2016 Volume 64 Issue 2 Pages 135-141
2016 Volume 64 Issue 2 Pages 135-141
Two N-benzenesulfonyl (BS) derivatives of 1,2,3,4-tetrahydroquinoline (THQ) were designed, prepared, and screened for antibacterial activity. This approach was based on combining the two privileged structures, BS and THQ, which are known to be active. The objective of this study was to evaluate the antibacterial activity of BS-THQ and its analogue 4-NH2BS-THQ, and to investigate the roles of reactive oxygen species and reactive nitrogen species in their lethality. Both showed bactericidal activity against Staphylococcus aureus ATCC 29213 and methicillin-resistant S. aureus (MRSA) ATCC 43300, with transmission electron microscopy revealing a disturbed membrane architecture. Furthermore, an increase of reactive oxygen species (ROS) in strains treated with BS-THQ with respect to the control was detected when fluorescent microscopy and spectrophotometric techniques were used. The analogue 4-NH2BS-THQ demonstrated a broader spectrum of activity than BS-THQ, with a minimum inhibitory concentration of 100 µg/mL against reference strains of S. aureus, Escherichia coli and Pseudomonas aeruginosa. The assayed compounds represent promising structures for the development of new synthetic classes of antimicrobials.
Bacterial antibiotic resistance has become a major public health problem, principally caused by great bacterial adaptability and the non-rational use of medicines. The steadily increasing prevalence of methicillin-resistant Staphylococcus aureus (MRSA) and multi-resistant Gram-negative pathogens, such as Enterobacteriaceae, and Pseudomonas aeruginosa, make it clear that new antibiotics against multi-resistant bacteria are urgently needed, and in particular,1–3) drugs with novel mechanisms of action. Therefore, the call for a greater effort to be applied to developing new classes of antibacterials is fully justified, with a number of strategies currently being employed in the search for new compounds.4)
As part of our present research project to discover new anti-infective compounds, we are investigating series of N-benzenesulfonyl derivatives of heterocycles (BS-Het), with fragment-based drug design,5) and especially the hybrid systems concept being selected as the techniques.6) In this study, the two privileged scaffolds are represented by 1,2,3,4-tetrahydroquinoline (THQ) and benzenesulfonyl moieties (BSs). The BS is a group frequently present in biologically active molecules, which leads to analogues with similar or better biological activities than their precursors, with the THQ7) moiety being present in compounds with diverse biological properties, such as antimalarial activity,8) anticancer activity,9) nonsteroidal glucocorticoid receptor ligands,10) agonists of β3 adrenergic receptors11) and histamine H3 receptor antagonists,12) among others.
Previously, we reported N-benzenesulfonyl-benzotriazol (BS-BZT) and its analogue N-4-acetamidobenzenesulfonyl-benzotriazol (AcNHBS-BZT) having activity against Escherichia coli and S. aureus. Moreover, BS-BZT generated stimuli of superoxide anion (O2·−) in the latter species, indicating that it influences the bacterial respiratory metabolism.13) These interesting results prompted us to go further with the exploration of the mechanism of action and the structure–activity relationship (SAR) analysis. However, the BS-BZT derivatives produced were very insoluble and also decomposed in dimethyl sulfoxide (DMSO) solution, where most of the biological experiments were performed. In an attempt to overcome these limitations, we selected other structurally related BS-Het with different heterocycles.
Bearing all above considerations in mind, the two analogue N-benzenesulfonyl-1,2,3,4-tetrahydroquinoline (BS-THQ) and N-4-aminobenzenesulfonyl-1,2,3,4-tetrahydroquinoline (4-NH2BS-THQ) were synthesized and assayed as antibacterials. Both compounds were found to be very stable and slightly more soluble than BS-BZT in most organic solvents compatible with microbiological assays, including DMSO. Furthermore, all the molecules were evaluated in a pre-screening examination of their drug-likeness profiles by using Molinspiration web JME Editor14) and OSIRIS Property Explorer.15)
It is known that different bactericidal compounds such as ciprofloxacin, gentamicin and chloramphenicol, among others, can generate deleterious reactive oxygen species (ROS) that contribute to cell killing, regardless of their drug–target interactions. Moreover, a relationship between this observable fact and antibacterial activity has been demostrated.16–18)
The objective of the present study was to evaluate the antibacterial profile of BS-THQ and 4-NH2BS-THQ by minimum inhibitory concentration (MIC) and time–kill curves, with a principal aim being to explore their activity against methicillin-resistant strains. Furthermore, the production of reactive species and their relationship with antibacterial action was also investigated.
