2016 Volume 64 Issue 2 Pages 161-170
2016 Volume 64 Issue 2 Pages 161-170
Tetradecyl 2,3-dihydroxybenzoate (ABG-001) has been designed and synthesised as a lead compound to treat Alzheimer’s disease, based on structure–activity relationships of gentisides. In this paper, the alkyl chain and ester linkage group of ABG-001 were modified. Consequently, several series of novel gentiside derivatives were designed and synthesised, and their neuritogenic activity was evaluated in PC12 cells. Among all the tested compounds, S-dodecyl 2,3-dihydroxybenzothioate (15d, named as ABG-199) was the most potent; the compound induced significant neurite outgrowth at 0.1 µM, which was comparable to that of nerve growth factor at the optimal concentration of 40 ng/mL and ABG-001 at 1 µM. A brief study on the mechanism of action of ABG-199 revealed that extracellular signal-regulated kinase phosphorylation was involved in ABG-199-induced neurite outgrowth in PC12 cells.
Alzheimer’s disease (AD) is the most prevalent neurodegenerative disease in aged populations, and the number of cases is rapidly increasing. As one of the most important neurotrophic factors, the nerve growth factor (NGF) is essential for neuronal differentiation, growth, survival, function maintenance and prevention of ageing in central and peripheral systems.1,2) However, NGF cannot pass through the blood–brain barrier because of its large molecular size and hydrophilic properties, thereby limiting its use in therapeutic drugs for the treatment of neurodegenerative diseases such as AD.3) Therefore, small molecular compounds that mimic or enhance the neuritogenic activity of NGF are promising candidates for drug discovery. To date, hundreds of neuritogenic substances have been isolated from natural products or synthesised by researchers; some of which are in clinical or pre-clinical studies for drug development.4) The PC12 cell line was cloned from rat pheochromocytoma; this cell line is one of the most important bioassay systems to evaluate the biological activity of neuritogenic substances in vitro.5–7)
Gentisides are a novel class of neuritogenic phenolic lipids, which we previously isolated from the traditional Chinese medicine Gentiana rigescens FRANCH8,9) (Fig. 1). These compounds are potent inducers of neurite outgrowth in PC12 cells. Subsequently, the effects of the alkyl chain length, number and position of hydroxy groups on benzene ring, as well as the type of linker between the benzene ring and the alkyl chain, on their neuritogenic activity were investigated.10) Results revealed important parts for the neuritogenic activity of gentiside derivatives including the following: (1) at least two nearby hydroxy groups on the benzene ring; (2) an ester linkage group; (3) the alkyl chain length (C10–C22). Among all the tested compounds, tetradecyl 2,3-dihydroxybenzoate (renamed as ABG-001) was the most important lead compound and exhibited significant neuritogenic activity at 1 µM (Fig. 1).
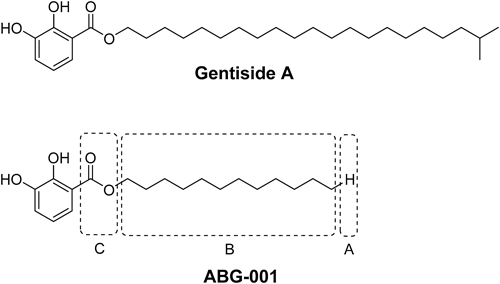
In the present structure–activity relationship (SAR) study of gentisides, we focused our interest mainly on the modification of alkyl chain, as well as the linkage between the benzene ring and the alkyl chain, based on previous results. The modified parts (Fig. 1) are (1) the end of the alkyl chain (part A), (2) the middle of the alkyl chain (part B) and (3) the linkage atom between the benzene ring and the alkyl chain (part C).
First, hydroxy, 2,3-dihydroxybenzoate, methoxy, acetate, methane sulphonate and halogen groups were introduced to the end of the ABG-001 alkyl chain to study effects of the end group in the alkyl chain on the neuritogenic activity. Tetradecyl-2,3-dihydroxybenzoate derivatives 3a–e were prepared using tetradecane 1,14-diol (1a) as a starting material (Chart 1). First, compound 1a was condensed with 2,3-dihydroxybenzoic acid (2), with 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC·HCl) as the condenser to afford compounds 3a (35%) and 3b (20%) simultaneously.11,12) Then one hydroxy group of 1a reacted with methyl iodide (MeI), Ac2O, and methylsulfonyl chloride (MsCl) to afford compounds 1b–d, respectively, subsequently compounds 1b–d condensed with 2 in the presence of EDC·HCl to yield compounds 3c–e, respectively.
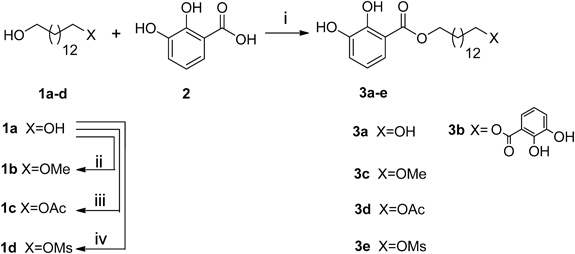
Reagents and condition: (i) EDC·HCl, DMAP, DCM, rt; (ii) NaH, MeI, DMF, rt; (iii) Ac2O, Et3N, DMAP, DCM, rt; (iv) MsCl, DMAP, pyridine, DCM, rt.
The synthetic route of compounds 3f–i is depicted in Chart 2. Treated 3e with KF in the presence of 4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8,8,8]hexacosane (K222) to afford 3f in low yield (10%). The reason was the nucleophilic ability of phenolic hydroxy was higher than fluoride ion, thereby yielding the intramolecular nucleophilic products as the main by-products. The same by-product was also afforded when 3e was treated with KCl in the presence of K222 to produce a low yield of 3g (13%). Compared with the fluoride and chloride ions, the nucleophilic ability of the bromide and iodide ions was much higher; these ions could react with 3e in the presence of K222 to afford 3h (yield: 88%) and 3i (yield: 92%), respectively, with high yield.
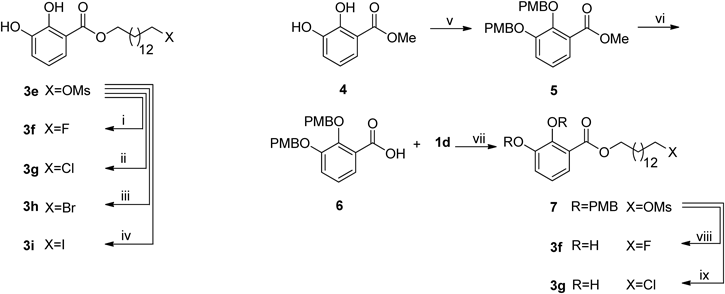
Reagents and condition: (i) KF, K222, CH3CN, 80°C; (ii) KCl, K222, CH3CN, 80°C; (iii) KBr, K222, CH3CN, 80°C; (iv) KI, K222, CH3CN, 80°C; (v) PMBCl, K2CO3, THF, rt; (vi) 2 M NaOH; (vii) EDC·HCl, DMAP, DCM, rt; (viii) (1) KF, K222, CH3CN, 80°C; (2) TFA, DCM, rt; (ix) (1) KCl, K222, CH3CN, 80°C; (2) TFA, DCM, rt.
Given that the yield of 3f and g was low and difficult to separate from mixture of reaction products, synthetic route was then modified. The phenolic hydroxy groups were protected. Compound 6 could be synthesised from 413) and condensed with 1d under EDC·HCl to afford compound 7. Subsequently, 7 reacted with potassium halogen in the presence of K222, and the p-methoxybenzyl (PMB) group was removed with trifluoroacetic acid (TFA) to afford 3f and g with high yield.14)
Second, an extra ether or ester group was introduced to the middle of the alkyl chain of ABG-001 to discover more active compound with better solubility.
The ABG-001 derivatives 11a–f were synthesised from the alkyl-diols 8a–f and the alkyl bromides 9a–f to yield intermediates 10a–f, which were condensed with 2 (Chart 3).
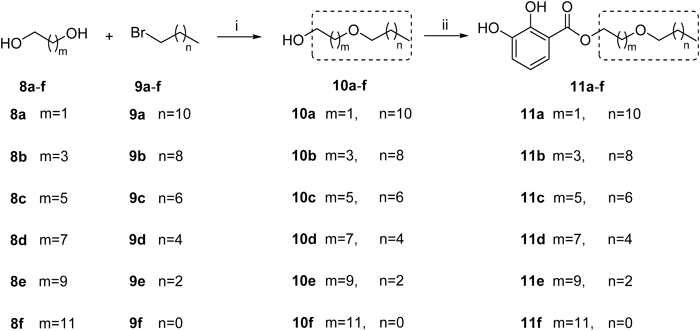
Reagents and condition: (i) NaH, TBAI, DMF, rt; (ii) Compound 2, EDC·HCl, DMAP, DCM, rt.
Compounds 14a–f were synthesised from the alkyl-diols 8a–f and the acyl chlorides 12a–f to yield intermediates 13a–f, which were condensed with 2 (Chart 4).
