2016 Volume 64 Issue 2 Pages 142-149
2016 Volume 64 Issue 2 Pages 142-149
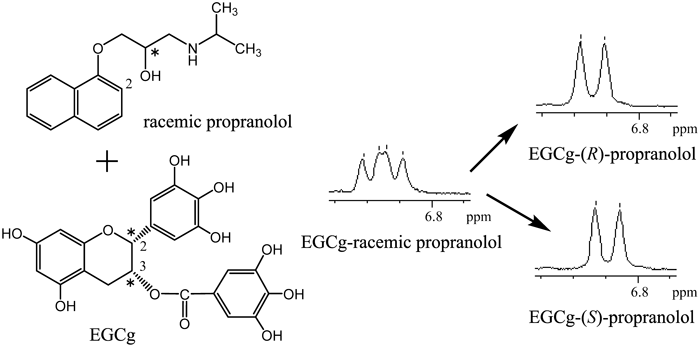
In the 1H-NMR spectrum of a solution containing an equimolecular amount of cyclo(L-Pro-Gly), cyclo(D-Pro-Gly) and (−)-epigallocatechin-3-O-gallate (EGCg) in a D2O, a difference in the chemical shift of 1H-NMR signal for H7α, H7β,8α of the Pro residue was observed. Judging from the crystal structures of the 2 : 2 complexes of EGCg and cyclo(L-Pro-Gly), cyclo(D-Pro-Gly), the difference in the chemical shift resulted mainly from a magnetic anisotropic shielding effect by the ring current from the B ring of EGCg. Therefore, it was considered that chirality of cyclo(Pro-Gly) was recognized by EGCg in the D2O solution. Furthermore, in the 1H-NMR spectrum of a solution containing an equimolecular amount of racemic propranolol ((R)- and (S)-propranolols) and EGCg in D2O, the 1H-NMR signal for H2 of the naphthalene group was observed as two doublets, suggesting that the racemic propranolol formed diastereomers of complexes with EGCg; as a result, chirality of propranolol was recognized by EGCg in the D2O solution.
When a hot tea beverage cools down, it becomes turbid and brown-white particles settle out. This phenomenon is called “creaming” or “a creaming-down reaction.” Previously, Ina and colleagues reported that creaming down eventually occurs when an aqueous caffeine solution is poured into an aqueous solution of gallated catechin (−)-epigallocatechin-3-O-gallate (EGCg), which is most abundant in tea catechins.1)
Then, we attempted crystallization of the precipitate formed by the creaming-down reaction made from an aqueous solution of tea gallated catechin EGCg and caffeine, and as a result obtained a crystal which was determined to be a 2 : 2 complex of EGCg and caffeine by X-ray crystallographic analysis2) (Fig. 1a).

As shown in Fig. 1b, the caffeine moieties of the 2 : 2 complex were located in the space surrounding the top and lower walls of the B′ rings of EGCg moieties and right and left walls of the A and B rings of EGCg moieties. As a result, caffeine molecules were captured by the hydrophobic space formed by the three aromatic A, B, B′ rings of the EGCg in the 2 : 2 complex. Water molecules existed outside the space formed by the three aromatic A, B, B′ rings of EGCg and were not observed in the space, suggesting that the space had high hydrophobicity. It was therefore thought that the sticky precipitate was formed by creaming precipitated from the aqueous solution of EGCg and caffeine due to its high hydrophobicity.
The hydrophobic space is available for capture in various compounds in substitution for caffeine. Furthermore, it was assumed that the space formed by the three aromatic A, B, B′ rings of EGCg could recognize the chirality of compounds included in the space because the C ring of EGCg has two chiral carbon atoms, C2 and C3, and the hydrophobic space formed by the three aromatic A, B, B′ rings of EGCg was a chiral space. Many medicines are currently used in racemic form; however, it is desirable to use a single enantiomer from the viewpoint of adverse effects and pharmacokinetics. EGCg, which easily forms a complex with various compounds and dissociates in an aqueous solution, may be available as a new optical resolving agent for natural products and medicines.
Thus, diketopiperazine cyclo(Pro-Gly) was selected as a chiral compound because the molecular size of cyclo(Pro-Gly) was about the same as that of caffeine3) (Fig. 1c). Subsequently, the chiral recognition of diketopiperazines cyclo(L-Pro-Gly) and cyclo(D-Pro-Gly) by EGCg was investigated using several NMR techniques and X-ray crystallographic analysis.4)
Therefore, as an application in medicine of the chiral recognition of EGCg, a sympathetic β-receptor blocker propranolol used for the treatment of hypertension as an ordinary racemic form was investigated (Fig. 1c).