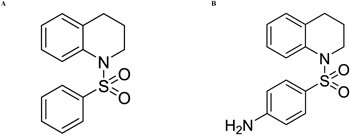
Both BS-THQ (Fig. 1A) and 4-NH2BS-THQ (Fig. 1B) were prepared, purified and their purity checked as previously reported.7,19) The molecular physicochemical properties relevant to drug design, including CLOGP, molecular polar surface area (PSA) and the Rule of 5 descriptors were calculated using the Molinspiration14) and OSIRIS Property Explorer15) free programs. By employing the former one, the compounds were found to obey Lipinski’s rule of 5 for an orally active drug.20) Of most interest, was the parameter predicting membrane penetration and drug transportation properties such as topological polar surface area (TPSA), C log P and molecular weight (M. Wt.). The drug score predicted by OSIRIS combines drug-likeness, lipophilicity, solubility, molecular weight and the risk of toxicity by qualifying the molecule through colour coding (green to red). This was found to be green for 4-NH2BS-THQ and orange for BS-THQ, which implies that the best performance was shown by the first molecule.
The effect of compounds (Fig. 1) on the growth of Gram-positive and Gram-negative strains was investigated by performing in vitro antimicrobial susceptibility experiments and calculating their MIC. Sulfisoxazole, a commercially available antimicrobial agent, was selected as a reference drug due to its structural similarity with the BS moiety, with BS-THQ and 4-NH2BS-THQ presenting antibacterial activity when tested against the reference strains S. aureus ATC C 29213, MRSA ATC C 43300, E. coli ATC C 25922 and P. aeruginosa ATC C 27853.
BS-THQ revealed MICs of 200 µg/mL (0.73 mM) against S. aureus ATC C 29213 and MRSA ATC C 43300; and MICs higher than 200 µg/mL against E. coli ATC C 25922 and P. aeruginosa ATC C 27853. Moreover, when its bactericidal effect in Gram-positive bacteria was evaluated, reductions of >3 log 10 against S. aureus ATC C 29213 (Fig. 2A) and MRSA ATC C 43300 (Fig. 2B) were observed.
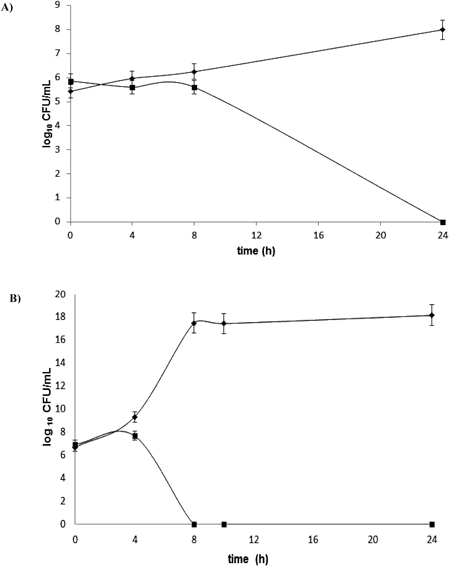
Control without BS-THQ (◆) and treated with 200 µg/mL BS-THQ (■). The treatment with BS-THQ shows a reduction of ≥3 log 10 against S. aureus ATCC 29213 and S. aureus ATCC 43300.
The MICs of the derivative 4-NH2BS-THQ were also measured in order to establish if the amine substitution could improve the antibacterial activity of BS-THQ, with the value of the MICs for all reference strains studied being 100 µg/mL. Therefore, these findings indicate that the amino substitution improved in vitro activity and widened the spectrum of action to Gram-negative bacteria.