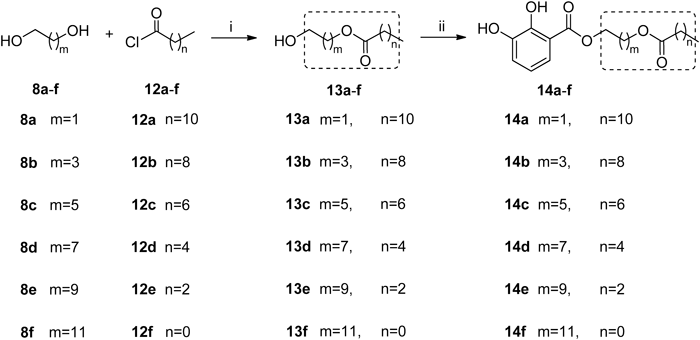
Reagents and condition: (i) Et3N, DMAP, DCM, rt; (ii) Compound 2, EDC·HCl, DMAP, DCM, rt.
Third, the oxygen atom between the benzene ring and the alkyl chain was replaced by a sulphur atom; a series of 2,3-dihydroxybenzothioates (Chart 5) with different alkyl chain lengths were synthesised. In our previous SAR studies, amide and ketone linkages decreased the biological activity; this effect may be attributed to their improved stability as compared with an ester group. In the present work, a thioester was used to replace the ester bond of ABG-001. The alkyl 2,3-dihydroxybenzothioates (15a–e) were prepared from compound 6 which involved of three steps in one pot. First, compound 6, pivaloyl chloride (PivCl), and triethylamine were added into dichloromethane at 0°C, warmed up to room temperature, and kept stirring until compound 6 disappeared (detected by thin-layer chromatography). Then the corresponding thiol and 4-dimethylaminopyridine were added into the mixture at 0°C, warmed up to room temperature, and kept stirring for 12 h.15) Finally, TFA (50% in water) was added into the mixture to remove protecting groups. The solubility of 2,3-dihydroxybenzothioates was reduced when alkyl chain length was longer than 14 carbon atoms. Therefore, 2,3-dihydroxybenzothioates with alkyl chain lengths between 6 to 14 carbon atoms were synthesised.

Reagents and condition: (i) (1) PivCl, Et3N, DCM, rt; (2) alkyl thiol, DMAP, rt; (3) 50% TFA, rt.
The neuritogenic activity of the synthesised gentiside derivatives (3a–i, 11a–f, 14a–f and 15a–e) was evaluated using PC12 cells as compared with the negative control (0.5% dimethyl sulfoxide (DMSO)) and the positive NGF control at an optimum concentration of 40 ng/mL, as well as ABG-001 (1 µM). Figure 2 shows the maximum neuritogenic activity of the gentiside derivatives 3a–i, 11a–f, 14a–f and 15a–e at their optimum concentration, respectively.
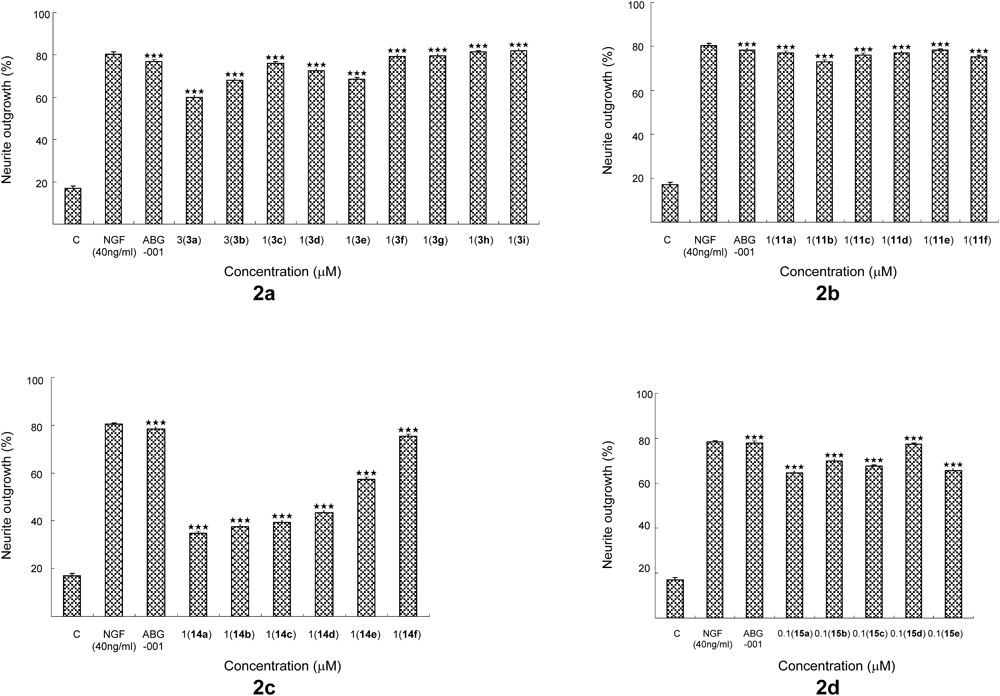
Value means are expressed as the average percentage of neurite outgrowth±S.D. (n=3). C: control (0.5% DMSO); positive control: NGF (40 ng/mL); ABG-001 (1.0 µM); (3a–i, 11a–f, 14a–f and 15a–e: *** p<0.001).
The biological activity of compounds 3a–i is depicted in Fig. 2a. Among compounds 3a–i, the 2,3-dihydroxybenzoate derivatives 3c–e exhibited a slight decrease in the percentage of neurite outgrowth, with a maximum activity of 75, 73 and 69%, respectively, at 1 µM as compared with ABG-001 (77%, 1 µM). The presence of a hydroxy group at the end of the alkyl chain (3a, 60%, 3 µM) decreased the activity. The introduction of 2,3-dihydroxybenzoate at the end of the alkyl chain (3b, 68%, 3 µM) also led to decreased activity. When one hydrogen atom of the terminal methyl was replaced by a halogen atom, the neuritogenic activity was slightly increased. Compounds 3f–i showed significant neuritogenic activity, with a maximum activity of 79, 80, 81 and 82% at 1 µM, respectively. Therefore, except for the hydroxy group, the introduction of other groups at the end of the alkyl chain led to minimal changes of biological activity in this study.
The biological activities of compounds 11a–f and 14a–f are presented in Figs. 2b and c, respectively. Compounds 11a–f, with an ether group in different positions of alkyl chain, did not show significant changes of biological activity, with a maximum activity of 77, 73, 76, 77, 78 and 75% at 1 µM, respectively, as compared with ABG-001 (78%, 1 µM). Compounds 14a–f had an ester group in different positions of the alkyl chain of ABG-001; these compounds showed a large change of biological activity, with a maximum activity of 34, 37, 39, 43, 57 and 75% at 1 µM, respectively, as compared with ABG-001. With the presence of carboxyl groups close to the aromatic ring, the decline in the biological activity was more obvious.
Compounds 15a–e showed neurite outgrowths of 65% (15a), 70% (15b), 68% (15c), 77% (15d) and 66% (15e) at 0.1 µM, respectively (Fig. 2d). Among these compounds, S-dodecyl 2,3-dihydroxybenzothioate (15d) was the most active and possessed a 12-carbon alkyl chain. The maximum activity of 15d was 77% at 0.1 µM, which was comparable to that of ABG-001 (78%) at 1.0 µM and NGF at the optimum concentration of 40 ng/mL (Fig. 2d).
Compound 15d showed a dose-dependent increase in the neuritogenic activity, which ranged from 0.03–0.1 µM, as shown in Fig. 3. Compound 15d exhibited neuritogenic activity of 50% even at a concentration of 0.03 µM and showed significant neuritogenic activity of 75% at 0.1 µM and higher doses. ABG-001 exhibited neuritogenic activity of 26, 33, 56 and 77% at doses of 0.03, 0.1, 0.3 and 1.0 µM, respectively. Figure 4 shows the morphological changes of PC12 cells after treatment with 15d at 0.1 µM as compared with the solvent control (0.5% DMSO), positive control (NGF at 40 ng/mL) and ABG-001 at 1 µM. Control cells (without adding compounds) showed few short neurite outgrowths (Fig. 4a). When treated with 15d at 0.1 µM, the cells extended long multipolar neurite outgrowths at 48 h after treatment (Fig. 4d), which was similar to those produced after treatment with ABG-001 at 1.0 µM (Fig. 4c) and NGF at 40 ng/mL (Fig. 4b). These results revealed that S-dodecyl 2,3-dihydroxybenzothioate (15d) is the best neuritogenic compound among all the gentisides and their derivatives in the present study and in previous studies. Subsequently, S-dodecyl 2,3-dihydroxybenzothioate (15d) was named as ABG-199.

C: control (0.5% DMSO); positive control: NGF (40 ng/mL), ABG-001 at 0.03, 0.1, 0.3 and 1 µM: *** p<0.001; 15d at 0.03, 0.1, 0.3 and 1 µM: *** p<0.001.

(a) Solvent control (0.5% DMSO), (b) NGF (40 ng/mL), (c) ABG-001 (1.0 µM), and (d) 15d (ABG-199, 0.1 µM).