EGCg (>90%) and (R)-propranolol, (S)-propranolol were purchased from Funakoshi Co., Ltd. and Sigma-Aldrich Co., respectively. EGCg was used with further purification.
NMR Experiments1H-NMR spectra were recorded at room temperature on a JEOL JMN-LA500 (Tokyo, Japan) operating at 500 MHz. D2O was used as a solvent (99.9 atom % D; Wako Pure Chemical Industries, Ltd., Osaka, Japan). Chemical shift values are expressed in ppm downfield using sodium 2,2-dimethyl-2-silapentane-5-sulfonate (DSS) as an internal standard. The nuclear Overhauser effect (NOE) difference experiments were typically conducted at 35°C with 32 K data points covering a spectral width of 10000 Hz and with ca. 5 s presaturation time.
Preparation of Creaming Precipitate Made from EGCg and Cyclo(Pro-Gly)A solution of cyclo(L-Pro-Gly) (1.68 mg, 1.09×10−2 mmol) in H2O (40 µL) was added to a solution of EGCg (5.00 mg, 1.09×10−2 mmol) in H2O (30 µL). After leaving the mixture at room temperature for 1 h, this was left at 10°C for about 24 h to afford a colorless block crystal (0.15×0.11×0.06 mm). A solution of cyclo(D-Pro-Gly) (1.68 mg, 1.09×10−2 mmol) in H2O (40 µL) was added to a solution of EGCg (5.00 mg, 1.09×10−2 mmol) in H2O (30 µL). By the same procedure as above, the mixture afforded a colorless block crystal (0.17×0.10×0.04 mm).
X-Ray Crystal Structure Analysis of the 2 : 2 Complex of EGCg and Cyclo(L-Pro-Gly)A crystal of the 2 : 2 complex of EGCg and cyclo(L-Pro-Gly) was determined by X-ray crystallographic analysis at 213 K. The X-ray intensity data of 14640 reflections (of which 8113 were unique) were collected on a Rigaku RAXIS RAPID II imaging plate area detector with graphite monochromated CuKα radiation (λ=1.54187 Å). The data were corrected for Lorentz and polarization effects. The structure was solved by direct methods using SIR20085) and expanded using Fourier techniques.6) The final cycle of full-matrix least-squares refinement on F2 was based on 8113 observed reflections and 851 variable parameters and converged with unweighted and weighted agreement factors of R=∑ ||Fo|−|Fc||/∑ |Fo|=0.0753 (I>2.00σ(I)), Rw=[∑ (w(Fo2−Fc2)2)/∑ w(Fo2)2]1/2=0.2288. The goodness of fit was 1.11. Unit weights were used. The maximum and minimum peaks on the final difference Fourier map corresponded to 0.33 and −0.38 e/Å3, respectively. The final Flack parameter was 0.2(4).7) All calculations were performed using the CrystalStructure8) crystallographic software package except for refinement, which was performed using SHELXL-97.9) Crystallographic data reported in this manuscript have been deposited with the Cambridge Crystallographic Data Center as supplementary publication No. 1017644 for the 2 : 2 complex of EGCg and cyclo(L-Pro-Gly).
X-Ray Crystal Structure Analysis of the 2 : 2 Complex of EGCg and Cyclo(D-Pro-Gly)A crystal of the 2 : 2 complex of EGCg and cyclo(D-Pro-Gly) was determined by X-ray crystallographic analysis at 213 K. X-Ray intensity data of 14231 reflections (of which 7918 were unique) were collected on a Rigaku RAXIS RAPID II imaging plate area detector with graphite monochromated CuKα radiation (λ=1.54187 Å). The data were corrected for Lorentz and polarization effects. The structure was solved by direct methods using SIR20085) and expanded using Fourier techniques.6) The final cycle of full-matrix least-squares refinement on F2 was based on 7918 observed reflections and 856 variable parameters and converged with unweighted and weighted agreement factors of R=∑ ||Fo|−|Fc||/∑ |Fo|=0.1138 (I>2.00σ(I)), Rw=[∑ (w(Fo2−Fc2)2)/∑ w(Fo2)2]1/2=0.3551. The goodness of fit was 1.13. Unit weights were used. The maximum and minimum peaks on the final difference Fourier map corresponded to 0.42 and −0.37 e/Å3, respectively. The final Flack parameter was 0.1(4).7) All calculations were performed using the CrystalStructure8) crystallographic software package except for refinement, which was performed using SHELXL-97.9) Crystallographic data reported in this manuscript have been deposited with the Cambridge Crystallographic Data Center as supplementary publication No. 1017643 for the 2 : 2 complex of EGCg and cyclo(D-Pro-Gly).
