2016 Volume 64 Issue 7 Pages 723-732
2016 Volume 64 Issue 7 Pages 723-732
Practical total syntheses of acromelic acids A (1) and B (2), which were scarce natural products isolated from toxic mushroom by Shirahama and Matsumoto, were accomplished in 13 (36% total yield) and 17 steps (6.9% total yield), respectively, from 2,6-dichloropyridine (8). Beginning with regioselective transformation of symmetric 8 by either ortho-lithiation or bromination, nitroalkenes 15 and 16 were provided. Stereoselective construction of the vicinal stereocenters at the C-3, 4 positions of 1 and 2 was performed by a Ni-catalyzed asymmetric conjugate addition of α-ketoesters to the nitroalkenes. Construction of the pyrrolidine ring was accomplished in a single operation via a sequence consisting of reduction of the nitro group, intramolecular condensation with the ketone, and reduction of the resulting ketimine.
In 1983, Shirahama and Matsumoto isolated 110 and 40 µg of acromelic acids A (1) and B (2), respectively, from 16 kg of Clitocybe acromelalga (Japanese name, Dokusasako) and determined their structures by means of extensive NMR analysis1) and total syntheses.2–4) These amino acids exhibit remarkably potent neuro-excitatory activity via activation of ionotropic glutamate receptors in the brain. Compound 1, for example, is almost 10 times more potent than domoic acid (3) and 100 times more potent than kainic acid (4).5–7) Thus, these compounds have the potential to be important biological research tools, because ionotropic glutamate receptors are involved in various neurophysiological processes, including memory and pain transmission. Allodynia induced by 1 is of interest because of its association with neuropathic pain transmission, but poor availability of 1 meant that recent biological investigations had to be performed with only simple synthetic analogues of 18–10) (Fig. 1).

To date, numerous total syntheses of 4 have been reported,11–33) but there are only a few reports of total synthesis of 134–37) and 2.38,39) In general, it is preferable to install polar and unstable heterocycles at a late stage during a total synthesis. Indeed, in the early syntheses, the relatively unstable pyridone ring was constructed from a methylpyridine unit,2–4,34–37) so that the difficulty in handling the compounds could be minimized. However, oxidative transformations with toxic heavy metal reagents had to be performed at the final stage, and these syntheses are unsuitable for providing sufficient amounts for detailed biological studies. In contrast, we planned to use methoxy picolinic acid ester as a pyridone precursor to improve the efficiency of the syntheses, even though the reaction of a pyridine substrate might be challenging, since such heterocyclic compounds tend to affect key catalytic and stereoselective reactions. As a part of our research program on kainoid chemistry,40–43) we herein describe the details of practical and scaleable total syntheses of acromelic acids A (1) and B (2).
The heart of our synthetic strategy is shown in Chart 1, in which substrates with the same oxidation state as the natural products serve as key intermediates. The pyrrolidine ring would be formed from δ-nitro keto compound 5 via reduction of the nitro group and intramolecular reductive amination. Thus, a crucial step of the total synthesis would be stereoselective coupling between nitroalkene 6 and α-ketoester 7, which we expected to achieve by the use of a Ni-catalyzed asymmetric reaction.40) Nitroalkene 6 would be synthesized from the corresponding pyridylaldehyde and nitromethane via Henry reaction and subsequent dehydration. Since 1 and 2 differ only in the substitution pattern on the pyridone ring, divergent synthesis of both regioisomeric pyridylaldehydes from the same compound is another key feature of the total synthesis.

As shown in Chart 2, aldehydes 11 and 14 could be readily prepared in a divergent manner from inexpensive 2,6-dichloropyridine (8). Mono-substitution of 8 with sodium methoxide proceeded smoothly to give 2-chloro-6-methoxypyridine (9) in high yield. For the preparation of aldehyde 11, the regioselective introduction of a formyl group was achieved via directed ortho-lithiation44) followed by reaction with N,N-dimethylformamide (DMF) to give 10. The carbonylation reaction45) of 10 proceeded smoothly in the presence of a catalytic amount of Pd(OAc)2–1,1′-bis(diphenylphosphino)ferrocene (dppf) and methanol under a CO atmosphere to afford the desired aldehyde 11. On the other hand, bromination of 9 with N-bromosuccinimide (NBS) occurred regioselectively at the para-position of the methoxy group to give a 3-bromopyridine derivative.46) The corresponding aldehyde 12 was obtained from the bromide according to Knochel’s protocol,47) by treatment with i-PrMgBr and DMF. Incorporation of an ester group into 12 was also accomplished by a Pd-catalyzed carbonylation reaction to provide n-butyl ester 13. As mentioned later, bulky tert-butyl ester was desirable for selective transformation in the advanced stage. But, direct conversion of 12 to bulky tert-butyl ester 14 in t-BuOH was difficult. Thus, we needed to convert the obtained n-butyl ester to the corresponding tert-butyl ester 14 in a four-step sequence including the protection and deprotection of the formyl group.