Subsequently, the key protein in the signalling pathway of neuritogenesis was investigated to understand the brief mechanism of action of neurite outgrowth induced by ABG-199. To date, the cellular mechanism of NGF-induced neuritogenesis has been well investigated.16–18) NGF first binds to the transmembrane-specific tyrosine receptor kinase A to induce phosphorylation of the specific tyrosine residues located at the intracellular domain, thereby leading to the recruitment and activation of a number of kinases. Among these kinases, the mitogen-activated protein kinase extracellular signal-regulated kinase (ERKl/2) is a key enzyme during NGF-induced neurite outgrowth.19) In our previous study, ERKl/2 similarly had an important role in ABG-001-induced neurite outgrowth.10) Therefore, the involvement of ERKl/2 in 15d-induced neurite outgrowth should be investigated. Results showed that the treatment with 0.1 µM of 15d led to a sustained and significant enhancement of ERKl/2 phosphorylation after 4 h (Fig. 5). Simultaneously, treatment with 40 ng/mL of NGF resulted in the sustained and strong increase of ERKl/2 phosphorylation over 10 h. The phosphorylation level induced by 15d was slightly lower and more delayed than those induced by NGF. The data revealed that the significant neuritogenesis induced by 15d was dependent on the sustained activation of ERKl/2 in PC12 cells. The phosphorylation of ERKl/2 was slow using 15d compared with NGF is due to different mechanism of action between these two substances on neurite outgrowth of PC12 cells. NGF directly bound to the transmembrane-specific tyrosine receptor kinase A, and resulted in phosphorylation of ERKl/2. However, we found that activation of insulin-like growth factor 1 receptor (IGF-1R) is involved in ABG-001, an analogue of 15d induced neurite outgrowth of PC12 cells in recent study,20) as the chemical structure of 15d is quite similar to that of ABG-001. Therefore, we presumed that the mechanism of 15d on neurite outgrowth is similar to ABG-001. Furthermore, we need confirm whether ABG-001 directly or indirectly targets IGF-1R in future study.

The phosphorylation level (%) was analysed with the Image J software and normalised to the control value at different time points (100%).
Several series of ABG-001 derivatives were prepared via convenient synthetic methods. Their structures were confirmed by spectroscopic data. The alkyl chain of ABG-001 was modified, as well as the ester linkage between benzene ring and alkyl chain was replaced by a thioester group. Results of SAR studies indicated that property of a group at the end of side chain had effects on the biological activity. Introduction of an ether group into the middle position of alkyl chain of ABG-001 did not significantly change neuritogenic activity. However, the introduction of an ester group into the alkyl chain of ABG-001 significantly decreased the neuritogenic activity of compounds, especially when the ester group was close to an aromatic ring. When ester linkage between benzene ring and alkyl chain was replaced by a thioester group, compounds showed significant neuritogenic activity even at a concentration of 0.03 µM. Among all the synthesised gentiside derivatives, the most promising compound was S-dodecyl 2,3-dihydroxybenzothioate (15d), which was named as ABG-199. ABG-199 exhibited significant neuritogenic activity at 0.1 µM, which was comparable to that of NGF at the optimum concentration of 40 ng/mL. The brief study of mechanism of action of ABG-199 revealed that ERKl/2 phosphorylation was involved in ABG-199-induced neurite outgrowth in PC12 cells. Intensive studies on neuritogenic activity of ABG-199 are underway in our laboratory.
TLC analyses were done using pre-coated silica gel plates. The NMR spectra were recorded on a Bruker AV III-500 spectrometer, the NMR chemical shifts in δ (ppm) were referenced to the solvent peaks of δC 77.0 and δH 7.26 for CDCl3, and chemical shifts were given in δ (ppm) and signals were described as singlet (s), doublet (d), triplet (t), double doublet (dd) and multiplet (m). The high-resolution (HR) electrospray ionization-time of flight (ESI-TOF)-MS was recorded on Agilent 6224 A (TOF) LC/MS. All the solvents used were analytical grade.
14-Methoxy-1-tetradecanol (1b)Tetradecane-1,14-diol (1a, 690 mg, 3.00 mmol) was dissolved in anhydrous N,N-dimethylformamide (DMF) (20 mL). NaH (180 mg, 4.5 mmol) was added in at 0°C, kept stirring for 30 min. Warmed up to room temperature for another 30 min. MeI (511 mg, 3.60 mmol) was added in at 0°C, kept stirring overnight. The reaction was stopped by adding 1 mL of methanol, the mixture was washed with 1 N HCl solution, water, saturated aqueous solution of NaHCO3 and brine, successively. The organic phase was dried over Na2SO4, filtered, and then concentrated. The crude mixture was purified by column chromatography (petroleum ether–EtOAc 20 : 1) on silica gel to afford 1b (424 mg, 58%) as a colorless powder. 1H-NMR (500 MHz, CDCl3) δ: 3.65 (2H, t, J=6.5 Hz), 3.36 (2H, t, J=6.5 Hz), 3.33 (3H, s), 1.57–1.53 (4H, m), 1.32–1.25 (20H, m).
1-Hydroxytetradecyl Acetate (1c)Tetradecane-1,14-diol (1a, 460 mg, 2.00 mmol), Et3N (1.01 g, 10.0 mmol) 4-(dimethylamino)pyridine (DMAP) (12 mg, 0.2 mmol) were dissolved in anhydrous CH2Cl2 (100 mL). Cooled down the mixture to 0°C and Ac2O (225 mg, 2.20 mmol) was added, and kept stirring overnight. The reaction was stopped by adding 1 mL of methanol, the mixture was washed with 1 N HCl solution, saturated aqueous solution of NaHCO3 and brine, successively. The organic phase was dried over Na2SO4, filtered, and then concentrated. The crude mixture was purified by column chromatography (petroleum ether–EtOAc 10 : 1) on silica gel to afford 1c (234 mg, 43%) as a colorless powder. 1H-NMR (500 MHz, CDCl3) δ: 4.05 (2H, t, J=7.0 Hz), 3.64 (2H, t, J=6.5 Hz), 2.04 (3H, s), 1.63–1.54 (4H, m), 1.34–1.26 (20H, m).
1-Hydroxytetradecyl Methanesulphonate (1d)Tetradecane-1,14-diol (1a, 2.30 g, 10.00 mmol) was dissolved in anhydrous pyridine (20 mL), DMAP (122 mg, 1.0 mmol), MsCl (0.77 mL, 10.00 mmol) were added into this mixture at 0°C. Then warmed up to room temperature and keep stirring for 6h. The reaction was stopped with 2 mL methanol, the mixture was washed with 1 N HCl solution, saturated aqueous solution of NaHCO3 and brine, successively. The organic phase was dried over Na2SO4, filtered, and then concentrated. The crude mixture was purified by column chromatography (CH2Cl2–MeOH 100 : 1) on silica gel to afford 1d (920 mg, 30%) as a colorless powder. 1H-NMR (500 MHz, CDCl3) 4.22 (2H, t, J=7.0 Hz), 3.64 (2H, t, J=6.5 Hz), 3.00 (3H, s), 1.75 (2H, m), 1.56 (2H, m), 1.40–1.26 (20H, m).
14-Hydroxytetradecyl 2,3-Dihydroxybenzoate (3a) and 1,14-(2,3-Dihydroxybenzoyloxy)tetradecane (3b)Tetradecane-1,14-diol (1a, 230 mg, 1.00 mmol) was dissolved in anhydrous CH2Cl2 (30 mL), DMAP (122 mg, 1.0 mmol), EDC·HCl (1.92 g, 10.0 mmol) and 2,3-dihydroxybenzoic acid (1.54 g, 10.00 mmol) was added into this mixture. Kept stirring overnight at room temperature, and then the mixture was concentrated under vacuum. The crude mixture was purified by column chromatography (petroleum ether–EtOAc 30 : 1) on silica gel to afford 3a (101 mg, 20%) as a colorless powder and 3b (181 mg, 48%) as a colorless powder. 3a: 1H-NMR (500 MHz, CDCl3) δ: 11.00 (1H, s), 7.37 (1H, d, J=8.0 Hz), 7.10 (1H, d, J=8.0 Hz), 6.80 (1H, t, J=8.0 Hz), 5.67 (1H, s), 4.34 (2H, t, J=7.0 Hz), 3.64 (2H, t, J=6.5 Hz), 1.77 (2H, m), 1.56 (2H, m), 1.43 (2H, m), 1.35–1.26 (18H, m). 13C-NMR (125 MHz, CDCl3) δ: 170.4, 148.9, 145.0, 120.5, 119.7, 119.1, 112.7, 65.7, 63.1, 32.8, 29.6, 29.5, 29.4, 29.2, 28.5, 25.9, 25.7. HR-ESI-MS m/z: 389.2311 (Calcd for C21H34O5Na [M+Na]+: 389.2298). 3b: 1H-NMR (500 MHz, CDCl3) δ: 10.99 (2H, s), 7.37 (2H, d, J=8.0 Hz), 7.10 (2H, d, J=8.0 Hz), 6.79 (2H, t, J=8.0 Hz), 5.65 (2H, s), 4.34 (4H, t, J=7.0 Hz), 1.79–1.75 (4H, m), 1.45–1.40 (4H, m), 1.37–1.27 (16H, m). 13C-NMR (125 MHz, CDCl3) δ: 170.4, 148.9, 145.0, 120.5, 119.7, 119.1, 112.7, 65.7, 29.6, 29.5, 29.2, 28.5, 25.9. HR-ESI-MS m/z: 525.2459 (Calcd for C28H38O8Na [M+Na]+: 525.2459).