Stoichiometry and Thermodynamic Parameters of EGCg Complexes with Cyclo(L-Pro-Gly) and Cyclo(D-Pro-Gly) in the D2O SolutionThe stoichiometry and stability constants Kc of the EGCg complexes with cyclo(L-Pro-Gly) and cyclo(D-Pro-Gly) were determined by monitoring the chemical shifts of cyclo(L-Pro-Gly) and cyclo(D-Pro-Gly) H9 proton signals in the 1H-NMR measurements when the concentration of EGCg was continuously increased from 0 to 54 mM in a constant concentration of cyclo(L-Pro-Gly) and cyclo(D-Pro-Gly) (10 mM) and using the equation Eq. 1 in the range 40–70°C.
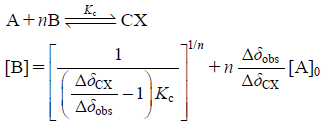 | (1) |
Also, the changes in free energy ΔG, enthalpy ΔH, entropy ΔS for formation of EGCg complexes with cyclo(L-Pro-Gly) and cyclo(D-Pro-Gly) on temperature were estimated by using the results of stability constants. Here, A, B and CX mean cyclo(L-Pro-Gly) or cyclo(D-Pro-Gly), EGCg and complexes of EGCg and cyclo(L-Pro-Gly) or cyclo(D-Pro-Gly), respectively, and Δδobs and ΔδCX represent (δA–δobs) and (δA–δCX), respectively. δA, δCX, and δobs represent the chemical shift (ppm) of the H9 proton of cyclo(L-Pro-Gly) or cyclo(D-Pro-Gly) in a free state, complexes of EGCg and cyclo(L-Pro-Gly) or cyclo(D-Pro-Gly), and the mixture of EGCg and cyclo(L-Pro-Gly) or cyclo(D-Pro-Gly) in 1H-NMR spectra, respectively.
Stoichiometry and Thermodynamic Parameters of EGCg Complexes with (R)-Propranolol and (S)-Propranolol in the D2O SolutionThe stoichiometry and stability constants Kc of EGCg complexes with (R)-propranolol and (S)-propranolol were determined by monitoring the chemical shifts of EGCg H2″,6″ proton signals in the 1H-NMR measurements when the concentration of (R)-propranolol and (S)-propranolol were continuously increased from 0 to 54 mM in a constant concentration of EGCg (10 mM) and using the equation of Eq. 1 in the range 35–70°C. Also, the changes in free energy ΔG, enthalpy ΔH, entropy ΔS for formation of EGCg complexes with (R)-propranolol and (S)-propranolol in temperature were estimated by using the results of stability constants. Here, A, B and CX mean EGCg, (R)-propranolol or (S)-propranolol and complexes of EGCg and (R)-propranolol or (S)-propranolol, respectively, and Δδobs and ΔδCX represent (δA–δobs) and (δA–δCX), respectively. δA, δCX, and δobs represent the chemical shift (ppm) of the H2″,6″ proton of EGCg in a free state, complexes of EGCg and (R)-propranolol or (S)-propranolol, and the mixture of EGCg and (R)-propranolol or (S)-propranolol in 1H-NMR spectra, respectively.
A solution of diketopiperazines cyclo(L-Pro-Gly), cyclo(D-Pro-Gly) in D2O was added to a solution of an equimolecular amount of EGCg in D2O. 1H-NMR spectra of the mixture are shown in Figs. 2b and c. All proton signals derived from cyclo(L-Pro-Gly) and cyclo(D-Pro-Gly) appeared as broad signals, as compared with the corresponding proton signals of the 1H-NMR spectra of cyclo(L-Pro-Gly), and cyclo(D-Pro-Gly) alone in D2O (Fig. 2a). It was thought that cyclo(L-Pro-Gly), cyclo(D-Pro-Gly) formed complexes with EGCg, and that the motion of their protons was restricted, leading to their signals being broadened.