Bacterial Stress Determined by Spectrophotometry and Fluorescence MicroscopyIn order to evaluate the ability of the compound to generate ROS, the nitro blue tetrazolium (NBT) assay was performed. As shown in Figs. 3A–C, there was a dose-dependent effect in every studied strain. In S. aureus, a remarkable difference in the generation of radicals between compounds was observed (Figs. 3A, B), with a clear increase in ROS at sub-MIC (12.5 µg/mL) with 4-NH2BS-THQ. It is worth mentioning that the induced stress in MRSA ATC C 43300 strain attained almost 80% of the ROS production (Fig. 3B). Furthermore, the amino derivative affected the oxidative metabolism in Gram-negative strains. At Sub-MIC (12.5 µg/mL), the percentage of ROS was nearly 70% and 20% for E. coli and P. aeruginosa, respectively (Fig. 3C).
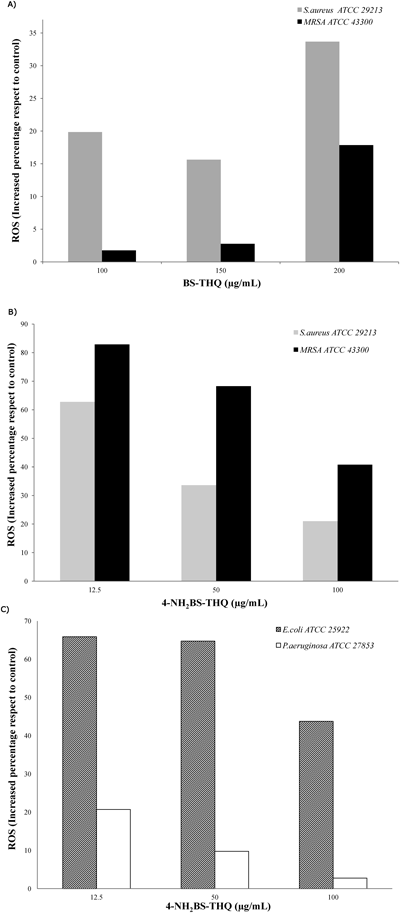
An increase in ROS was founded in treated strains with respect to the untreated control.
In summary, the results obtained from the NBT assays demonstrated that there were evident differences in ROS generation and in the respiratory metabolism between compounds against the same strains and also among strains with the same compounds.
To add to the results obtained by the NBT assays, the method of fluorescence microscopy with a sensitive probe to ROS was applied. Figure 4A shows untreated S. aureus ATC C 29213 and the image corresponding to MRSA is presented in Fig. 4B, with ciprofloxacin being employed as a positive control (Figs. 4E, F). The enhanced fluorescence intensity was easily visualized, which displayed an intracellular production of ROS in S. aureus by BS-THQ (Fig. 4C). Furthermore, MRSA ATC C 43300 revealed a lower fluorescence (Fig. 4D) than the sensitive strain, in agreement with the NBT findings.
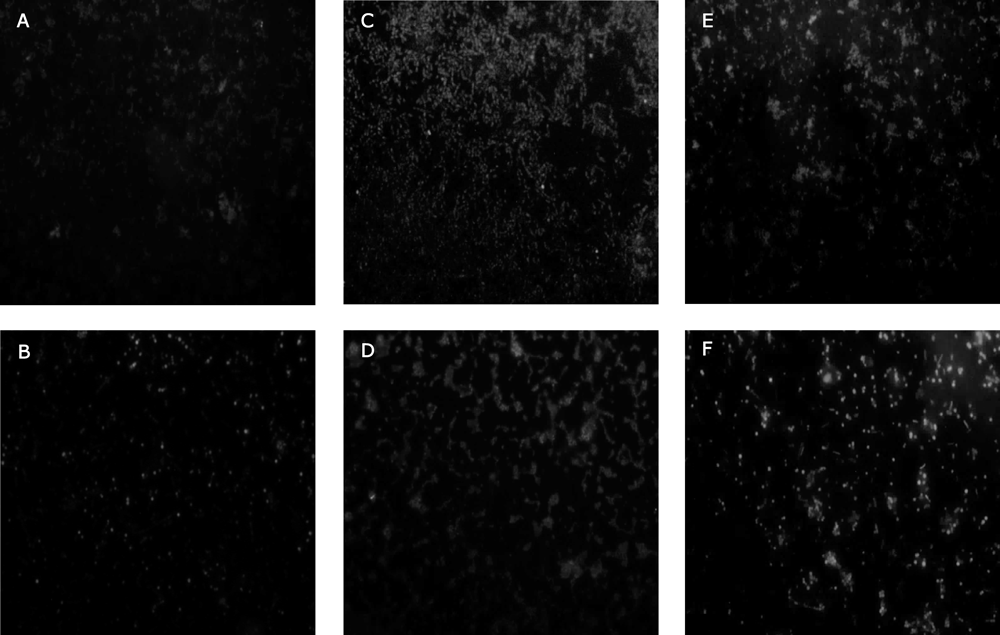
Untreated cells of S. aureus ATCC 29213 (A) and MRSA (B). Treated samples after 2 h incubation with BS-THQ, S. aureus ATCC 29213 (C) and MRSA (D). Strains treated as a positive control with ciprofloxacin, S. aureus ATCC 29213 (E) and MRSA (F).