14-Methoxytetradecyl 2,3-Dihydroxybenzoate (3c)Compound 1b (244 mg, 1.00 mmol) was dissolved in anhydrous CH2Cl2 (20 mL), DMAP (12 mg, 0.1 mmol), EDC·HCl (575 mg, 3.0 mmol) and 2,3-dihydroxybenzoic acid (308 mg, 2.00 mmol) were added into this mixture. Kept stirring overnight at room temperature, and then the mixture was concentrated under vacuum. The crude mixture was purified by column chromatography (petroleum ether–EtOAc 20 : 1) on silica gel to afford 3c (181 mg, 48%) as a colorless powder. 1H-NMR (500 MHz, CDCl3) δ: 10.99 (1H, s), 7.37 (1H, d, J=8.0 Hz), 7.10 (1H, d, J=8.0 Hz), 6.79 (1H, t, J=8.0 Hz), 5.68 (1H, s), 4.34 (2H, t, J=7.0 Hz), 3.36 (2H, t, J=6.5 Hz), 3.33 (3H, s), 1.78 (2H, m), 1.59–1.55 (2H, m), 1.43–1.26 (20H, m). 13C-NMR (125 MHz, CDCl3) δ: 170.4, 148.9, 145.0, 120.5, 119.7, 119.1, 112.7, 73.0, 65.7, 58.5, 29.6, 29.5, 29.2, 28.5, 26.1, 25.9. HR-ESI-MS m/z: 403.2451 (Calcd for C22H36O5Na [M+Na]+: 403.2455).
14-Acetoxytetradecyl 2,3-Dihydroxybenzoate (3d)The synthesis and purification methods were the same as for 3c, condensing 1c (100 mg, 0.37 mmol) with 2,3-dihydroxybenzoic acid (113 mg, 0.73 mmol). The pure 3d was obtained as a colorless powder (72 mg, 48%). 1H-NMR (500 MHz, CDCl3) δ: 11.00 (1H, s), 7.37 (1H, d, J=8.0 Hz), 7.10 (1H, d, J=8.0 Hz), 6.80 (1H, t, J=8.0 Hz), 5.65 (1H, s), 4.34 (2H, t, J=6.5 Hz), 4.05 (2H, t, J=7.0 Hz), 2.05 (3H, s), 1.78 (2H, m), 1.60 (2H, m), 1.45–1.26 (20H, m). 13C-NMR (125 MHz, CDCl3) δ: 171.3, 170.4, 148.9, 145.0, 120.5, 119.7, 119.1, 112.7, 65.7, 64.7, 29.6, 29.5, 29.3, 29.2, 28.6, 28.5, 25.9, 21.0. HR-ESI-MS m/z: 431.2372 (Calcd for C23H36O6Na [M+Na]+: 431.2404).
14-(Methylsulphonyloxy)tetradecyl 2,3-Dihydroxybenzoate (3e)Compound 1d (920 mg, 2.98 mmol) was dissolved in anhydrous CH2Cl2 (20 mL), DMAP (37 mg, 0.3 mmol), EDC·HCl (1.14 g, 6.0 mmol) were added into this mixture. The crude mixture was purified by column chromatography (petroleum ether–EtOAc 5 : 1) on silica gel to afford 3e (540 mg, 41%) as a colorless powder. 1H-NMR (500 MHz, CDCl3) 10.99 (1H, s), 7.37 (1H, d, J=8.0 Hz), 7.10 (1H, d, J=8.0 Hz), 6.80 (1H, t, J=8.0 Hz), 5.64 (1H, s), 4.35 (2H, t, J=6.5 Hz), 4.22 (2H, t, J=6.5 Hz), 3.00 (3H, s), 1.79–1.73 (4H, m), 1.44–1.26 (20H, m). 13C-NMR (125 MHz, CDCl3) δ: 170.4, 148.9, 145.0, 120.5, 119.7, 119.1, 112.7, 70.2, 65.7, 37.4, 29.6, 29.5, 29.4, 29.2, 29.1, 29.0, 28.5, 25.9, 25.4. HR-ESI-MS m/z: 467.2081 (Calcd for C22H36O7SNa [M+Na]+: 467.2074).
Methyl 2,3-Bis(4-methoxybenzyloxy)benzoate (5)Methyl 2,3-dihydroxybenzoate (4, 1.00 g, 5.94 mmol) was dissolved in anhydrous tetrahydrofuran (THF) (50 mL), K2CO3 (4.92 g, 35.6 mmol), tetrabutylammonium iodide (TBAI) (0.22 g, 0.6 mmol), and PMBCl (2.42 mL, 17.82 mmol) were added into the solution. The mixture was refluxed for 24 h, and then filtered. The filtrate was concentrated, and purified by column chromatography (petroleum ether–EtOAc 50 : 1) to afford 5 (1.70 g, 70%) as a colorless powder. 1H-NMR (500 MHz, CDCl3) δ: 7.40–7.35 (5H, m), 7.14 (1H, dd, J=8.5, 1.5 Hz), 7.07 (1H, t, J=8.0 Hz), 6.92 (2H, d, J=8.5 Hz), 6.85 (2H, d, J=8.5 Hz), 5.06 (2H, s), 5.04 (2H, s), 3.87 (3H, s), 3.82 (3H, s), 3.80 (3H, s).
2,3-Bis(4-methoxybenzyloxy)benzoic Acid (6)A solution of 5 (0.94 g, 1.83 mmol) in dioxane (9 mL) and 2 N NaOH (4.6 mL) was stirred for 24 h at room temperature. The reaction mixture was concentrated. The residue was stirred with H2O (5 mL) and then acidified to pH 2 with 2 N HCl. The colorless powder was filtered, washed with hexane, and recrystallized from EtOAc–hexane to yield 500 mg (69%) of 6 as a colorless crystal. 1H-NMR (500 MHz, CDCl3) δ: 7.72 (1H, dd, J=8.0, 2.0 Hz), 7.41 (2H, d, J=9.0 Hz), 7.27–7.22 (3H, m), 7.18 (1H, t, J=8.0 Hz), 6.97–6.95 (2H, m), 6.84–6.82 (2H, m), 5.20 (2H, s), 5.12 (2H, s), 3.85 (3H, s), 3.80 (3H, s). 13C-NMR (125 MHz, CDCl3) δ: 165.2, 160.3, 159.8, 151.3, 147.0, 131.1, 129.6, 127.9, 126.7, 124.9, 124.3, 122.9, 119.0, 114.1, 76.8, 71.3, 55.3, 55.2.
14-(Methylsulphonyloxy)tetradecyl 2,3-Dihydroxybenzoate (7)Compound 1d (270 mg, 0.88 mmol) was dissolved in anhydrous CH2Cl2 (20 mL), DMAP (11 mg, 0.1 mmol) and EDC·HCl (503 mg, 2.6 mmol), and 6 (686 mg, 1.75 mmol) were added into this mixture. The crude mixture was purified by column chromatography (petroleum ether–EtOAc 5 : 1) on silica gel to yield 7 (259 mg, 43%) as a colorless powder. 1H-NMR (500 MHz, CDCl3) δ: 7.37–7.32 (5H, m), 7.12 (1H, d, J=8.0 Hz), 7.06 (1H, t, J=8.0 Hz), 6.91 (2H, m), 6.82 (2H, m), 5.05 (2H, s), 5.02 (2H, s), 4.26 (2H, t, J=7.0 Hz), 4.22 (2H, t, J=7.0 Hz), 3.83 (3H, s), 3.80 (3H, s), 2.99 (3H, s), 1.77–1.67 (4H, m), 1.41–1.35 (4H, m), 1.31–1.23 (16H, m). 13C-NMR (125 MHz, CDCl3) δ: 166.5, 159.5, 159.3, 152.8, 148.2, 130.2, 129.8, 129.3, 128.7, 127.3, 123.8, 122.6, 117.9, 113.9, 113.6, 77.2, 75.2, 71.1, 70.2, 65.3, 55.3, 55.2, 37.3, 29.6, 29.5, 29.4, 29.3, 29.1, 29.0, 28.7, 26.0, 25.4.