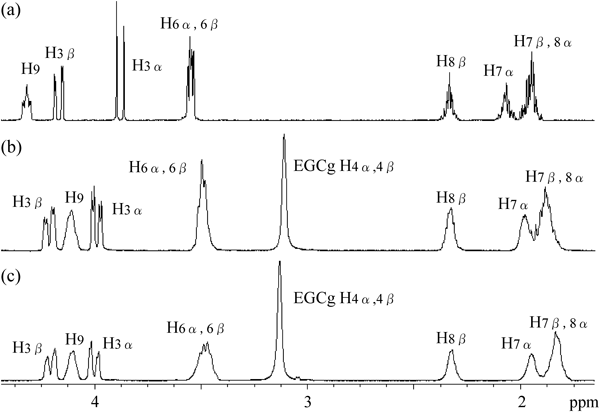
Table 1 shows the chemical shift of 1H-NMR signals of a solution containing equimolecular amounts of EGCg and cyclo(L-Pro-Gly), cyclo(D-Pro-Gly) in D2O, and indicates the shift values started from the chemical shift of 1H-NMR signals of a solution containing cyclo(L-Pro-Gly) and position of the Pro residue of cyclo(L-Pro-Gly) and cyclo(D-Pro-Gly) was observed, while a downfield shift of the proton signal H3α in the α position of the Gly residue was also observed. Upfield shifts in proton signals H7α,β and H8α in the β and γ positions of the Pro residue of cyclo(D-Pro-Gly) were more marked than those of cyclo(L-Pro-Gly).
 | |||
|---|---|---|---|
| Chemical shift (ppm) of cyclo(L-Pro-Gly) | |||
| Proton | δ (ppm) | δ (ppm) in the presence of EGCg | Shift value (ppm) |
| 9 | 4.319 | 4.108 | −0.211 |
| 3β | 4.170 | 4.215 | +0.045 |
| 3α | 3.881 | 3.989 | +0.108 |
| 6α,β | 3.549 | 3.497 | −0.052 |
| 8β | 2.332 | 2.325 | −0.007 |
| 7α | 2.064 | 1.977 | −0.087 |
| 7β,8α | 1.947 | 1.882 | −0.065 |
 | |||
| Chemical shift (ppm) of cyclo(D-Pro-Gly) | |||
| Proton | δ (ppm) | δ (ppm) in the presence of EGCg | Shift value (ppm) |
| 9 | 4.320 | 4.100 | −0.220 |
| 3β | 4.170 | 4.207 | +0.037 |
| 3α | 3.880 | 4.001 | +0.121 |
| 6α,β | 3.548 | 3.480 | −0.068 |
| 8β | 2.333 | 2.319 | −0.014 |
| 7α | 2.065 | 1.947 | −0.118 |
| 7β,8α | 1.947 | 1.834 | −0.113 |
Thermodynamic data on the complex formation of EGCg and cyclo(L-Pro-Gly), cyclo(D-Pro-Gly) were investigated. The stability constants Kc of the complexes of EGCg and cyclo(L-Pro-Gly), cyclo(D-Pro-Gly) at 40°C were 996.3 and 1072.1 M−1, respectively (Table 2).
| EGCg-cyclo(L-Pro-Gly) | |||||
|---|---|---|---|---|---|
| T (°C) | Kc (M−1) | ΔG (kJ mol−1) | ΔH (kJ mol−1) | ΔS (J mol−1 K−1) | n |
| 40 | 996.3 | −18.0 | −31.8 | −44.1 | 0.81 |
| 45 | 846.9 | −17.8 | 0.89 | ||
| 50 | 716.7 | −17.7 | 0.91 | ||
| 55 | 583.7 | −17.4 | 1.05 | ||
| 60 | 479.5 | −17.1 | 1.24 | ||
| 70 | 348.7 | −16.7 | 1.34 | ||
| EGCg-cyclo(D-Pro-Gly) | |||||
| T (°C) | Kc (M−1) | ΔG (kJ mol−1) | ΔH (kJ mol−1) | ΔS (J mol−1 K−1) | n |
| 40 | 1072.1 | −18.2 | −31.7 | −43.8 | 0.83 |
| 45 | 762.2 | −17.5 | 0.93 | ||
| 50 | 659.8 | −17.4 | 0.98 | ||
| 55 | 570.0 | −17.3 | 1.01 | ||
| 60 | 477.8 | −17.1 | 1.24 | ||
| 70 | 350.0 | −16.9 | 1.05 | ||
From the dependency of Kc on temperature, the change in free energy ΔG, enthalpy ΔH and entropy ΔS of the complex formation were estimated as shown in Table 2. The thermodynamic data for the complex formation of EGCg and cyclo(L-Pro-Gly) were very similar to those of EGCg and cyclo(D-Pro-Gly). The entropy (ΔS) for the formation of the complex of EGCg and cyclo(L-Pro-Gly), cyclo(D-Pro-Gly) had large negative values of −44.1 and −43.8 J mol−1 K−1, respectively, suggesting that cyclo(L-Pro-Gly) and cyclo(D-Pro-Gly) were fixed tightly in the complex with EGCg.