Reagents and conditions: (a) NaOMe, MeOH, 60°C, 24 h, quant; (b) t-BuLi, THF, −78°C, 1 h then DMF, −78°C to rt, 1 h; (c) CO (balloon), Pd(OAc)2, dppf, NaOAc, MeOH, toluene, 50°C, 23 h, 97% over 2 steps; (d) NBS, CH3CN, reflux, 24 h, 63%; (e) i-PrMgCl, LiCl, THF, −20°C, 2 h then DMF, −20°C, 30 min; (f) CO (balloon), Pd(dppf)Cl2, NaOAc, n-BuOH, toluene, 100°C, 18 h, 65% over 2 steps; (g) CSA, HC(OMe)3, MeOH, reflux, 24 h, 70%; (h) KOH, H2O, THF, 40°C, 3 h; (i) N,N′-diisopropyl-O-tert-butylisourea, NH4Cl, CH2Cl2, rt, 15 h; (j) 1 M HCl, THF, rt, 1.5 h, 80% over 3 steps.
As shown in Chart 3, the coupling of aldehyde 11 or 14 with nitromethane, followed by dehydration, provided the nitroalkenes 15 and 16 in high yield, respectively. In contrast, condensation reaction of 13 with nitromethane provided the double alkylated product 17. In this nitro-aldol reaction, the generated hydroxyl group was readily captured by the n-butyl ester to give a five-membered lactone 18. Subsequent β-elimination and conjugate addition of nitromethane to 19 afforded 17 predominantly. However, the bulky tert-butyl ester 14 did not undergo such a side reaction. Thus, ester exchange from 13 to 14 was necessary.

Reagents and conditions: (a) CH3NO2, Et3N, rt, 1.5 h for 15, 20 h for 16; (b) MsCl, Et3N, CH2Cl2 0°C, 3 h, quant for 15 over 2 steps, 64% for 16 over 2 steps.
For the synthesis of acromelic acid A (1), we next investigated the key step needed to construct the vicinal stereocenters at the C-3, 4 positions. Firstly, asymmetric conjugate addition of α-ketoester 7a with nitroalkene 15 was examined, since this reaction worked well in our previous 4-(2-methoxyphenyl)-2-carboxy-3-pyrrolidineacetic acid (MFPA) synthesis.40) Although nitroalkene 15 had a Lewis-basic pyridine ring that potentially interferes with the Ni catalyst, the desired reaction proceeded smoothly without siginificant side reactions in the presence of 5 mol% of Ni(OAc)2–diamine catalyst 20 (Table 1, entry 1). In place of 7a, we next examined the reaction of 7b with the same oxidation state at the C4 side chain in 1 and 2. Although the desired reaction proceeded smoothly, the diastereoselectivity was decreased when a methyl ester was employed (entry 2). As shown in Table 1, as the size of the ester group at the γ-position of the nucleophile was increased, higher diastereoselectivity was observed, probably because epimerization at the α-position of the keto group was minimized. Although the excellent results were obtained when bulky t-Bu and diphenylmethyl (Dpm) esters 7c and d43) were used, we selected the Dpm ester for selective deprotection in the advanced stage.
| |||||||
|---|---|---|---|---|---|---|---|
| Entry | R | T (°C) | Yield (%)a) | dr | ee (%) | ||
| 1 | –CH2OPMB | rt | 81 | 25 : 1 | 93 | ||
| (7a) | |||||||
| 2 | –CO2Me | rt | 71 | 2 : 1 | 93 | ||
| (7b) | |||||||
| 3 | –CO2t-Bu | rt | Quant | 10 : 1 | 79 | ||
| (7c) | |||||||
| 4 | –CO2t-Bu | 0 | 75 | 27 : 1 | 94 |
| |
| (7c) | |||||||
| 5 | –CO2CHPh2 | 0 | 93 | 10 : 1 | ndb) | ||
| (7d) | |||||||
| 6 | CO2CHPh2 | −10 | 89 | 25 : 1 | 95 | ||
| (7d) | |||||||
a) Isolated yield. b) Not determined.
This asymmetric conjugate reaction could be carried out on a large scale (10 g scale) without difficulty. In the presence of 5 mol% of Ni-catalyst, the desired reaction between nitroalkene 15 and α-ketoester 7d proceeded smoothly to give the desired coupling product 21 with the correct stereochemistry in high yield with excellent stereoselectivity (dr=>25 : 1, 95% enantiomeric excess (ee) for the major isomer).
