ESI): 494.3 (C27H29FN3O3P, [M+H]+). Anal. Calcd for C27H29FN3O3P: C, 65.75; H, 6.02; N, 8.45. Found: C, 65.71; H, 5.92; N, 8.51%.
Diethyl (((4-Chlorophenyl)amino)(1-phenyl-3-(p-tolyl)-1H-pyrazol-4-yl)methyl)phosphonate (4h)White crystal, yield 85%; mp 194–196°C; 1H-NMR (300 MHz, CDCl3, δ ppm): 1.16 (t, J=7.2 Hz, 3H), 1.25 (t, J=7.2 Hz, 3H), 2.44 (s, 3H), 3.70–4.20 (m, 4H), 4.89 (d, J=21 Hz, 1H), 6.45 (d, J=8.4 Hz, 2H), 7.02 (d, J=8.4 Hz, 2H), 7.30–7.75 (m, 10H), 8.31 (d, J=2.04 Hz, 1H). MS (ESI): 510.4 (C27H29ClN3O3P, [M+H]+). Anal. Calcd for C27H29ClN3O3P: C, 63.57; H, 5.82; N, 8.15. Found: C, 63.59; H, 5.73; N, 8.24%.
Diethyl ((3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)(p-tolylamino)methyl)phosphonate (4i)White crystal, yield 83%; mp 182–184°C; 1H-NMR (300 MHz, CDCl3, δ ppm): 1.08 (t, J=7.2 Hz, 3H), 1.21 (t, J=7.2 Hz, 3H), 2.11 (s, 3H), 3.84–4.15 (m, 4H), 4.79 (d, J=21 Hz, 1H), 6.33 (d, J=8.4 Hz, 2H), 6.81 (d, J=8.4 Hz, 2H), 7.19–7.66 (m, 10H), 8.21 (d, J=2.04 Hz, 1H). MS (ESI): 510.9 (C27H29ClN3O3P, [M+H]+). Anal. Calcd for C27H29ClN3O3P: C, 63.51; H, 5.66; N, 8.29. Found: C, 63.59; H, 5.73; N, 8.24%.
Diethyl ((3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)((4-methoxyphenyl)amino)methyl)phosphonate (4j)White crystal, yield 87%; mp 146–148°C; 1H-NMR (300 MHz, CDCl3, δ ppm): 1.15 (t, J=7.2 Hz, 3H), 1.28 (t, J=7.2 Hz, 3H), 3.78 (s, 3H), 3.86–4.25 (m, 4H), 4.80 (d, J=21 Hz, 1H), 6.46 (d, J=8.4 Hz, 2H), 6.68 (d, J=8.4 Hz, 2H), 7.16–7.63 (m, 10H), 8.30 (d, J=2.04 Hz,1H). MS (ESI): 527.2 (C27H29ClN3O4P, [M+H]+). Anal. Calcd for C27H29ClN3O4P: C, 61.72; H, 5.67; N, 8.05. Found: C, 61.66; H, 5.56; N, 7.99%.
Diethyl ((3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)((4-fluorophenyl)amino)methyl)phosphonate (4k)White crystal, yield 78%; mp 164–166°C; 1H-NMR (300 MHz, CDCl3, δ ppm): 1.16 (t, J=7.2 Hz, 3H), 1.37 (t, J=7.2 Hz, 3H), 3.90–4.25 (m, 4H), 4.80 (d, J=21 Hz, 1H), 6.43 (d, J=8.4 Hz, 2H), 6.78 (d, J=8.4 Hz, 2H), 7.29–7.73 (m, 10H), 8.30 (d, J=2.04 Hz, 1H). MS (ESI): 514.7 (C26H26ClFN3O3P, [M+H]+). Anal. Calcd for C26H26ClFN3O3P: C, 60.87; H, 5.17; N, 8.25. Found: C, 60.76; H, 5.10; N, 8.18%.
Diethyl ((3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)((4-chlorophenyl)amino)methyl)phosphonate (4l)White crystal, yield 80%; mp 163–165°C; 1H-NMR (300 MHz, CDCl3, δ ppm): 1.16 (t, J=7.2 Hz, 3H), 1.37 (t, J=7.2 Hz, 3H), 3.94–4.18 (m, 4H), 4.82 (d, J=21 Hz, 1H), 6.44 (d, J=8.4 Hz, 2H), 7.03 (d, J=8.4 Hz, 2H), 7.29–7.73 (m, 10H), 8.30 (d, J=2.04 Hz, 1H). MS (ESI): 531.4 (C26H26Cl2N3O3P, [M+H]+). Anal. Calcd for C26H26Cl2N3O3P: C, 58.97; H, 5.01; N, 7.85. Found: C, 58.88; H, 4.94; N, 7.92%.