There is no great difference between the thermodynamic data of the complex formation of EGCg with cyclo(L-Pro-Gly) and those with cyclo(D-Pro-Gly), indicating that the physical properties of the complex of EGCg with cyclo(L-Pro-Gly) and those with cyclo(D-Pro-Gly) are very similar.
Determination of the Stereochemical Structure of the Complex of EGCg and Cyclo(Pro-Gly)A solution of diketopiperazine cyclo(L-Pro-Gly) in H2O was added to a solution of an equimolecular amount of EGCg in H2O. The mixture afforded a colorless block crystal, which contained EGCg and cyclo(L-Pro-Gly) at a molar ratio of 1 : 1, based on measurement of the integral volume of 1H-NMR signals. A single crystal was determined to be a 2 : 2 complex of EGCg and cyclo(L-Pro-Gly) by X-ray crystallographic analysis.3)
Using the same method as for crystallization of the complex of EGCg and cyclo(D-Pro-Gly), a single crystal of a complex of EGCg and cyclo(D-Pro-Gly) was prepared and determined to be a 2 : 2 complex of EGCg and cyclo(D-Pro-Gly) by X-ray crystallographic analysis.3)
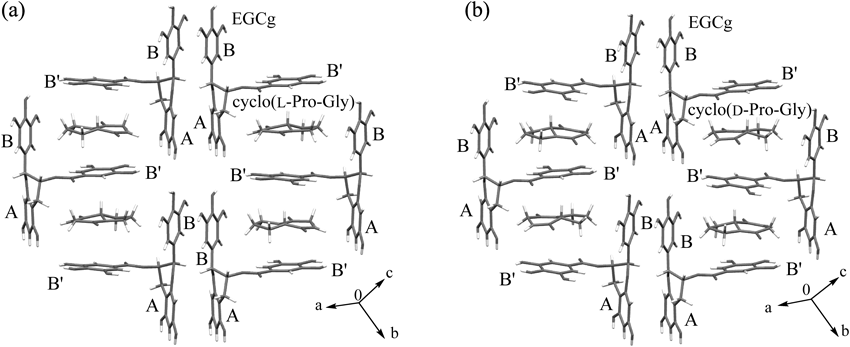
ORTEP drawings of 2 : 2 complexes of EGCg and cyclo(L-Pro-Gly), EGCg and cyclo(D-Pro-Gly) are shown in Figs. 3a and b. The 2 : 2 complexes were formed from two crystallographically different EGCgs (EGCg-A and EGCg-B) and two cyclo(L-Pro-Gly)s, two cyclo(D-Pro-Gly)s.
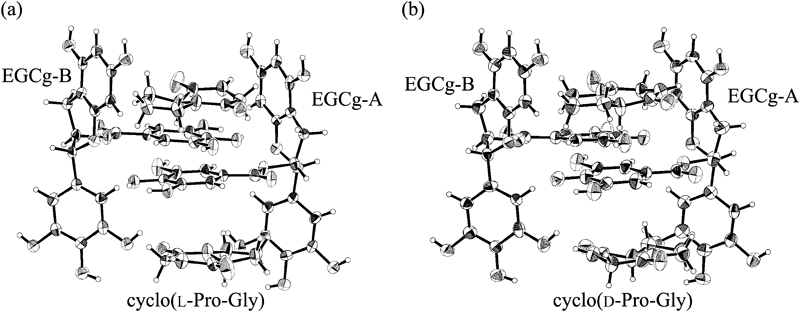
(a) 2 : 2 complex of EGCg and cyclo(L-Pro-Gly) (b) 2 : 2 complex of EGCg and cyclo(D-Pro-Gly).
One unit cell contained one unit of the 2 : 2 complex of EGCg and cyclo(L-Pro-Gly), cyclo(D-Pro-Gly) and seven water molecules as a crystal solvent (Figs. 4a, b).