With the key intermediate in hand, we next examined the formation of the pyrrolidine ring. Upon treatment of 21 with Raney Ni under 900 psi of hydrogen, reduction of the nitro group, intramolecular condensation with the ketone, and stereoselective reduction of the resulting ketimine 22 could be performed in a single operation, and the pyrrolidine compound 23 was obtained in high yield. The next task was inversion of the stereochemistry at the C-2 position. Since t-BuOK-promoted inversion gave the excellent results in our previous synthesis of MFPA,40–43) the same condition was applied to N-Cbz-protected 23. However, treatment with t-BuOK resulted in marked decomposition of the substrate (vide infra). Therefore, we tried epimerization via formation of an active ester derivative. Thus, conversion of 23 to methyl ester 24 was performed via a two-step sequence consisting of removal of the Dpm group and methyl ester formation. After incorporation of a Cbz-group into 24 followed by deprotection with trifluoroacetic acid (TFA), treatment of 25 with NaOAc in Ac2O underwent complete epimerization to give 27 by way of the formation of a mixed anhydride 26 as an activated ester. Finally, simultaneous removal of the Cbz group and the methyl ether on the pyridine ring, and concomitant hydrolysis of the methyl esters were carried out by treatment with HBr in acetic acid to provide acromelic acid A (1), which gave spectral data (1H-NMR, 13C-NMR, IR and high resolution (HR)-MS) in full agreement with those of the natural product.1–4) Thus, the total synthesis of 1 has been accomplished in 13 steps from 2,6-dichloropyridine (8) in 36% total yield. Furthermore our synthesis was applicable to the gram scale preparation of 1 (Chart 4).

Reagents and conditions: (a) 20 (5 mol %), DME, −10°C, 48 h, 89%, dr>25 : 1, 95% ee; (b) H2 (900 psi), Raney Ni, MeOH, 75°C, 2 h; (c) H2 (balloon), Pd/C, MeOH, rt, 1.5 h; (d) SOCl2, MeOH, rt, 20 h, 68% over 3 steps; (e) CbzCl, Et3N, CH2Cl2, rt, 5 h 89%; (f) TFA, CH2Cl2, rt, 19 h; (g) NaOAc, Ac2O, 110°C, 25 h, 70% over 2 steps; (h) HBr, AcOH, H2O, 100°C, 12 h, quant.
Next, we envisaged that the established route to 1 would also be applicable to acromelic acid B (2) (Chart 5). In the case of 16 as a Michael acceptor, however, the reaction of 7d gave an almost 1 : 1 mixture of the diastereoisomers, which might be attributed to epimerization during the reaction. To address this issue, we examined the reaction of α-ketoester 7a.40,43) The Ni-catalyzed reaction between 16 and 7a produced the desired product 28 quantitatively with excellent stereoselectivity (dr=20 : 1, 91% ee). Subsequent hydrogenation with Raney Ni also proceeded smoothly, but a 1 : 2 mixture of 29a and b were isolated after protection with a Cbz group at the secondary amine. In contrast to 23, epimerization from 29b to a could be performed by simple treatment with t-BuOK in t-BuOH. The difference in the reactivity between 29b and 23 could be rationalized as follows: Deprotonation at the C-4 position of 23 would be possible, whereas such deprotonation did not occur in the case of 29b. Although the exact reason was unclear, steric repulsion between the tert-butyl ester and the pyrrolidine ring might prevent the proton at the C-4 position from taking a perpendicular position with respect to the pyridine ring, so that its acidity could be decreased (Chart 5). After removal of the p-methoxybenzyl (PMB) group, direct conversion of the resulting primary alcohol to the corresponding acid 30 was achieved by Iwabuchi oxidation.48) Finally, treatment with HBr in acetic acid enabled simultaneous cleavage of the remaining protecting groups to furnish acromelic acid B (2). All the spectroscopic data of the synthetic 2 were in good agreement with those of the natural product.1–4) Thus, the total synthesis of 2 has been accomplished in 17 steps from 2,6-dichloropyridine (8) in 6.9% total yield.
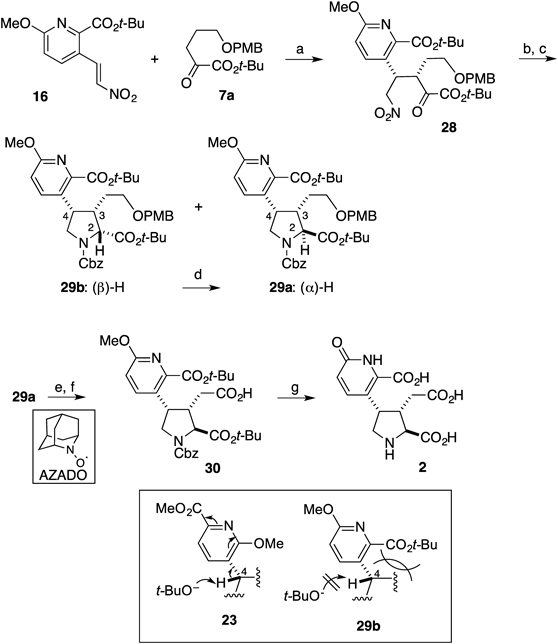
Reagents and conditions: (a) 20 (5 mol%), Et3N, IPA, −10°C, 14 h, quant, dr>20 : 1, 91% ee; (b) H2 (700 psi), Raney Ni, EtOH, 75°C, 1.5 h; (c) CbzCl, Et3N, CH2Cl2, rt, 30 min 26% for 27a over 2 steps, 47% for 27b over 2 steps; (d) t-BuOK, t-BuOH, benzene, rt, 6 h, 62%; (e) DDQ, CH2Cl2, H2O, rt, 1 h, 99%; (f) AZADO, PhI(OAc)2, CH2Cl2, phosphate buffer (pH 7.6), 0°C, 87%; (g) HBr, AcOH, H2O, 100°C, 36 h, 99%.