The results obtained support the hypothesis that BS-THQ is a redox-cycling active compound able to cause bacterial killing through ROS production as part of its lethality. The analogue 4-NH2BS-THQ generated higher levels of ROS compared to BS-THQ, indicating a major perturbation of the respiratory metabolism in both S. aureus strains, with 4-NH2BS-THQ inducing a 60% and 80% increase of ROS at the lowest Sub-MIC (12.5 µg/mL) in S. aureus ATC C 29213 and MRSA ATC C 43300, respectively. From a structural point of view, these findings may be attributed to the unique amine substitution in the BS moiety (Fig. 1).
It was of interest to determine whether the metabolism of nitrogen was also affected in strains treated with 4-NH2BS-THQ. Even though the level of ROS in P. aeruginosa induced by 12.5 µg/mL of 4-NH2BS-THQ was only around 20% (Fig. 3C), the quantification of reactive nitrogen species (RNS) was around 200-fold higher compared to the control (Fig. 5). When the same measurements were performed in S. aureus strains, a similar behaviour was observed. In contrast, for E. coli, the production of RNS at Sub-MIC was almost the same as the control (Fig. 5).
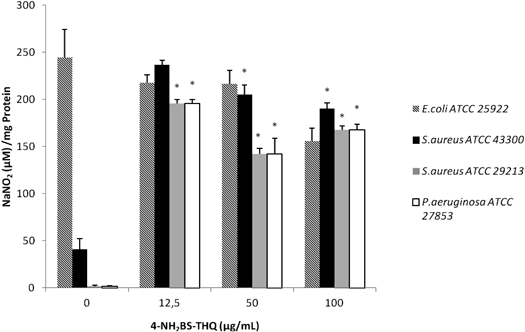
An increase of RNS was observed in treated strains respect to the untreated control. * p<0.05 respect to the control.
Reactive nitrogen intermediates can modify proteins, DNA and lipids in a number of ways. For example, the product of the combination reaction between nitric oxide (NO·) and superoxide (O2·−), which is peroxynitrite, promotes oxidative injury.21) Thus, the high levels of RNS obtained in S. aureus and P. aeruginosa treated with 4-NH2BS-THQ could be the consequence of the above-mentioned chemical reaction. Nevertheless, in E. coli there was no induction of RNS, which may be attributed to the fact that the production of these species is related to the metabolism and the detoxifying activities of each pathogen.22)
The effect of oxidative and nitrosative stress is one of the most important sources of metabolic disturbances and cellular damage. Previously published studies have shown that antibiotics with different mechanisms of action can stimulate the induction of ROS and RNS, leading to macromolecular damage of the cells in Gram-positive and Gram-negative bacteria.17,23–25) These agents are also involved in changes in the plasmatic membrane, and consequently, produce alterations at the beginning of cellular death.26,27) In this regard, Wenzel et al. reported antibiotic properties of new heterocyclic compounds, which led to activity against S. aureus, including MRSA and an associated structural membrane alteration with increased in ROS.1) Moreover, Desai et al. described a new compound with activity against E. coli, which induces oxidative stress associated with cell killing.28)
Transmission Electron Microscopy (TEM) of BS-THQ and 4-NH2BS-THQ Treated BacteriaAs activity was shown by BS-THQ in S. aureus ATC C 29213, the TEM technique was selected to investigate how the compound affected the bacterial cell morphology. Untreated cells revealed a normal structure (Figs. 6A, B), but large changes in the membrane, amorphous shape and bright electron areas in the centre of the treated cells were observed (Figs. 6C–F). The formation of vacuole can be seen in Figs. 6C and D, while Fig. 6E shows several ghost cells and others without a wall. Additionally, cells containing septa are also shown in Figs. 6E and F.