Diethyl ((3-(4-Methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)(p-tolylamino)methyl)phosphonate (4m)White crystal, yield 85%; mp 181–183°C; 1H-NMR (300 MHz, CDCl3, δ ppm): 1.15 (t, J=7.2 Hz, 3H), 1.28(t, J=7.2 Hz, 3H), 2.31 (s, 3H), 3.88 (s, 3H), 4.02–4.21 (m, 4H), 4.89 (d, J=21 Hz, 1H), 6.41 (d, J=8.4 Hz, 2H), 6.87 (d, J=8.4 Hz, 2H), 7.28–7.73 (m, 10H), 8.26 (d, J=2.04 Hz, 1H). MS (ESI): 506.9 (C28H32N3O4P, [M+H]+). Anal. Calcd for C28H32N3O4P: C, 66.43; H, 6.45; N, 8.29. Found: C, 66.52; H, 6.38; N, 8.31%.
Diethyl ((3-(4-Methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)((4-methoxyphenyl)amino)methyl)phosphonate (4n)White crystal, yield 86%; mp 148–150°C; 1H-NMR (300 MHz, CDCl3, δ ppm): 1.15 (t, J=7.2 Hz, 3H), 1.26 (t, J=7.2 Hz, 3H), 3.68 (s, 3H), 3.86 (s, 3H), 4.02–4.21 (m, 4H), 4.83 (d, J=21 Hz, 1H), 6.45 (d, J=8.4 Hz, 2H), 6.64 (d, J=8.4 Hz, 2H), 6.97–7.73 (m, 10H), 8.26 (d, J=2.04 Hz, 1H). MS (ESI): 522.6 (C28H32N3O5P, [M+H]+). Anal. Calcd for C28H32N3O5P: C, 64.54; H, 6.21; N, 8.12. Found: C, 64.48; H, 6.18; N, 8.06%.
Diethyl (((4-Fluorophenyl)amino)(3-(4-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)methyl)phosphonate (4o)White crystal, yield 78%; mp 180–181°C; 1H-NMR (300 MHz, CDCl3, δ ppm): 1.15 (t, J=7.2 Hz, 3H), 1.30 (t, J=7.2 Hz, 3H), 3.87(s, 3H), 3.91–4.11 (m, 4H), 4.83 (d, J=21 Hz, 1H), 6.46 (d, J=8.4 Hz, 2H), 6.76(d, J=8.4 Hz, 2H), 6.97–7.74 (m, 10H), 8.29 (d, J=2.04 Hz, 1H). MS (ESI): 510.6 (C27H29FN3O4P, [M+H]+). Anal. Calcd for C27H29FN3O4P: C, 63.74; H, 5.82; N, 8.21. Found: C, 63.65; H, 5.74; N, 8.25%.
Diethyl (((4-Chlorophenyl)amino)(3-(4-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)methyl)phosphonate (4p)White crystal, yield 81%; mp 186–188°C; 1H-NMR (300 MHz, CDCl3, δ ppm): 1.16 (t, J=7.2 Hz, 3H), 1.28 (t, J=7.2 Hz, 3H), 3.87 (s, 3H), 3.91–4.21 (m, 4H), 4.85 (d, J=21 Hz, 1H), 6.43 (d, J=8.4 Hz, 2H), 6.99 (d, J=8.4 Hz, 2H), 7.27–7.74 (m, 10H), 8.28 (d, J=2.04 Hz, 1H). MS (ESI): 527.1 (C27H29ClN3O4P, [M+H]+). Anal. Calcd for C27H29ClN3O4P: C, 61.57; H, 5.62; N, 8.02. Found: C, 61.66; H, 5.56; N, 7.99%.
Diethyl ((3-(4-Fluorophenyl)-1-phenyl-1H-pyrazol-4-yl)(p-tolylamino)methyl)phosphonate (4q)White crystal, yield 79%; mp 172–174°C; 1H-NMR (300 MHz, CDCl3, δ ppm): 1.17 (t, J=7.2 Hz, 3H), 1.30 (t, J=7.2 Hz, 3H), 2.20 (s, 3H), 3.88–4.28 (m, 4H), 4.85 (d, J=21 Hz, 1H), 6.43 (d, J=8.4 Hz, 2H), 6.89 (d, J=8.4 Hz, 2H), 7.14–7.78 (m, 10H), 8.31 (d, J=2.04 Hz, 1H). MS (ESI): 510.9 (C27H29FN3O3P, [M+H]+). Anal. Calcd for C27H29FN3O3P: C, 65.71; H, 5.92; N, 8.51. Found: C, 65.84; H, 5.74; N, 8.65%.
Diethyl ((3-(4-Fluorophenyl)-1-phenyl-1H-pyrazol-4-yl)((4-methoxyphenyl)amino)methyl)phosphonate (4r)White crystal, yield 84%; mp 155–157°C; 1H-NMR (300 MHz, CDCl3, δ ppm): 1.16 (t, J=7.2 Hz, 3H), 1.30 (t, J=7.2 Hz, 3H), 3.68 (s, 3H), 3.84–4.23 (m, 4H), 4.84 (d, J=21 Hz, 1H), 6.46 (d, J=8.4 Hz, 2H), 6.78 (d, J=8.4 Hz, 2H), 7.17–7.74 (m, 10H), 8.31 (d, J=2.04 Hz,1H). MS (ESI): 527.2 (C27H29FN3O4P, [M+H]+). Anal. Calcd for C27H29FN3O4P: C, 63.65; H, 5.74; N, 8.25. Found: C, 63.74; H, 5.68; N, 8.14%.