In conclusion, we have developed practical total syntheses of acromelic acids A and B (1, 2). Our syntheses feature a regioselective synthesis of the methoxypicolinic acid derivatives 11 and 14 from 2,6-dichloropyridine (8), efficient construction of the pyrrolidine rings via a sequence involving Ni-catalyzed asymmetric conjugate addition, intramolecular reductive amination under hydrogenation conditions, and basic epimerization of the C-2 position. Our approach is suitable for the large-scale synthesis to provide sufficient amount of 1 and 2 for detailed biological studies. The biological activities of these compounds in mice and the binding behavior to glutamate receptors are under investigation.
1H-NMR (500 MHz), 13C-NMR (125 MHz) spectra were determined on JEOL ECA-500 instrument. Chemical shifts for 1H-NMR were reported in parts per million downfields from tetramethylsilane (δ) as the internal standard and coupling constants were in hertz (Hz). The following abbreviations are used for spin multiplicity: s=singlet, d=doublet, t=triplet, q=quartet, m=multiplet, br=broad. Chemical shifts for 13C-NMR were reported in ppm relative to the centerline of a triplet at 77.0 ppm for deuteriochloroform. HR-MS were obtained on a BRUKER DALTONICS micrOTOF (electrospray ionization (ESI)). IR spectra were recorded on a SHIMADZU IRPrestige-21. Optical rotations were measured on a JASCO P-1030 Polarimeter at RT using the sodium D line. Analytical TLC was performed on Merck precoated analytical plates, 0.25 mm thick, silica gel 60 F254. Preparative TLC separations were made on 7×20 cm plates prepared with a 0.25 mm layer of Merck silica gel 60 F254. Compounds were eluted from the adsorbent with 10% MeOH in chloroform. Column chromatography separations were performed on KANTO CHEMICAL Silica Gel 60 (spherical) 40–50 µm, Silica Gel 60 (spherical) 63–210 µm or Silica Gel 60 N (spherical, neutral) 63–210 µm. Reagents and solvents were commercial grades and were used as supplied with the following exceptions. 1) Dichloromethane, tetrahydrofuran and toluene: dried over molecular sieves 4A. 2) MeOH and acetonitrile: dried over molecular sieves 3A. All reactions sensitive to oxygen and/or moisture were conducted under an argon atmosphere.
2-Chloro-6-methoxypyridine (9)To a stirred solution of 2,6-dichloropyridine (8) (68.9 g, 466 mmol) in MeOH (500 mL) was added NaOMe (100 g, 1.86 mol) at room temperature. The resulting mixture was stirred at 60°C for 24 h. After cooling, the mixture was quenched with 2 M aqueous HCl, and extracted with CH2Cl2. The organic layer was dried over anhydrous MgSO4, filtered and concentrated under reduced pressure to afford 9 (66.9 g, quant) as a colorless oil.
9: IR (film, cm−1): 1599, 1585, 1560, 1468, 1410, 1302, 1265, 1152, 1024, 876, 789; 1H-NMR (CDCl3, 500 MHz) δ: 7.51 (t, J=7.37 Hz, 1H), 6.90 (d, J=7.37 Hz, 1H), 6.65 (d, J=7.37 Hz, 1H), 3.94 (s, 3H); 13C-NMR (CDCl3, 125 MHz) δ: 163.9, 148.4, 140.5, 116.2, 109.1, 54.0; HR-MS (ESI-time-of-flight (TOF)): Calcd for C6H7ClNO [(M+H)+] 144.0211. Found 144.0211.
Methyl 5-Formyl-6-methoxypicolinate (11)To a stirred solution of 9 (20.8 g, 145 mmol) in tetrahydrofuran (THF) (400 mL) was added t-BuLi (ca. 1.6 M solution in n-heptane, 100 mL, 160 mmol) at −78°C. After 1 h, DMF (33.8 mL, 435 mmol) was added at –78°C. After 30 min, the resulting mixture was stirred at room temperature for 30 min. Then the reaction mixture was quenched with 2 M aqueous HCl, and extracted with EtOAc. The organic layer was dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The crude material including 10 was applied to the following reaction without further purification.