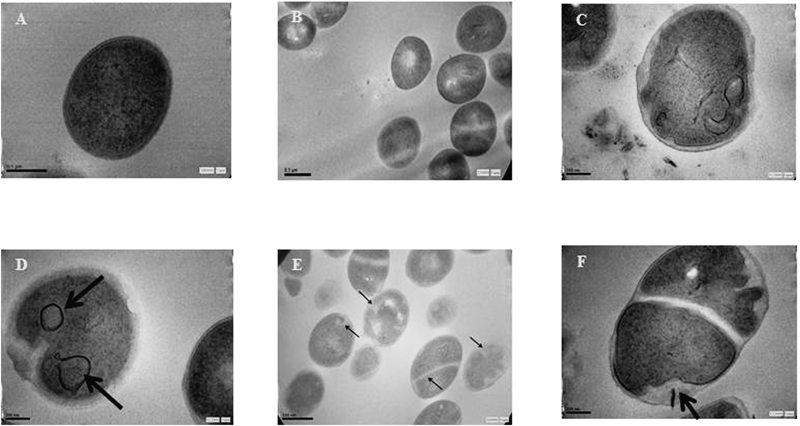
Untreated cells of S. aureus showed a normal cell shape with an undamaged structure of the membrane (Figs. A, B). A disturbed cell integrity was evident in BS-THQ treated samples after 24 h of incubation (Figs. C–F). Scale bar=0.5 µm.
As the amino substituted analogue improved the activity and broadened the spectrum of action compared to BS-THQ, the morphological damage in E. coli was assessed by TEM. In contrast with untreated cells (Figs. 7A, B), E. coli exposed to 4-NH2BS-THQ displayed several morphological changes, such as a rough surface (Figs. 7C–F), with the inner membrane boundaries becoming unclear (Figs. 7C–E). The density of the intracellular protoplasm was visibly lower than the untreated cell (Figs. 7A, B). Furthermore, cell lysis (Fig. 7D) and a disruption and detachment of the outer membrane (Figs. 7E, F) were identified.
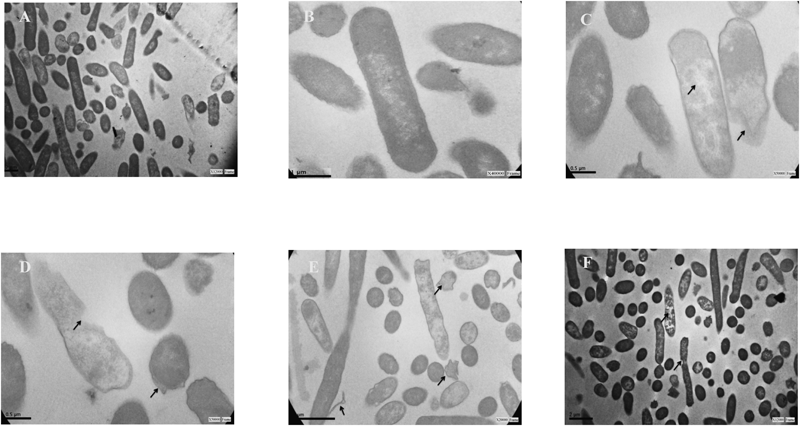
Untreated cells of E. coli displayed a normal cell shape with an undamaged structure of the membrane (Figs. A, B). A disturbed outer membrane integrity was evident in 4-NH2BS-THQ treated samples after 24 h of incubation (Figs. C–F). Scale bar=0.5 µm.