14-Fluorotetradecyl 2,3-Dihydroxybenzoate (3f)Compound 7 (10 mg, 0.015 mmol) was dissolved in 2 mL anhydrous acetonitrile, KF (1 mg, 0.2 mmol), K222 (20 mg, 0.1 mmol) were added, the reaction mixture was refluxed for 15 min. Solvent was evaporated under vacuum to give a crude product. The crude mixture was dissolved in 1 mL CH2Cl2, then 1 mL TFA was added in with stirring at room temperature, kept stirring for 10 min. Then the mixture was diluted with 10 mL distilled water, and subjected to C18 column, washed with 2 mL distilled water, 2 mL EtOH (50%) to yield 3f (4.1 mg, 76%) as a colorless powder. 1H-NMR (500 MHz, CDCl3) δ: 11.00 (1H, s), 7.37 (1H, d, J=8.0 Hz), 7.10 (1H, d, J=8.0 Hz), 6.80 (1H, t, J=8.0 Hz), 5.63 (1H, s), 4.44 (2H, dt, J=47.0, 6.5 Hz), 4.34 (2H, t, J=6.5 Hz), 1.80–1.63 (4H, m), 1.45–1.27 (20H, m). 13C-NMR (125 MHz, CDCl3) δ: 170.4, 148.9, 145.9, 120.5, 119.6, 119.1, 112.7, 84.3 (d, J=162.9 Hz), 65.7, 30.4, 30.3, 29.6, 29.5, 29.2, 28.5, 25.9, 25.1. HR-ESI-MS m/z: 391.2265 (Calcd for C21H33O4FNa [M+Na]+: 391.2255).
14-Chlorotetradecyl 2,3-Dihydroxybenzoate (3g)The synthesis and purification methods were the same as for 3f, starting from compound 7 (68 mg, 0.10 mmol), K222 (75 mg, 0.2 mmol) and KCl (37 mg, 0.5 mmol). The pure 3g was obtained as a colorless powder (30 mg, 79%). 1H-NMR (500 MHz, CDCl3) δ: 11.00 (1H, s), 7.37 (1H, d, J=8.0 Hz), 7.10 (1H, d, J=8.0 Hz), 6.80 (1H, t, J=8.0 Hz), 5.64 (1H, s), 4.35 (2H, t, J=6.5 Hz), 3.53 (2H, t, J=6.5 Hz), 1.81–1.74 (4H, m), 1.45–1.41 (4H, m), 1.37–1.27 (16H, m). 13C-NMR (125 MHz, CDCl3) δ: 170.4, 148.9, 145.0, 120.5, 119.7, 119.1, 112.7, 65.7, 45.2, 32.6, 29.6, 29.5, 29.4, 29.2, 28.9, 28.5, 26.9, 25.9. HR-ESI-MS m/z: 407.1956 (Calcd for C21H33O4ClNa [M+Na]+: 407.1960).
14-Bromotetradecyl 2,3-Dihydroxybenzoate (3h)Compound 3e (44 mg, 0.10 mmol) was dissolved in 2 mL anhydrous acetonitrile, KBr (36 mg, 0.3 mmol), K222 (38 mg, 0.1 mmol) were added, the reaction mixture was refluxed for 30 min. Solvent was evaporated under vacuum to give the crude product which was purified by column chromatography (petroleum ether–EtOAc 5 : 1) on silica gel to afford 3h (38 mg, 88%) as a colorless powder. 1H-NMR (500 MHz, CDCl3) δ: 10.99 (1H, s), 7.37 (1H, d, J=8.0 Hz), 7.10 (1H, d, J=8.0 Hz), 6.80 (1H, t, J=8.0 Hz), 5.64 (1H, s), 4.34 (2H, t, J=6.5 Hz), 3.41 (2H, t, J=6.5 Hz), 1.87–1.75 (4H, m), 1.45–1.27 (20H, m). 13C-NMR (125 MHz, CDCl3) δ: 170.4, 148.9, 145.0, 120.5, 119.6, 119.1, 112.7, 65.7, 34.0, 32.8, 29.6, 29.5, 29.4, 29.2, 28.8, 28.5, 28.2, 25.9. HR-ESI-MS m/z: 451.1457 (Calcd for C21H33O479BrNa [M+Na]+: 451.1454).
14-Iodotetradecyl 2,3-Dihydroxybenzoate (3i)Compound 3e (44 mg, 0.10 mmol) was dissolved in anhydrous acetonitrile, KI (33 mg, 0.2 mmol), K222 (38 mg, 0.1 mmol) were added, the reaction mixture was refluxed for 30 min. Solvent was evaporated to give the crude which was purified by column chromatography (hexane–EtOAc 30 : 1) on silica gel to afford 3i (44 mg, 92%) as a colorless powder. 1H-NMR (500 MHz, CDCl3) δ: 11.00 (1H, s), 7.37 (1H, d, J=8.0 Hz), 7.10 (1H, d, J=8.0 Hz), 6.80 (1H, t, J=8.0 Hz), 5.66 (1H, s), 4.34 (2H, t, J=6.5 Hz), 3.19 (2H, t, J=7.0 Hz), 1.85–1.75 (4H, m), 1.46–1.26 (20H, m). 13C-NMR (125 MHz, CDCl3) δ: 170.4, 148.9, 145.0, 120.5, 119.6, 119.1, 112.7, 65.7, 33.6, 30.5, 29.6, 29.5, 29.4, 29.2, 28.5, 25.9, 7.3. HR-ESI-MS m/z: 499.1330 (Calcd for C21H33O4INa [M+Na]+: 499.1316).
2-(Dodecyloxy)ethanol (10a)Ethanediol (1.1 mL, 20.00 mmol) was dissolved in 200 mL anhydrous DMF, NaH (480 mg, 12.0 mmol) was added in at 0°C. The mixture was warmed up to room temperature and stirred for 30 min. Then 1-bromododecane (2.4 mL, 10.00 mmol), TBAI (369 mg, 1.0 mmol) were added in at 0°C, kept stirring overnight. The reaction mixture was diluted with 20 mL methanol, then washed with 1 N HCl solution, water, saturated aqueous solution of NaHCO3 and brine, successively. The organic phase was dried over Na2SO4, filtered, and then concentrated. The crude mixture was purified by column chromatography (petroleum ether–EtOAc 30 : 1) on silica gel to afford 10a as a colorless oil (1.12 g, 49%). 1H-NMR (500 MHz, CDCl3) δ: 3.73 (2H, t, J=4.5 Hz), 3.53 (2H, t, J=4.5 Hz), 3.47 (2H, t, J=6.5 Hz), 1.59 (2H, m), 1.29–1.26 (18H, m), 0.88 (3H, t, J=6.5 Hz).
4-(Decyloxy)butan-1-ol (10b)The synthesis and purification methods were the same as for 10a. The pure 10b was obtained as a colorless oil (1.01 g, 44%). 1H-NMR (500 MHz, CDCl3) δ: 3.64 (2H, t, J=5.5 Hz), 3.45 (2H, t, J=5.5 Hz), 3.42 (2H, t, J=6.5 Hz), 1.70–1.65 (4H, m), 1.56 (2H, m), 1.30–1.25 (14H, m), 0.87 (3H, t, J=7.0 Hz).
6-(Octyloxy)hexan-1-ol (10c)The synthesis and purification methods were the same as for 10a. The pure 10c was obtained as a colorless oil (1.09 g, 47%). 1H-NMR (500 MHz, CDCl3) δ: 3.60 (2H, t, J=6.5 Hz), 3.39–3.35 (4H, m), 1.58–1.51 (6H, m), 1.35–1.25 (14H, m), 0.85 (3H, t, J=6.5 Hz).
8-(Hexyloxy)octan-1-ol (10d)The synthesis and purification methods were the same as for 10a. The pure 10d was obtained as a colorless oil (0.91 g, 39%). 1H-NMR (500 MHz, CDCl3) δ: 3.63 (2H, t, J=6.5 Hz), 3.39 (4H, t, J=6.5 Hz), 1.57–1.53 (6H, m), 1.36–1.25 (14H, m), 0.88 (3H, t, J=6.5 Hz).
10-(Butoxy)decan-1-ol (10e)The synthesis and purification methods were the same as for 10a. The pure 10e was obtained as a colorless oil (1.11 g, 48%). 1H-NMR (500 MHz, CDCl3) δ: 3.64 (2H, t, J=6.5 Hz), 3.41–3.37 (4H, m), 1.58–1.52 (6H, m), 1.40–1.28 (14H, m), 0.92 (3H, t, J=7.0 Hz).
12-(Ethoxy)dodecan-1-ol (10f)The synthesis and purification methods were the same as for 10a. The pure 10f was obtained as a colorless oil (0.99 g, 43%). 1H-NMR (500 MHz, CDCl3) δ: 3.63 (2H, t, J=6.5 Hz), 3.46 (2H, m, J=7.0 Hz), 3.40 (2H, t, J=7.0 Hz), 1.58–1.52 (4H, m), 1.33–1.26 (16H, m), 1.19 (3H, t, J=7.0 Hz).