To a stirred suspension of the crude material including 10 and NaOAc (17.8 g, 218 mmol) in the 2 : 1 mixture of MeOH and toluene (total 450 mL) were added Pd(OAc)2 (651 mg, 2.90 mmol) and DPPF (2.41 g, 4.35 mmol) at room temperature. The resulting mixture was stirred under ordinary CO pressure (balloon) at 50°C for 23 h. Then the reaction mixture was quenched with 1 M aqueous HCl, and extracted with EtOAc. The organic layer was dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The residue was purified by column chromatography (SiO2, n-hexane–EtOAc=2 : 1) to afford 11 (27.5 g, 97%, 2 steps) as a colorless solid.
11: mp: 87–88°C; IR (film, cm−1): 1726, 1694, 1591, 1456, 1381, 1254, 1134; 1H-NMR (CDCl3, 500 MHz) δ: 10.4 (s, 1H), 8.22 (d, J=7.94 Hz, 1H), 7.79 (d, J=7.94 Hz, 1H), 4.17 (s, 3H), 4.00 (s, 3H); 13C-NMR (CDCl3, 125 MHz) δ: 188.6, 164.7, 164.0, 149.8, 138.6, 121.2, 118.5, 54.3, 53.0; HR-MS (ESI-TOF): Calcd for C9H9NO4Na [(M+Na)+] 218.0424. Found 218.0428.
3-Bromo-2-chloro-6-methoxypyridine (31)To a stirred solution of 9 (7.00 g, 48.8 mmol) in CH3CN (25 mL) was added NBS (13.0 g, 73.1 mmol) at room temperature. The resulting mixture was refluxed for 24 h. Then the reaction was quenched with saturated aqueous Na2S2O3 and extracted with EtOAc. The organic layer was dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The residue was purified by column chromatography (SiO2, n-hexane–EtOAc=98 : 2) to afford methoxypyridine 31 (6.83 g, 63%) as a colorless solid.
31: mp 64–65°C; IR (film, cm−1): 1584, 1551, 1466, 1408, 1344, 1306, 1256, 1155, 1121, 1022, 1009; 1H-NMR (CDCl3, 500 MHz) δ: 7.72 (d, J=8.50 Hz, 1H), 6.58 (d, J=8.50 Hz, 1H), 3.92 (s, 3H); 13C-NMR (CDCl3, 125 MHz) δ: 162.4, 147.3, 143.8, 110.9, 110.1, 54.3; HR-MS (ESI-TOF): Calcd for C6H6BrClNO [(M+H)+] 221.9316. Found 221.9314.
Butyl 3-Formyl-6-methoxypicolinate (13)To a stirred solution of 31 (6.83 g, 30.7 mmol) in THF (120 mL) was added i-PrMgCl·LiCl (ca. 1.0 M solution in THF, 32.2 mL, 32.2 mmol) at −20°C. After 2 h, DMF (7.2 mL, 92.1 mmol) was added dropwise at −20°C. The resulting mixture was stirred at room temperature for 30 min. The reaction mixture was quenched with saturated aqueous NH4Cl and extracted with EtOAc. The organic layer was dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The crude material including 12 was applied to the following reaction without further purification.
To a suspension of the crude material including 12 and NaOAc (3.78 g, 46.1 mmol) in the 1 : 1 mixture of n-BuOH and toluene (total 120 mL) was added Pd(dppf)Cl2 (1.12 g, 1.54 mmol)at room temperature. The resulting mixture was stirred under ordinary CO pressure (balloon) at 100°C for 18 h. The reaction was quenched with saturated aqueous NH4Cl, filtered through a pad of Celite and extracted with EtOAc. The organic layer was dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The residue was purified by column chromatography (SiO2, n-hexane–EtOAc=2 : 1) to afford 13 (4.75 g, 65% 2 steps) as a yellow oil.
13: IR (film, cm−1): 2963, 2876, 1721, 1692, 1595, 1481, 1337, 1277, 1261, 1219, 1138, 1072, 1022; 1H-NMR (CDCl3, 500 MHz) δ: 10.39 (s, 1H), 8.18 (d, J=8.50 Hz, 1H), 6.94 (d, J=8.50 Hz, 1H), 4.44 (t, J=6.80 Hz, 2H), 4.06 (s, 3H), 1.83–1.76 (m, 2H), 1.54–1.45 (m, 2H), 0.99 (t, J=7.37 Hz, 3H); 13C-NMR (CDCl3, 125 MHz) δ: 189.1, 166.1, 165.2, 150.7, 138.6, 125.9, 114.1, 66.3, 54.5, 30.5, 19.2, 13.7; HR-MS (ESI-TOF): Calcd for C12H15NO4Na [(M+Na)+] 260.0893. Found 260.0891.