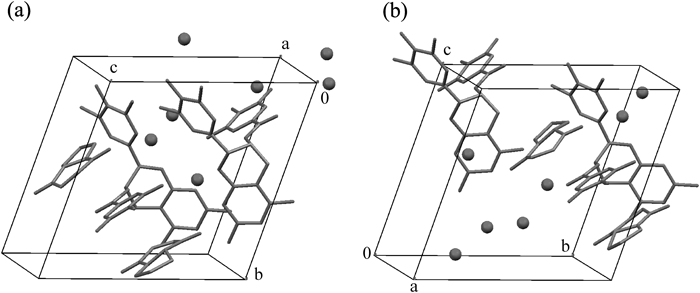
Hydrogen atoms are omitted for clarity. (a) 2 : 2 complex of EGCg and cyclo(L-Pro-Gly) (b) 2 : 2 complex of EGCg and cyclo(D-Pro-Gly).
The torsion angles of EGCg moieties (EGCg-A and EGCg-B) of the 2 : 2 complexes of EGCg and cyclo(L-Pro-Gly), cyclo(D-Pro-Gly) indicated that the B rings of EGCg-A and EGCg-B were both at equatorial positions, while the B′ rings of EGCg-A and EGCg-B were both in axial positions with respect to the C rings of the EGCg molecules (Table 3).
| Torsion angle | EGCg in cyclo(L-Pro-Gly) complex | EGCg in cyclo(D-Pro-Gly) complex | ||
|---|---|---|---|---|
| A | B | A | B | |
| ∠H2-C2-C3-3O | 169° | 168° | 169° | 170° |
| ∠C1′-C2-C3-C4 | 172.0(6)° | 173.6(6)° | 171.8(7)° | 169.1(8)° |
| ∠C1′-C2-C1-C8a | 173.7(6)° | 173.7(6)° | 174.1(7)° | 176.0(7)° |
In the layer structures of the 2 : 2 complexes of EGCg and cyclo(L-Pro-Gly), cyclo(D-Pro-Gly), cyclo(L-Pro-Gly) and cyclo(D-Pro-Gly) were captured by the space formed by the three aromatic A, B, B′ rings of EGCg and were located almost in the middle of the two B′ rings of EGCg-A or EGCg-B (Figs. 5a, b), the same as caffeine in the 2 : 2 complex of EGCg (Fig. 1a).
In the 2 : 2 complexes of EGCg and cyclo(L-Pro-Gly), cyclo(D-Pro-Gly), intermolecular interactions forming between EGCg and cyclo(L-Pro-Gly), cyclo(D-Pro-Gly) were elucidated. In the 2 : 2 complex of EGCg and cyclo(L-Pro-Gly), CH–π interactions formed between methine C9-H of cyclo(L-Pro-Gly) and the B’ rings of EGCg, methylene C7-H of cyclo(L-Pro-Gly) and the B rings of EGCg, and two O–H…O intermolecular hydrogen bonds were observed between EGCg and cyclo(L-Pro-Gly) (Fig. 6a). In the 2 : 2 complex of EGCg and cyclo(D-Pro-Gly), CH–π interactions formed between methine C9–H of cyclo(D-Pro-Gly) and the B′ rings of EGCg, methylene C7–H of cyclo(D-Pro-Gly) and the B rings of EGCg. In addition, CH–π interactions formed between methylene C3–H of cyclo(D-Pro-Gly) and the A rings of EGCg. Furthermore, in the same way as the 2 : 2 complex of cyclo(L-Pro-Gly), two O–H…O intermolecular hydrogen bonds were observed (Fig. 6b).

(a) 2 : 2 complex of EGCg and cyclo(L-Pro-Gly) (b) 2 : 2 complex of EGCg and cyclo(D-Pro-Gly). Black arrows and dotted lines indicate CH–π interactions and hydrogen bonds, respectively.
Judging from the crystal structures of 2 : 2 complexes of EGCg and cyclo(L-Pro-Gly), cyclo(D-Pro-Gly), the upfield shift in the proton signal H9 resulted from magnetic anisotropic shielding by the ring current from the B′ ring of the EGCg moieties (Fig. 6). Upon the formation of the 2 : 2 complex, the upfield shift values of the proton signals H7α, H7β,8α of cyclo(L-Pro-Gly) were 0.087 and 0.065 ppm, respectively, and those of cyclo(D-Pro-Gly) were 0.118 and 0.113 ppm, respectively (Table 1). Such a difference between the upfield shift values of the proton signals of cyclo(L-Pro-Gly) and cyclo(D-Pro-Gly) were thought to result mainly from the magnitude of the magnetic anisotropic shielding by the ring current from the B ring of EGCg.