2-(Dodecyloxy)ethyl 2,3-Dihydroxybenzoate (11a)The synthesis and purification methods were the same as for 3a, condensing from 10a (230 mg, 1.00 mmol) and 2,3-dihydroxybenzoic acid (308 mg, 2.00 mmol). The pure 11a was obtained as a colorless powder (181 mg, 49%). 1H-NMR (500 MHz, CDCl3) δ: 10.83 (1H, s), 7.41 (1H, d, J=8.0 Hz), 7.11 (1H, d, J=8.0 Hz), 6.80 (1H, t, J=8.0 Hz), 5.64 (1H, s), 4.49 (2H, t, J=5.0 Hz), 3.76 (2H, t, J=5.0 Hz), 3.51 (2H, t, J=6.5 Hz), 1.58 (2H, m), 1.34–1.25 (18H, m), 0.88 (3H, t, J=7.0 Hz). 13C-NMR (125 MHz, CDCl3) δ: 170.2, 148.8, 145.0, 120.8, 119.8, 119.2, 112.5, 71.6, 68.2, 64.6, 31.9, 29.7, 29.6, 29.4, 29.3, 26.1, 22.7, 14.1. HR-ESI-MS m/z: 389.2291 (Calcd for C21H34O5Na [M+Na]+: 389.2298).
4-(Decyloxy)butyl 2,3-Dihydroxybenzoate (11b)The synthesis and purification methods were the same as for 3a, condensing from 10b (230 mg, 1.00 mmol) and 2,3-dihydroxybenzoic acid (308 mg, 2.00 mmol). The pure 11b was obtained as a colorless powder (180 mg, 47%). 1H-NMR (500 MHz, CDCl3) δ: 10.97 (1H, s), 7.37 (1H, d, J=8.0 Hz), 7.10 (1H, d, J=8.0 Hz), 6.79 (1H, t, J=8.0 Hz), 5.64 (1H, s), 4.38 (2H, t, J=6.5 Hz), 3.47 (2H, t, J=6.0 Hz), 3.41 (2H, t, J=6.5 Hz), 1.88 (2H, m), 1.73 (2H, m), 1.56 (2H, m), 1.34–1.23 (14H, m), 0.88 (3H, t, J=7.0 Hz). 13C-NMR (125 MHz, CDCl3) δ: 170.4, 148.9, 145.0, 120.5, 119.7, 119.1, 112.6, 71.1, 70.1, 65.5, 31.9, 29.7, 29.6, 29.5, 29.3, 26.3, 26.2, 25.5, 22.7, 14.1. HR-ESI-MS m/z: 389.2311 (Calcd for C21H34O5Na [M+Na]+: 389.2298).
6-(Octyloxy)hexyl 2,3-Dihydroxybenzoate (11c)The synthesis and purification methods were the same as for 3a, condensing from 10c (230 mg, 1.00 mmol) and 2,3-dihydroxybenzoic acid (308 mg, 2.00 mmol). The pure 11c was obtained as a colorless solid (170 mg, 44%). 1H-NMR (500 MHz, CDCl3) δ: 10.99 (1H, s), 7.37 (1H, d, J=8.0 Hz), 7.10 (1H, d, J=8.0 Hz), 6.79 (1H, t, J=8.0 Hz), 5.64 (1H, s), 4.35 (2H, t, J=6.5 Hz), 3.42–3.38 (4H, m), 1.79 (2H, m), 1.63–1.27 (18H, m), 0.88 (3H, t, J=7.0 Hz). 13C-NMR (125 MHz, CDCl3) δ: 170.4, 148.9, 145.0, 120.5, 119.7, 119.1, 112.7, 71.0, 70.7, 65.6, 31.8, 29.8, 29.6, 29.5, 29.3, 28.5, 26.2, 25.9, 25.8, 22.6, 14.1. HR-ESI-MS m/z: 389.2316 (Calcd for C21H34O5Na [M+Na]+: 389.2298).
8-(Hexyloxy)octyl 2,3-Dihydroxybenzoate (11d)The synthesis and purification methods were the same as for 3a, condensing from 10d (230 mg, 1.00 mmol) and 2,3-dihydroxybenzoic acid (308 mg, 2.00 mmol). The pure 11d was obtained as a colorless powder (178 mg, 48%). 1H-NMR (500 MHz, CDCl3) δ: 10.99 (1H, s), 7.37 (1H, d, J=8.0 Hz), 7.10 (1H, d, J=8.0 Hz), 6.79 (1H, t, J=8.0 Hz), 5.67 (1H, s), 4.34 (2H, t, J=6.5 Hz), 3.40–3.38 (4H, m), 1.77 (2H, m), 1.59–1.53 (4H, m), 1.45–1.27 (14H, m), 0.88 (3H, t, J=7.0 Hz). 13C-NMR (125 MHz, CDCl3) δ: 170.4, 148.9, 145.3, 120.5, 120.0, 119.1, 112.7, 71.0, 70.9, 65.7, 31.7, 29.7, 29.3, 29.2, 28.5, 26.1, 25.9, 22.6, 14.0. HR-ESI-MS m/z: 389.2324 (Calcd for C21H34O5Na [M+Na]+: 389.2298).
10-(Butoxy)decyl 2,3-Dihydroxybenzoate (11e)The synthesis and purification methods were the same as for 3a, condensing from 10e (230 mg, 1.00 mmol) and 2,3-dihydroxybenzoic acid (308 mg, 2.00 mmol). The pure 11e was obtained as a colorless powder (190 mg, 52%). 1H-NMR (500 MHz, CDCl3) δ: 11.00 (1H, s), 7.37 (1H, d, J=8.0 Hz), 7.10 (1H, d, J=8.0 Hz), 6.80 (1H, t, J=8.0 Hz), 5.67 (1H, s), 4.34 (2H, t, J=6.5 Hz), 3.41–3.78 (4H, m), 1.77 (2H, m), 1.59–1.52 (4H, m), 1.45–1.30 (14H, m), 0.92 (3H, t, J=7.5 Hz). 13C-NMR (125 MHz, CDCl3) δ: 170.4, 148.9, 145.0, 120.5, 119.6, 119.1, 112.7, 70.9, 70.6, 65.7, 31.9, 29.8, 29.5, 29.4, 29.2, 28.5, 26.2, 25.9, 19.4, 13.9. HR-ESI-MS m/z: 389.2293 (Calcd for C21H34O5Na [M+Na]+: 389.2298).
12-(Ethoxy)dodecyl 2,3-Dihydroxybenzoate (11f)The synthesis and purification methods were the same as for 3a, condensing from 10f (230 mg, 1.00 mmol) and 2,3-dihydroxybenzoic acid (308 mg, 2.00 mmol). The pure 11f was obtained as a colorless powder (197 mg, 55%). 1H-NMR (500 MHz, CDCl3) δ: 11.00 (1H, s), 7.37 (1H, d, J=8.0 Hz), 7.10 (1H, d, J=8.0 Hz), 6.80 (1H, t, J=8.0 Hz), 5.66 (1H, s), 4.34 (2H, t, J=6.5 Hz), 3.47 (2H, m), 3.40 (2H, t, J=6.5 Hz), 1.77 (2H, m), 1.57 (2H, m), 1.43 (2H, m), 1.34–1.27 (14H, m), 1.20 (3H, t, J=7.0 Hz). 13C-NMR (125 MHz, CDCl3) δ: 170.4, 148.9, 145.0, 120.5, 119.6, 119.1, 112.7, 70.8, 66.0, 65.7, 29.8, 29.6, 29.5, 29.2, 28.5, 26.2, 25.9, 15.2. HR-ESI-MS m/z: 389.2323 (Calcd for C21H34O5Na [M+Na]+: 389.2298).
2-Hydroxyethyl Dodecanoate (13a)Ethanediol (1.1 mL, 20.00 mmol), Et3N (5.10 g, 50.0 mmol) and DMAP (122 mg, 1.0 mmol) were dissolved in 200 mL anhydrous CH2Cl2. The C11H23COCl (2.18 g, 10.00 mmol) was added dropwise at 0°C. The mixture was warmed up to room temperature and kept stirring overnight then washed with 1 N HCl solution, water, saturated aqueous solution of NaHCO3 and brine, successively. The organic phase was dried over Na2SO4, filtered, and then concentrated. The crude mixture was purified by column chromatography (petroleum ether–EtOAc 30 : 1) on silica gel to afford 13a as a colorless oil (1.51 g, 62%). 1H-NMR (500 MHz, CDCl3) δ: 4.20 (2H, m), 3.82 (2H, m), 2.34 (2H, t, J=7.5 Hz), 1.62 (2H, m), 1.32–1.25 (16H, m), 0.87 (3H, t, J=6.5 Hz).
4-Hydroxybutyl Decanoate (13b)The synthesis and purification methods were the same as for 13a. The pure 13b was obtained as a colorless oil (1.05 g, 43%). 1H-NMR (500 MHz, CDCl3) δ: 4.10 (2H, t, J=6.5 Hz), 3.68 (2H, t, J=6.5 Hz), 2.29 (2H, t, J=7.5 Hz), 1.72–1.62 (6H, m), 1.30–1.26 (12H, m), 0.87 (3H, t, J=7.0 Hz).