Methyl 3-(Dimethoxymethyl)-6-methoxypicolinate (32)To a stirred solution of 13 (4.75 g, 20.0 mmol) in MeOH (100 mL) were added CH(OMe)3 (11 mL, 100 mmol) and 10-camphorsulfonic acid (CSA) (465 mg, 2.00 mmol) at room temperature. The resulting mixture was refluxed for 24 h. Then the reaction mixture was concentrated under reduced pressure. The residue was diluted with saturated aqueous NaHCO3 and extracted with EtOAc. The organic layer was dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The residue was purified by column chromatography (SiO2, n-hexane–EtOAc=9 : 1) to afford methoxypicolinate 32 (3.40 g, 70%) as a yellow oil.
32: IR (attenuated total reflectance (ATR), cm−1) 2951, 2832, 1730, 1597, 1479, 1321, 1250, 1217, 1109, 1070, 1051, 1026, 974, 831; 1H-NMR (CDCl3, 500 MHz) δ: 7.90 (d, J=8.50 Hz, 1H), 6.86 (d, J=8.50 Hz, 1H), 5.85 (s, 1H), 3.97 (s, 3H), 3.96 (s, 3H), 3.34 (s, 6H); 13C-NMR (CDCl3, 125 MHz) δ: 166.9, 163.3, 145.5, 138.3, 127.1, 113.0, 100.0, 53.8, 53.6, 52.6; HR-MS (ESI-TOF): Calcd for C11H15NO5Na [(M+Na)+] 264.0842. Found 264.0842.
tert-Butyl 3-Formyl-6-methoxypicolinate (14)To a stirred solution of 32 (500 mg, 2.07 mmol) in THF (4 mL) was added 1 M aqueous KOH (4.15 mL, 4.15 mmol). The resulting mixture was stirred at 40°C for 3 h. Then the mixture was concentrated under reduced pressure. The crude material including carboxylic acid (33) was applied to the following reaction without further purification.
To a stirred suspension of the crude material including 33 in CH2Cl2 (5 mL) were added NH4Cl (277 mg, 5.18 mmol) and N,N′-diisopropyl-O-tert-butylisourea (1.63 mL, 7.25 mmol) at room temperature. The resulting mixture was stirred at room temperature for 15 h. Then the reaction mixture was filtered and concentrated under reduced pressure. The crude material including tert-butyl ester 34 was applied to the following reaction without further purification.
To a stirred solution of the crude material including 34 in THF (2 mL) was added 1 M aqueous HCl (2 mL) at room temperature. The resulting mixture was stirred at the same temperature for 1.5 h. Then the reaction mixture was diluted with water and extracted with EtOAc. The organic layer was dried over anhydrous MgSO4, filtered and concentrated under reduced pressure. The residue was purified by column chromatography (SiO2, n-hexane–EtOAc=4 : 1) to afford 14 (391 mg, 80%, 3 steps) as a colorless oil.
14: IR (film, cm−1): 2982, 1736, 1595, 1481, 1335, 1279, 1223, 1167, 1138, 1072, 1020, 845; 1H-NMR (CDCl3, 500 MHz) δ: 10.35 (s, 1H), 8.14 (d, J=8.50 Hz, 1H), 6.91 (d, J=8.50 Hz, 1H), 4.06 (s, 3H), 1.65 (s, 9H); 13C-NMR (CDCl3, 125 MHz) δ: 189.1, 166.1, 164.2, 152.3, 138.5, 125.1, 113.6, 83.9, 54.4, 28.1; HR-MS (ESI-TOF): Calcd for C12H15NO4Na [(M+Na)+] 260.0893. Found 260.0881.
(E)-Methyl 6-Methoxy-5-(2-nitrovinyl)picolinate (15)To a stirred solution of 11 (616 mg, 3.16 mmol) in CH3NO2 (10 mL) was added Et3N (319 mg, 3.16 mmol) at room temperature. The resulting mixture was stirring at the same temperature for 1.5 h. Then the reaction mixture was concentrated under reduced pressure. The crude material including nitroalcohol 35 was applied to the following reaction without further purification.
To a stirred solution of the crude material including 35 in CH2Cl2 (10 mL) were added Et3N (319 mg, 3.16 mmol) and MsCl (996 mg, 4.74 mmol) at 0°C. The resulting mixture was stirred at the same temperature for 3 h. Then the reaction mixture was quenched with saturated aqueous NaHCO3, and extracted with CH2Cl2. The organic layer was dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The residue was purified by column chromatography (SiO2, CHCl3–EtOAc=9 : 1) to afford 15 (750 mg, quant) as a pale yellow solid.
15: IR (film, cm−1): 1726, 1632, 1516, 1342, 1271; 1H-NMR (CDCl3, 500 MHz) δ: 8.02 (d, J=13.8 Hz, 1H), 7.98 (d, J=13.8 Hz, 1H), 7.87 (d, J=7.45 Hz, 1H), 7.79 (d, J=7.45 Hz, 1H), 4.19 (s, 3H), 3.99 (s, 3H); 13C-NMR (CDCl3, 125 MHz) δ: 164.8, 161.9, 147.3, 141.9, 140.9, 132.7, 118.8, 117.6, 54.6, 53.0; HR-MS (ESI-TOF): Calcd for C10H10N2O5Na [(M+Na)+] 261.0482. Found 261.0494.