Therefore, it was considered that upon formation of the 2 : 2 complex, a chirality of cyclo(Pro-Gly) was recognized by the magnetic anisotropic shielding effect of the ring current from the B ring of EGCg.
Chiral Recognition of Propranolol Using EGCgA solution of racemic propranolol ((R)- and (S)-propranolols) in D2O was added to a solution of an equimolecular amount of EGCg in D2O. In the 1H-NMR spectrum of the mixture, the H2 proton signal of racemic propranolol was observed as two doublets (Fig. 7), suggesting that (R)- and (S)-propranolols each formed complexes with EGCg, which are diastereomers of each other. By adding EGCg, the H2 proton signal of (R)- and (S)-propranolols was observed as two doublets, showing that EGCg recognized the chirality of propranolol.
Measurement condition of 1H-NMR spectra: EGCg and racemic propranolol (each 40 mM) in D2O at room temperature.
For assignment of the two doublets, (R)- and (S)-propranolols were added to a solution containing an equimolecular amount of EGCg and racemic propranolol in D2O (Fig. 8). As a result, doublets of 6.850 and 6.841 ppm were assigned to the H2 of (R)- and (S)-propranolols, respectively.
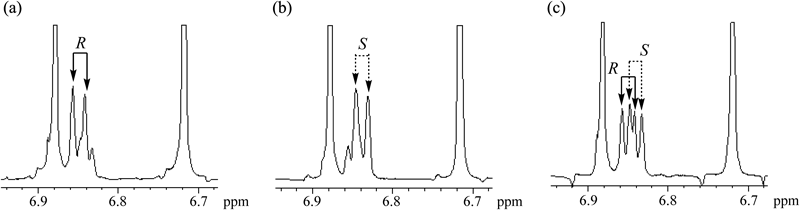
Concentration of (R)- and (S)-propranolol: (a) 30 and 10 mM, (b) 10 and 30 mM, (c) 20 and 20 mM.
A kinetic on the formation of complexes of (R)- and (S)-propranolols and EGCg was performed. The stability constants for the formation of complexes of EGCg and (R)-, (S)-propranolol Kc at 35–70°C were estimated, which assumed the order of the reaction n. The reaction n of the complexes of EGCg and (R)-, (S)-propranolol at 35°C were 0.86 and 0.87, respectively (Table 4), suggesting that (R)- and (S)-propranolol each formed a 1 : 1 complex of EGCg.
| EGCg-(R)-propranolol | |||||
|---|---|---|---|---|---|
| T (°C) | Kc (M−1) | ΔG (kJ mol−1) | ΔH (kJ mol−1) | ΔS (J mol−1 K−1) | n |
| 35 | 943.7 | −17.5 | −24.4 | −21.7 | 0.86 |
| 40 | 835.2 | −17.5 | 0.87 | ||
| 45 | 893.8 | −18.0 | 0.89 | ||
| 50 | 664.9 | −17.5 | 0.89 | ||
| 55 | 579.6 | −17.3 | 0.91 | ||
| 60 | 490.8 | −17.2 | 0.91 | ||
| 70 | 369.5 | −16.9 | 0.93 | ||
| EGCg-(S)-propranolol | |||||
| T (°C) | Kc (M−1) | ΔG (kJ mol−1) | ΔH (kJ mol−1) | ΔS (J mol−1 K−1) | n |
| 35 | 871.8 | −17.3 | −19.6 | −6.7 | 0.87 |
| 40 | 838.1 | −17.5 | 0.89 | ||
| 45 | 793.2 | −17.7 | 0.91 | ||
| 50 | 699.1 | −17.6 | 0.92 | ||
| 55 | 608.4 | −17.5 | 0.91 | ||
| 60 | 495.0 | −17.2 | 0.94 | ||
| 70 | 424.8 | −17.3 | 1.00 | ||
From the dependency of Kc on temperature, the change in free energy ΔG, enthalpy ΔH and entropy ΔS of the complex formation were estimated (Table 4). The entropy (ΔS) for the formation of the complex of EGCg and (R)-, (S)-propranolols had large negative values of −21.7 and −6.7 J mol−1 K−1, respectively. It was suggested that (R)-propranolol was fixed tightly in the complex of EGCg and (R)-propranolol, while (S)-propranolol fitted loosely in the complex of EGCg and (S)-propranolol.