6-Hydroxyhexyl Octanoate (13c)The synthesis and purification methods were the same as for 13a. The pure 13c was obtained as a colorless oil (1.00 g, 41%). 1H-NMR (500 MHz, CDCl3) δ: 4.05 (2H, t, J=7.0 Hz), 3.64 (2H, t, J=7.0 Hz), 2.28 (2H, t, J=7.5 Hz), 1.63–1.55 (6H, m), 1.35–1.29 (12H, m), 0.89 (3H, t, J=7.0 Hz).
8-Hydroxyoctyl Hexanoate (13d)The synthesis and purification methods were the same as for 13a. The pure 13d was obtained as a colorless oil (1.30 g, 53%). 1H-NMR (500 MHz, CDCl3) δ: 4.05 (2H, t, J=7.0 Hz), 3.64 (2H, t, J=7.0 Hz), 2.28 (2H, t, J=7.5 Hz), 1.63–1.55 (6H, m), 1.35–1.29 (12H, m), 0.89 (3H, t, J=7.0 Hz).
10-Hydroxydecyl Butyrate (13e)The synthesis and purification methods were the same as for 13a. The pure 13e was obtained as a colorless oil (1.01 g, 41%). 1H-NMR (500 MHz, CDCl3) δ: 4.05 (2H, t, J=6.5 Hz), 3.63 (2H, t, J=6.5 Hz), 2.27 (2H, t, J=7.5 Hz), 1.67–1.54 (6H, m), 1.38–1.24 (12H, m), 0.94 (3H, t, J=7.5 Hz).
12-Hydroxydodecyl Acetate (13f)The synthesis and purification methods were the same as for 13a. The pure 13f was obtained as a colorless oil (1.10 g, 45%). 1H-NMR (500 MHz, CDCl3) δ: 4.04 (2H, t, J=6.5 Hz), 3.63 (2H, m), 2.04 (3H, s), 1.64–1.53 (4H, m), 1.34–1.26 (16H, m).
2-(Dodecanoyloxy)ethyl 2,3-Dihydroxybenzoate (14a)The synthesis and purification methods were the same as for 3a, condensing from 13a (230 mg, 0.94 mmol) and 2,3-dihydroxybenzoic acid (308 mg, 2.00 mmol). The pure 14a was obtained as a colorless powder (190 mg, 53%). 1H-NMR (500 MHz, CDCl3) δ: 10.75 (1H, s), 7.38 (1H, d, J=8.0 Hz), 7.12 (1H, d, J=8.0 Hz), 6.81 (1H, t, J=8.0 Hz), 4.55 (2H, m), 4.43 (2H, m), 2.34 (2H, t, J=7.5 Hz), 1.61 (2H, m), 1.29–1.24 (16H, m), 0.88 (3H, t, J=7.0 Hz). 13C-NMR (125 MHz, CDCl3) δ: 173.6, 170.0, 148.9, 145.0, 120.7, 120.0, 119.3, 112.1, 63.2, 61.5, 34.1, 31.9, 29.6, 29.4, 29.3, 29.2, 29.1, 24.9, 22.7, 14.1. HR-ESI-MS m/z: 403.2089 (Calcd for C21H32O6Na [M+Na]+: 403.2091).
4-(Decanoyloxy)butyl 2,3-Dihydroxybenzoate (14b)The synthesis and purification methods were the same as for 3a, condensing from 13b (230 mg, 0.94 mmol) and 2,3-dihydroxybenzoic acid (308 mg, 2.00 mmol). The pure 14b was obtained as a colorless powder (180 mg, 50%). 1H-NMR (500 MHz, CDCl3) δ: 10.93 (1H, s), 7.36 (1H, d, J=8.0 Hz), 7.11 (1H, d, J=8.0 Hz), 6.80 (1H, t, J=8.0 Hz), 5.64 (1H, s), 4.39 (2H, t, J=6.5 Hz), 4.14 (2H, t, J=6.5 Hz), 2.30 (2H, t, J=7.5 Hz), 1.90–1.77 (4H, m), 1.62 (2H, m), 1.32–1.26 (12H, m), 0.87 (3H, t, J=7.0 Hz). 13C-NMR (125 MHz, CDCl3) δ: 173.9, 170.3, 148.9, 145.1, 120.5, 119.8, 119.1, 112.4, 65.0, 63.5, 34.3, 31.8, 29.4, 29.2, 29.1, 25.3, 25.0, 22.6, 14.1. HR-ESI-MS m/z: 403.2128 (Calcd for C21H32O6Na [M+Na]+: 403.2091).
6-(Octanoyloxy)hexyl 2,3-Dihydroxybenzoate (14c)The synthesis and purification methods were the same as for 3a, condensing from 13c (230 mg, 0.94 mmol) and 2,3-dihydroxybenzoic acid (308 mg, 2.00 mmol). The pure 14c was obtained as a colorless powder (199 mg, 56%). 1H-NMR (500 MHz, CDCl3) δ: 10.97 (1H, s), 7.36 (1H, d, J=8.0 Hz), 7.11 (1H, d, J=8.0 Hz), 6.80 (1H, t, J=8.0 Hz), 5.65 (1H, s), 4.35 (2H, t, J=6.5 Hz), 4.08 (2H, t, J=6.5 Hz), 2.29 (2H, t, J=7.0 Hz), 1.79 (2H, m), 1.68–1.57 (4H, m), 1.50–1.41 (4H, m), 1.30–1.27 (8H, m), 0.87 (3H, t, J=7.0 Hz). 13C-NMR (125 MHz, CDCl3) δ: 174.0, 170.4, 148.9, 145.0, 120.5, 119.7, 119.1, 112.6, 65.5, 64.1, 34.4, 31.7, 29.1, 28.9, 28.5, 28.4, 25.6, 25.0, 22.6, 14.0. HR-ESI-MS m/z: 403.2129 (Calcd for C21H32O6Na [M+Na]+: 403.2091).
8-(Hexanoyloxy)octyl 2,3-Dihydroxybenzoate (14d)The synthesis and purification methods were the same as for 3a, condensing from 13d (230 mg, 0.94 mmol) and 2,3-dihydroxybenzoic acid (308 mg, 2.00 mmol). The pure 14d was obtained as a colorless powder (177 mg, 49%). 1H-NMR (500 MHz, CDCl3) δ: 10.99 (1H, s), 7.37 (1H, d, J=8.0 Hz), 7.10 (1H, d, J=8.0 Hz), 6.80 (1H, t, J=8.0 Hz), 5.65 (1H, s), 4.34 (2H, t, J=7.0 Hz), 4.06 (2H, t, J=7.0 Hz), 2.29 (2H, t, J=7.5 Hz), 1.78 (2H, m), 1.64–1.61 (4H, m), 1.44–1.29 (12H, m), 0.89 (3H, t, J=7.0 Hz). 13C-NMR (125 MHz, CDCl3) δ: 174.0, 170.4, 148.9, 145.0, 120.5, 119.7, 119.1, 112.6, 65.6, 64.3, 34.4, 31.3, 29.1, 28.6, 28.5, 25.8, 24.7, 22.3, 13.9. HR-ESI-MS m/z: 403.2118 (Calcd for C21H32O6Na [M+Na]+: 403.2091).
10-(Butyryloxy)decyl 2,3-Dihydroxybenzoate (14e)The synthesis and purification methods were the same as for 3a, condensing from 13e (230 mg, 0.94 mmol) and 2,3-dihydroxybenzoic acid (308 mg, 2.00 mmol). The pure 14e was obtained as a colorless powder (190 mg, 53%). 1H-NMR (500 MHz, CDCl3) δ: 10.98 (1H, s), 7.37 (1H, d, J=8.0 Hz), 7.10 (1H, d, J=8.0 Hz), 6.79 (1H, t, J=8.0 Hz), 5.69 (1H, s), 4.34 (2H, t, J=6.5 Hz), 4.06 (2H, t, J=6.5 Hz), 2.27 (2H, t, J=7.5 Hz), 1.78 (2H, m), 1.67–1.26 (16H, m), 0.95 (3H, t, J=7.5 Hz). 13C-NMR (125 MHz, CDCl3) δ: 173.8, 170.4, 148.9, 145.1, 120.5, 119.7, 119.1, 112.7, 65.7, 64.3, 36.3, 29.4, 29.2, 28.6, 28.5, 25.9, 18.5, 13.7. HR-ESI-MS m/z: 403.2092 (Calcd for C21H32O6Na [M+Na]+: 403.2091).
12-(Acetoxy)dodecyl 2,3-Dihydroxybenzoate (14f)The synthesis and purification methods were the same as for 3a, condensing from 13f (230 mg, 0.94 mmol) and 2,3-dihydroxybenzoic acid (308 mg, 2.00 mmol). The pure 14f was obtained as a colorless powder (203 mg, 57%). 1H-NMR (500 MHz, CDCl3) δ: 11.00 (1H, s), 7.37 (1H, d, J=8.0 Hz), 7.10 (1H, d, J=8.0 Hz), 6.79 (1H, t, J=8.0 Hz), 5.78 (1H, s), 4.34 (2H, t, J=6.5 Hz), 4.05 (2H, t, J=6.5 Hz), 2.05 (3H, s), 1.77 (2H, m), 1.61 (2H, m), 1.45–1.27 (16H, m). 13C-NMR (125 MHz, CDCl3) δ: 171.2, 170.4, 148.9, 145.0, 120.5, 119.6, 119.1, 112.7, 65.7, 64.7, 29.5, 29.2, 28.6, 28.5, 25.9, 21.0. HR-ESI-MS m/z: 403.2094 (Calcd for C21H32O6Na [M+Na]+: 403.2091).