(E)-tert-Butyl 6-Methoxy-3-(2-nitrovinyl)picolinate (16)To a stirred solution of 14 (1.48 g, 6.22 mmol) in CH3NO2 (30 mL) was added Et3N (1.72 mL, 12.4 mmol) at room temperature. The reaction mixture was stirred at the same temperature for 20 h. Then the reaction mixture was concentrated under reduced pressure. The crude material including nitroalcohol 36 was applied to the following reaction without further purification.
To a stirred solution of the crude material including 36 in CH2Cl2 (30 mL) were added Et3N (1.29 mL, 9.33 mmol) and MsCl (963 µL, 12.4 mmol) at 0°C. The resulting mixture was stirred at the same temperature for 3 h. Then the reaction mixture was quenched with saturated aqueous NH4Cl and extracted with CH2Cl2. The organic layer was dried over anhydrous MgSO4, filtered and concentrated under reduced pressure. The residue was purified by column chromatography (SiO2, CHCl3–EtOAc=9 : 1) to afford 16 (1.12 g, 64%, 2 steps) as a pale yellow solid.
16: IR (film, cm−1): 3115, 2978, 2943, 1734, 1630, 1595, 1560, 1508, 1481, 1425, 1395, 1370, 1331, 1275, 1260, 1171, 1144, 1074, 1020, 966, 957, 833, 596; 1H-NMR (CDCl3, 500 MHz) δ: 8.59 (d, J=13.6 Hz, 1H), 7.76 (d, J=8.50 Hz, 1H), 7.42 (d, J=13.6 Hz, 1H), 6.91 (d, J=8.50 Hz, 1H), 4.04 (s, 3H), 1.66 (s, 9H); 13C-NMR (CDCl3, 125 MHz) δ: 165.2, 164.1, 149.1, 137.7, 137.4, 135.5, 118.9, 114.2, 84.0, 54.2, 28.1; HR-MS (ESI-TOF): Calcd for C13H16N2O5Na [(M+Na)+] 303.0954. Found 303.0958.
(S)-5-Benzhydryl 1-tert-Butyl 3-((S)-1-(2-Methoxy-6-(methoxycarbonyl)pyridin-3-yl)-2-nitroethyl)-2-oxopentanedioate (21)To a stirred solution of 15 (5.40 g, 22.7 mmol) in 1,2-dimethoxyethane (DME) (230 mL) were added α-ketoester 7d43) (8.77 g, 23.8 mmol) and Ni–diamine complex 20 (576 mg, 1.14 mmol) at −10°C. The resulting mixture was stirred at the same temperature for 48 h. The mixture was diluted with n-hexane, filtered through a pad of SiO2 and concentrated under reduced pressure. The residue was purified by column chromatography (SiO2, n-hexane–EtOAc=2 : 1) to afford 21 (12.1 g, 88%, dr>25 : 1) as a colorless oil. The ee value was determined by chiral HPLC analysis.
21: HPLC: DAICEL CHIRALCEL OD-H, n-hexane–isopropylalcohol (IPA)=9 : 1, 1.0 mL/min, 254 nm, τmajor 25.1 min, τminor 29.2 min; [α]D25 +4.4 (c=1.0, CHCl3, 95% ee); IR (film, cm−1): 1724, 1584, 1555, 1460, 1371, 1267, 1167, 1022, 982, 760, 702; 1H-NMR (CDCl3, 500 MHz) δ: 7.61 (d, J=7.37 Hz, 1H), 7.45 (d, J=7.37 Hz, 1H), 7.20–7.38 (m, 10H), 6.82 (s, 1H), 4.92 (dd, J=13.0, 9.07 Hz, 1H), 4.78 (dd, J=13.0, 4.53 Hz, 1H), 4.31–4.25 (m, 1H), 4.02 (s, 3H), 4.01–3.95 (m, 1H), 3.93 (s, 3H), 2.95 (dd, J=17.0, 9.64 Hz, 1H), 2.73 (dd, J=17.0, 4.53 Hz, 1H), 1.43 (s, 9H); 13C-NMR (CDCl3, 125 MHz) δ: 193.8, 170.0, 165.0, 161.0, 159.3, 145.3, 140.0, 139.4, 139.3, 128.5, 128.4, 128.1, 128.0, 127.2, 126.9, 122.5, 118.7, 84.4, 77.9, 75.1, 53.8, 52.6, 42.9, 41.6, 35.0, 27.5; HR-MS (ESI-TOF): Calcd for C32H35N2O10 [(M+H)+] 607.2286. Found 607.2312.