TEM studies are a useful tool to reveal more clearly structural alterations such as membrane damage induced by antibacterial compounds.29–31) A good knowledge of bacterial cell morphology and metabolic assays is required for understanding the probable mechanisms of action of antibacterials. Our results revealed a clear disorganization of the membrane caused by BS-THQ in S. aureus ATC C 29213 and by 4-NH2BS-THQ in E. coli. Both these drugs, and in particular the latter one, have advantageous molecular properties (CLOGP, MW and TPSA) that might explain their good interaction with the bacterial membrane. This interaction may facilitate the generation of ROS, thereby altering the bacterial physiology and the oxidative stress balance.
The synthesis, purification, and structural characterization of BS-THQ and 4-NH2BS-THQ have been described in a previous report7) and the structures are presented in Fig. 1. All the solvents were purchased from Cicarelli (Argentina), and were of p.a. grade and used without further purification.
Bacterial Strains and Culture ConditionsThe microorganisms used for this study included the reference strains S. aureus ATC C 29213, MRSA ATC C 43300, E. coli ATC C 25922, and P. aeruginosa ATC C 27853. Stock cultures were preserved at −70°C using glycerol 10% (v/v) as the cryoprotectant, and the strains were grown in tryptic soy broth (TSB) at 37°C for 18 h.
MIC DeterminationMIC of BS-THQ and 4-NH2BS-THQ was determined by using the standard tube dilution method, according to the guidelines of the Clinical Laboratories Standard Institute (CLSI).32) Overnight cultures in Mueller–Hinton broth (MHB) of four reference strains and three clinical isolates were diluted to 106 colony forming units (CFU)/mL. The stock solution of BS-THQ (400 µg/mL; 1.46 mM) was prepared in a solvent mixture of DMSO : polyethylene glycol (PEG) 400 : MHB at a proportion of 20 : 10 : 70. The stock solution of 4-NH2BS-THQ (200 µg/mL; 0.69 mM) was prepared in 20% (v/v) methanol and phosphate buffer saline (PBS) pH 7, with the final concentration of solvent being less than 10% for all experiments. All drugs were freshly diluted prior to use and suitable solvent controls were also run simultaneously. Sulfisoxazole was used as the reference drug, with the drug solution being prepared according to the guidelines of CLSI. Bacterial suspensions were inoculated to 2-fold diluted solutions of the compound in MHB, and the MIC was determined as the lowest antibiotic concentration at which growth was completely inhibited after overnight incubation of the tubes at 37°C. All MIC determinations were performed in triplicate.
Time–Kill CurvesAliquots of exponentially growing bacteria (S. aureus ATC C 29213, MRSA ATC C 43300) were suspended in fresh MHB at approximately 106 CFU/mL and exposed to BS-THQ at 1×MIC. Viability counts were performed at 0, 4, 8, and 24 h of incubation at 37°C by plating 0.1 mL of 10-fold serial diluted samples onto Mueller–Hinton agar plates in duplicate. The experiments were performed three times on different days, with time–kill curves being constructed by plotting the CFU/mL surviving at each time point in the presence and absence of the compound. Bactericidal activity (99.9% kill) was defined as a ≥3 log 10 CFU/mL reduction in the colony count from the initial inoculum.
Oxidative Stress Determined by Nitroblue Tetrazolium ReductionBacterial suspensions of S. aureus ATC C 29213, MRSA ATC C 43300, E. coli ATC C 25922, and P. aeruginosa ATC C 27853 (OD600 1.0) were prepared from overnight cultures. Then, 50 µL of bacterial suspensions in PBS were incubated with 50 µL of BS-THQ or 4-NH2BS-THQ at MIC and Sub-MICs for 2 h, after which, 50 µL of 1 mg/mL NBT was added for 30 min at 37°C. After adding 10 µL of 0.1 M HCl, the tubes were centrifuged at 1500×g for 10 min. The pellets were treated with 40 µL of DMSO to extract the reduced NBT, and the optical density was determined at 540 nm.33)
NO DeterminationOvernight cultures of S. aureus ATC C 29213, MRSA ATC C 43300, E. coli ATC C 25922, and P. aeruginosa ATC C 27853 were incubated for 2 h with 4-NH2BS-THQ at equal volumes. Then, the supernatant (0.1 mL) was mixed with Griess reagent (0.1 mL), sulfanilamide 1.5% in 1N HCL, and N-1-naphthyl ethylenediamine dihydrochloride 0.13% in sterile distilled water. A standard curve was generated with sodium nitrite in concentrations from 0.1 to 1 mM in PBS, and the spectrophotometry was performed at 540 nm.34)
Determination of Intracellular Oxidation Levels by Fluorescence MicroscopyBacterial cells were grown aerobically in MHB with BS-THQ at a final concentration of 150 µg/mL for 18 h at 37°C. Then, 10 mL of this bacterial suspension were washed with PBS (pH 7.0) and incubated for 30 min in the same buffer containing 15 µM of 2′,7′-dichlorodihydrofluorescein diacetate (DCFH2-DA) dissolved in DMSO. After washing, the cells were suspended in the same buffer, with bacterial suspensions (0.1 mL) being mixed with 0.9 mL of PBS (pH 7.0) and the fluorescence intensity measured using a NIKON TE-2000U microscope, (excitation, 490 nm; emission, 519 nm; mirror, 500 nm; emission, LP 515 nm).35) Also, a positive control of fluorescence (suspensions treated with ciprofloxacin) and a negative one (untreated strains) were run in parallel.