Next, changes in chemical shifts of proton signals of (R)- and (S)-propranolols in 1H-NMR spectra by adding regular amount of EGCg were observed (Fig. 9). Upfield shifts in proton signals for H9 in the side chain and H2, H3, H4, H5, H6, H7, H8 in the naphthalene ring of (R)- and (S)-propranolols were observed. It was considered that the upfield shifts of proton signals resulted from the magnetic anisotropic shielding by the ring current from the B′ ring of EGCg when the naphthalene ring of propranolol was captured by the hydrophobic space formed by EGCg as with the caffeine moieties of the 2 : 2 complex of EGCg and caffeine shown in Fig. 1a.
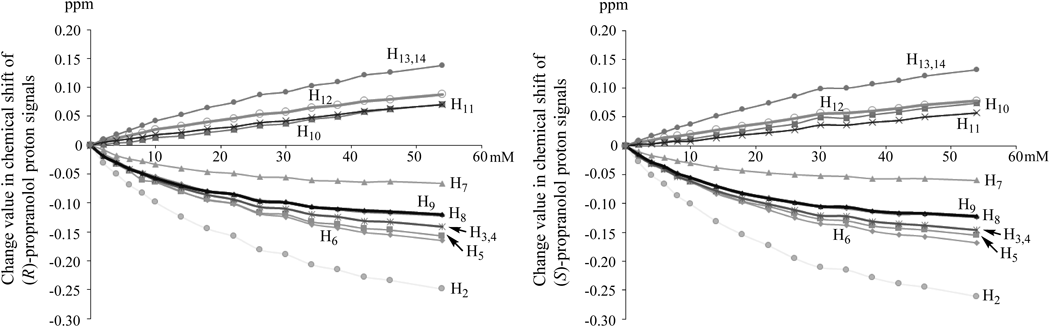
Measurement conditions of 1H-NMR spectra: (R)- and (S)-propranolol (each 10 mM) in D2O at room temperature.
Upon the complex formation, intermolecular interactions between EGCg and propranolol moieties were investigated. The naphthalene ring of propranolol was considered to form a π–π interaction with the B′ ring of EGCg.
Judging from the entropy (ΔS) value (Table 4), the complexes of EGCg and (R)-propranolol were expected to form not only the π–π interaction between the naphthalene ring of (R)-propranolol and the B’ ring of EGCg, but also an intermolecular hydrogen bond between the side chain of (R)-propranolol and hydroxyl group of EGCg.
Measurements of the intramolecular nuclear Overhauser effect (NOE) of (R)- and (S)-propranolols in the complexes with EGCg in D2O were performed (Figs. 10b, c). For a comparison, the intramolecular NOE of (S)-propranolol alone in D2O was measured (Fig. 10a). A large number of NOEs were observed in (S)-propranolol in a free state, but not in (R)- and (S)-propranolols in the complex with EGCg, suggesting that their conformers were fixed by forming complexes with EGCg.
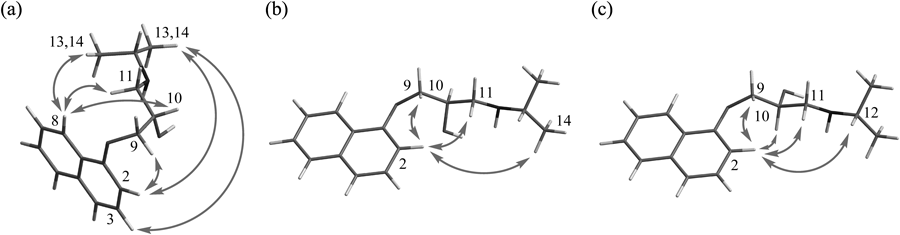
Both arrows indicate intramolecular NOEs.
A characteristic intramolecular NOE in the solution of EGCg and (S)-propranolol was observed between H2 in the naphthalene ring and H10 in the side chain of (S)-propranolol, but was not observed in the solution of (R)-propranolol. It was considered that the H2 proton signal of (R)-propranolol was observed in a lower field than that of (S)-propranolol due to the influence of the oxygen atom of the hydroxyl group of (R)-propranolol in the neighborhood.
It was therefore concluded that a difference in the chemical shift of the H2 proton signal between (R)- and (S)-propranolols resulted from a difference in their conformations in the complexes with EGCg, and then EGCg recognized a chirality of racemic propranolol in the D2O solution.
This study was supported by JSPS KAKENHI Grant Number 26860074.
The authors declare no conflict of interest.