Diethyl ((3-(4-Fluorophenyl)-1-phenyl-1H-pyrazol-4-yl)((4-fluorophenyl)amino)methyl)phosphonate (4s)White crystal, yield 74%; mp 143–145°C; 1H-NMR (300 MHz, CDCl3, δ ppm): 1.14 (t, J=7.2 Hz, 3H), 1.36 (t, J=7.2 Hz, 3H), 3.94–4.23 (m, 4H), 4.84 (d, J=21 Hz, 1H), 6.45 (d, J=8.4 Hz, 2H), 6.73 (d, J=8.4 Hz, 2H), 7.24–7.71 (m, 10H), 8.31 (d, J=2.04 Hz, 1H). MS (ESI): 514.7 (C26H26F2N3O3P, [M+H]+). Anal. Calcd for C26H26F2N3O3P: C, 62.77; H, 5.27; N, 8.45. Found: C, 62.85; H, 5.19; N, 8.37%.
Diethyl (((4-Chlorophenyl)amino)(3-(4-fluorophenyl)-1-phenyl-1H-pyrazol-4-yl)methyl)phosphonate (4t)White crystal, yield 82%; mp 168–169°C; 1H-NMR (300 MHz, CDCl3, δ ppm): 1.18 (t, J=7.2 Hz, 3H), 1.27 (t, J=7.2 Hz, 3H), 3.90–4.25 (m, 4H), 4.83 (d, J=21 Hz, 1H), 6.44 (d, J=8.4 Hz, 2H), 7.03 (d, J=8.4 Hz, 2H), 7.17–7.71 (m, 10H), 8.31 (d, J=2.04 Hz, 1H). MS (ESI): 531.4 (C26H26ClFN3O3P, [M+H]+). Anal. Calcd for C26H26ClFN3O3P: C, 60.76; H, 5.10; N, 8.18. Found: C, 50.81; H, 4.95; N, 7.99%.
Insecticidal ActivityThe larvicidal activity of the title compounds and contrast compound fipronil and chlorantraniliprole against P. xylostella was tested by the leaf-dip method using the reported procedure. The bioassay was replicated at 25±1°C according to statistical requirements. Assessments were made on a dead/alive basis, and mortality rates were corrected applying Abbott’s formula.
Fresh cabbage discs were dipped into the test solutions containing title compounds and the control reagent of for 10 s, after air-drying, and the treated leaf disks were placed individually in a petri dish lined with filter paper. Thirty larvae of second-instar P. xylostella were carefully transferred to the dried treated leaf disk. Percentage mortalities were evaluated 3 d after treatment. Each treatment was performed three times. The insecticidal activity is summarized in Table 2.
AChE Inhibitory ActivityThe activities of AChE was measured according to the instructions of the acetyl cholinesterase assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).29) The optical density (OD) of AChE was monitored at 412 nm using a Thermo Fisher Multiskan GO (Thermo Scientific Inc., U.S.A.). Three replicates were maintained for each treatment and per replicate was performed thrice.
Crystal Structure DeterminationCrystal structure determination of title compounds was carried out on a Bruker D8 VENTURE PHOTON equipped with graphitemonochromated MoKα (1 1/4 0.71073 Å) radiation. The structure was solved by direct methods and refined on F2 by full-matrix leastsquares methods using SHELXTL.23) All non-hydrogen atoms were refined with anisotropic thermal parameters. All hydrogen atoms with the exception of those on nitrogen atoms were geometrically fixed and refined using a riding model.
Molecular DockingThe crystal structures of DmAChE (PDB code: 1QO9) were retrieved from the Protein Data Bank. The molecular docking procedure was performed by using CDOCKER protocol for receptor-ligand interactions section of DS 3.1 (Discovery Studio 3.5, Accelrys, Inc., San Diego, CA, U.S.A.).30)
To sum up, a series of α-aminophosphonate derivatives containing a pyrazole moiety has been designed and the target compounds 4a–t have been evaluated for their insecticidal activity and AChE inhibitory activity. The biological evaluation showed that some of compounds exhibit excellent larvicidal activities against P. xylostella. Specifically, compound 4e shows the significant inhibitory effect for AChE. For the binding model of 4e an H-bond (2.32 Å, 103.31°) is established between its oxygen atomic and ARG17, and it also forms a Pi–cation interaction (4.5 Å). In short, the results obtained from this study suggest that compound 4e may serve as a novel AChE inhibitor used as a new insecticidal drug.
This work was supported by National Natural Science Foundation of China (No. 21302002) and Anhui Natural Science Foundation (1408085QB33). This work has been checked by Dr. Deepak Kumar.
The authors declare no conflict of interest.