TEMThe S. aureus ATC C 29213 strain was grown overnight in MHB at 37°C. Bacterial suspensions at 106 CFU/mL were treated with BS-THQ at sub-MIC for 24 h and E. coli ATC C 25922 was treated with 4-NH2BS-THQ.
BS-THQ was dissolved in a solvent mixture of DMSO and PEG 400 (proportion 2 : 1), with 4-NH2BS-THQ being dissolved in methanol. Controls without the compounds prepared in the same solvent mixture were run in parallel. Then, samples were centrifuged for 5 min at 1000 rpm, before being withdrawn and fixed at 1 h in 300 mL of 2% glutaraldehyde and 4% formaldehyde solutions (1 : 1) in a 0.1 M cacodylate buffer for 2 h at room temperature. These were then post-fixed with osmium tetroxide at 1% in the same buffer, dehydrated, and embedded in Araldite. Thin sections were cut with a diamond knife on a JEOL JUM-7 ultramicrotome, mounted on nickel grids, and examined in a Zeiss LEO 906E electron microscope.29)
Statistical AnalysisThe Student’s t-test was used to perform the statistical analysis. A p-value <0.05 was considered to be significant. The results shown are these means of triplicates.
These in vitro studies have shown that 4-NH2BS-THQ has a broader spectrum of action than BS-THQ. Interestingly, the drug-resistant organism (MRSA) showed a particularly good susceptibility, which makes the former compound a promising agent. In this regard, it may be possible to demonstrate a relationship between the antimicrobial activity of the afore-mentioned compounds and the production of redox species, which depend on several factors including concentration, chemical stability, ability to cross hydrophobic membranes, and alteration of the respiratory metabolism.
Considering the results obtained, the 4-NH2 substitution not only provided a better antibacterial activity and widened the spectrum compared to BS-THQ, but also revealed the best predicted drug score as a drug-like molecule. Therefore, the assayed compounds represent promising structures for the development of new synthetic classes of antimicrobials. Further studies on new structurally related molecules, which are focused on the mechanism of action, are currently in progress.
Moreover, future research on new structures with novel targets of action should lead to the development of chemically-derived synthetic derivatives, thus guaranteeing consistent efficacy.
The authors wish to acknowledge the assistance of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Secretaría de Ciencia y Tecnología de la Universidad Nacional de Córdoba (SeCyT-UNC) and Ministerio de Ciencia y Tecnología (MINCyT), all of whom provided the facilities used in this investigation. We are grateful to Dr. Cristina Maldonado (Centro de Microscopía Electrónica. Fac. Cs. Médicas, UNC) for her assistance in processing the samples for TEM assays and to Dr. Pilar Crespo from Dpto. Bioquímica Clínica, Fac. Cs. Químicas, UNC. CIBICI-CONICET for her help with the fluorescence microscopy assays. We also thank Dr. Claudia Sola from Dpto. Bioquímica Clínica, Fac. Cs. Químicas, UNC. CIBICI-CONICET for the strain provided; and Pharmacist Natalia Villegas Venencia for her help with the chemical handling of the compounds. SRM and GEM are supported by the CONICET fellowship program (Argentina). MCB is a career research member of CONICET.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.