S-Hexyl 2,3-Dihydroxybenzothioate (15a)To a solution of compound 6 (79 mg, 0.20 mmol), triethylamine (202 mg, 2.0 mmol) in dry CH2Cl2 (5 mL) at 0°C was added pivaloyl chloride (24 mg, 0.20 mmol). The mixture was warmed to room temperature and was stirred until compound 6 disappeared (detected by TLC). Then 1-hexanethiol (47 mg, 0.40 mmol) and 4-dimethylaminopyridine (2.4 mg, 0.02 mmol) were added into the mixture at 0°C, warmed up to room temperature, and kept stirring for 12 h. Finally, trifluoroacetic acid (50% in water, 5 mL) was added into the mixture, and kept stirring for 12 h. The mixture was extracted with CH2Cl2, and washed with water, saturated aqueous solution of NaHCO3 and brine, successively. The organic phase was dried over Na2SO4, filtered, and then concentrated. The crude mixture was purified by column chromatography (n-hexane–EtOAc 300 : 1) on silica gel to afford 15a as a colorless oil (27 mg, 53%). 1H-NMR (500 MHz, CDCl3) δ: 11.26 (1H, s), 7.42 (1H, d, J=8.0 Hz), 7.10 (1H, d, J=8.0 Hz), 6.81 (1H, t, J=8.0 Hz), 5.69 (1H, s), 3.07 (2H, t, J=7.0 Hz), 1.68 (2H, m), 1.44 (2H, m), 1.33–1.31 (4H, m), 0.90 (3H, t, J=7.0 Hz). 13C-NMR (125 MHz, CDCl3) δ: 197.9, 146.6, 145.3, 120.0, 119.7, 119.5, 119.4, 31.8, 29.3, 29.1, 28.9, 22.6, 14.1. HR-ESI-MS m/z: 255.1063 (Calcd for C13H19O3S [M+H]+: 255.1049).
S-Octyl 2,3-Dihydroxybenzothioate (15b)The synthesis and purification methods were the same as for 15a using 1-octanethiol (29 mg, 0.20 mmol) as corresponding thiol, the pure 15b was obtained as a colorless oil (30 mg, 53%). 1H-NMR (500 MHz, CDCl3) δ: 11.26 (1H, s), 7.42 (1H, d, J=8.0 Hz), 7.10 (1H, d, J=8.0 Hz), 6.81 (1H, t, J=8.0 Hz), 5.69 (1H, s), 3.07 (2H, t, J=7.0 Hz), 1.68 (2H, m), 1.41–1.40 (2H, m), 1.34–1.28 (8H, m), 0.89 (3H, t, J=7.0 Hz). 13C-NMR (125 MHz, CDCl3) δ: 197.9, 146.6, 145.3, 120.0, 119.7, 119.5, 119.4, 31.8, 29.3, 29.1, 28.9, 22.6, 14.1. HR-ESI-MS m/z: 283.1369 (Calcd for C15H23O3S [M+H]+: 283.1362).
S-Decyl 2,3-Dihydroxybenzothioate (15c)The synthesis and purification methods were the same as for 15a using 1-decanethiol (35 mg, 0.20 mmol) as corresponding thiol, the pure 15c was obtained as a colorless oil (35 mg, 56%). 1H-NMR (500 MHz, CDCl3) δ: 11.26 (1H, s), 7.42 (1H, d, J=8.0 Hz), 7.10 (1H, d, J=8.0 Hz), 6.81 (1H, t, J=8.0 Hz), 5.69 (1H, s), 3.07 (2H, t, J=7.5 Hz), 1.68 (2H, m), 1.42 (2H, m), 1.34–1.27 (12H, m), 0.88 (3H, t, J=7.0 Hz). 13C-NMR (125 MHz, CDCl3) δ: 197.9, 146.6, 145.3, 120.0, 119.7, 119.5, 119.4, 31.9, 29.5, 29.3, 29.1, 28.9, 22.7, 14.1. HR-ESI-MS m/z: 311.1687 (Calcd for C17H27O3S [M+H]+: 311.1675).
S-Dodecyl 2,3-Dihydroxybenzothioate (15d)The synthesis and purification methods were the same as for 15a using 1-dodecanethiol (40 mg, 0.20 mmol) as corresponding thiol, the pure 15d was obtained as a colorless oil (39 mg, 58%). 1H-NMR (500 MHz, CDCl3) δ: 11.23 (1H, s), 7.42 (1H, d, J=8.0 Hz), 7.10 (1H, d, J=8.0 Hz), 6.81 (1H, t, J=8.0 Hz), 5.67 (1H, s), 3.07 (2H, t, J=7.5 Hz), 1.68 (2H, m), 1.42 (2H, m), 1.34–1.26 (16H, m), 0.88 (3H, t, J=6.5 Hz). 13C-NMR (125 MHz, CDCl3) δ: 197.880, 146.642, 145.300, 120.001, 119.689, 119.492, 119.350, 31.904, 29.620, 29.557, 29.462, 29.330, 29.287, 29.104, 28.891, 22.676, 14.098. HR-ESI-MS m/z: 339.1989 (Calcd for C19H31O3S [M+H]+: 339.1988).
S-Tetradecyl 2,3-Dihydroxybenzothioate (15e)The synthesis and purification methods were the same as for 15a using 1-tetradecanethiol (46 mg, 0.20 mmol) as corresponding thiol, the pure 15e was obtained as a colorless powder (37 mg, 50%). 1H-NMR (500 MHz, CDCl3) δ: 11.27 (1H, s), 7.42 (1H, d, J=8.0 Hz), 7.10 (1H, d, J=8.0 Hz), 6.81 (1H, t, J=8.0 Hz), 5.67 (1H, s), 3.07 (2H, t, J=6.5 Hz), 1.68 (2H, m), 1.42 (2H, m), 1.34–1.26 (20H, m), 0.88 (3H, t, J=6.5 Hz). 13C-NMR (125 MHz, CDCl3) δ: 197.881, 146.643, 145.299, 120.000, 119.690, 119.490, 119.350, 31.914, 29.643, 29.559, 29.463, 29.346, 29.288, 29.104, 28.883, 22.680, 14.101. HR-ESI-MS m/z: 367.2300 (Calcd for C21H35O3S [M+H]+: 367.2301).
BioassayBiological activity was done using the methods described in our previous study.6) PC12 cells (20000) were seeded in each well of a 24-well microplate and incubated under a humidified atmosphere of 5% CO2 at 37°C. After 24 h, the medium was replaced with 1 mL of serum-free Dulbecco’s modified Eagle’s medium (DMEM) containing a sample or DMSO (0.5%). NGF (40 ng/mL) was used as a positive control. The morphological changes of the cells were observed under a microscope. Approximately, 100 cells were watched from a randomly chosen area. A cell having neurite outgrowth longer than the diameter of cell body was determined as a positive cell. Significant differences between each groups were tested by ANOVA, followed by two-tailed multiple Student–Newman–Keuls t-tests using SPSS biostatistics software (IBM; Armonk, NY, U.S.A.). Values of p<0.05 were considered significant. Independent experiments were repeated for three times. Each value represents as the mean±standard error of the mean (S.E.M.) of three replicates. ** or *** indicates significant differences relative to the control at p<0.01 or p<0.001, respectively.
Western BlotWestern blot analysis was performed as previously described.10) Briefly, 1.5×106 cells were incubated in 6 cm dish with 15d (ABG-199) at a concentration of 0.1 µM for 0, 2, 4, 6, 8 or 10 h. The cells were lysed in 150 µL lysis buffer and then sonicated. The supernatant was removed after centrifugation at 12000 rpm for 15 min and protein concentration was determined by Bio-Rad protein assay kit (Bio-Rad Lab.; CA, U.S.A.). The cells were treated with 40 ng/mL NGF as positive control. A 15 µg of protein was loaded into sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and electrophoresised, then protein was transferred onto the polyvinylidene difluoride (PVDF) membrane. The membrane was blocked and incubated with anti-phospho-p44/42 mitogen-activated protein kinase (MAPK) (Thr202/Tyr204) rabbit polyclonal antibody (Cell Signaling Technology; MA, U.S.A.) for one hour at room temperature. The horseradish peroxidase-conjugated secondary antibody (Beijing ComWin Biotech Co., Ltd., Beijing, China) was incubated with membrane subsequently. The bands were developed with enhanced chemiluminescent reaction (Beijing ComWin Biotechnology) and analyzed by the software-Image J (National Institutes of Health, MD, U.S.A.).
This work was financially supported by International Science and Technology Cooperation Program of China (No. 2014DFG32690), NSFC (81072536 and 81273385). We thank Pharmaceutical Informatics Institute of Zhejiang University for performing NMR spectrometry.
The authors declare no conflict of interest.