Abstract
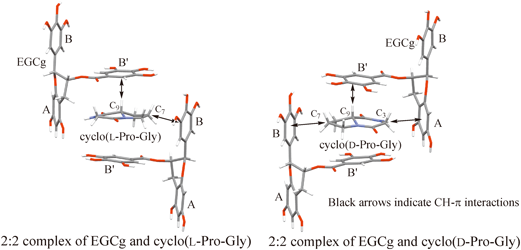
In the 1H-NMR spectrum of a solution containing an equimolecular amount of cyclo(L-Pro-Gly), cyclo(D-Pro-Gly) and (−)-epigallocatechin-3-O-gallate (EGCg) in a D2O, a difference in the chemical shift of 1H-NMR signal for H7α, H7β,8α of the Pro residue was observed. Judging from the crystal structures of the 2 : 2 complexes of EGCg and cyclo(L-Pro-Gly), cyclo(D-Pro-Gly), the difference in the chemical shift resulted mainly from a magnetic anisotropic shielding effect by the ring current from the B ring of EGCg. Therefore, it was considered that chirality of cyclo(Pro-Gly) was recognized by EGCg in the D2O solution. Furthermore, in the 1H-NMR spectrum of a solution containing an equimolecular amount of racemic propranolol ((R)- and (S)-propranolols) and EGCg in D2O, the 1H-NMR signal for H2 of the naphthalene group was observed as two doublets, suggesting that the racemic propranolol formed diastereomers of complexes with EGCg; as a result, chirality of propranolol was recognized by EGCg in the D2O solution.