Methyl 5-((3S,4S,5R)-5-(tert-Butoxycarbonyl)-4-(2-methoxy-2-oxoethyl)pyrrolidin-3-yl)-6-methoxy Picolinate (24)To a stirred solution of 21 (2.33 g, 3.84 mmol) in MeOH (80 mL) was added Raney nickel (6.0 g, purchased from Aldrich, washed with water and MeOH) at room temperature. The resulting mixture was stirred under hydrogen pressure (900 psi) at 75°C for 2 h. Then, the reaction mixture was filtered through a pad of Celite and concentrated under reduced pressure. The crude material including 23 was applied to the following reaction without further purification.
To a stirred solution of the crude material including 23 in MeOH (40 mL) was added 10% Pd/C (1.0 g) at room temperature. The resulting mixture was stirred under ordinary hydrogen pressure (balloon) at the same temperature for 1.5 h. The reaction mixture was filtered through a pad of Celite and concentrated under reduced pressure. The crude material including carboxylic acid 37 was applied to the following reaction without further purification.
To a stirred solution of the crude material including 37 in MeOH (40 mL) was added SOCl2 (0.28 mL, 3.8 mmol) at 0°C. The resulting mixture was stirred at room temperature for 20 h. Then the reaction mixture was diluted with toluene and concentrated under reduced pressure. The residue was purified by column chromatography (SiO2, CHCl3–MeOH=96 : 4) to afford 24 (1.07 g, 68%, 3 steps) as a yellow oil.
24: [α]D25 −89.5 (c=0.97, CHCl3); IR (film, cm−1): 1740, 1462, 1263, 1211, 1159; 1H-NMR (CDCl3, 500 MHz) δ: 7.68 (br s, 2H), 4.69–4.63 (m, 1H), 4.15–4.08 (m, 2H), 4.07 (s, 3H), 3.96 (s, 3H), 3.89–3.82 (m, 1H), 3.74–3.66 (m, 1H), 3.34 (s, 3H), 2.36 (dd, J=8.00, 17.8 Hz, 1H), 2.18 (dd, J=5.75, 17.8 Hz, 1H), 1.46 (s, 9H); 13C-NMR (CDCl3, 125 MHz) δ: 170.8, 165.8, 165.3, 161.6, 144.7, 137.3, 122.3, 118.4, 85.6, 63.3, 54.1, 52.7, 51.7, 46.1, 40.6, 38.9, 30.5, 27.9; HR-MS (ESI-TOF): Calcd for C20H29N2O7 [(M+H)+] 409.1969. Found 409.1968.
(2R,3S,4S)-1-Benzyl 2-tert-Butyl 3-(2-Methoxy-2-oxoethyl)-4-(2-methoxy-6-(methoxycarbonyl)pyridin-3-yl)pyrrolidine-1,2-dicarboxylate (25)To a stirred solution of 24 (540 mg, 1.22 mmol) in CH2Cl2 (5 mL) were added Et3N (0.51 mL, 3.66 mmol) and CbzCl (0.26 mL, 1.83 mmol) at 0°C. The resulting mixture was stirred at room temperature for 5 h. Then the reaction was quenched with saturated aqueous NH4Cl and extracted with CH2Cl2. The organic layer was dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The residue was purified by column chromatography (SiO2, n-hexane–EtOAc=2 : 1) to afford 25 (589 mg, 89%) as a colorless amorphous solid. 25 exists as a mixture of rotamers in CDCl3 at 25°C.
25: [α]D25 −63.2 (c=0.90, CHCl3); IR (film, cm−1): 1742, 1721, 1709, 1460, 1412, 1368, 1287, 1265, 1213, 1155; 1H-NMR (CDCl3, 500 MHz) δ: 7.86 (d, J=7.37 Hz, 0.55H), 7.79 (d, J=7.37 Hz, 0.45H), 7.70–7.66 (m, 1H), 7.40–7.25 (m, 5H), 5.26–5.10 (m, 2H), 4.55 (d, J=8.50 Hz, 0.45H), 4.51 (d, J=8.50 Hz, 0.55H), 4.03 (s, 3H), 3.97–3.86 (m, 3H), 3.95 (s, 3H), 3.55 (s, 1.65H), 3.52 (s, 1.35H), 3.59–3.49 (m, 1H), 2.35 (dd, J=18.0, 6.24 Hz, 0.55H), 2.24 (dd, J=17.6, 6.80 Hz, 0.45H), 2.18 (dd, J=17.6, 8.50 Hz, 0.45H), 2.09 (dd, J=18.0, 8.50 Hz, 0.55H), 1.37 (s, 4H), 1.21 (s, 5H); 13C-NMR (CDCl3, 125 MHz) δ: 117.6, 171.5, 169.1, 168.9, 165.4, 161.4, 154.5, 154.4, 143.7, 143.6, 138.5, 138.1, 136.3, 136.1, 128.4, 128.3, 127.9, 127.8, 125.8, 125.5, 118.5, 118.4, 82.1, 82.0, 67.2, 67.1, 62.3, 61.5, 53.7, 52.5, 51.4, 50.5, 49.5, 40.5, 39.2, 39.0, 37.8, 31.2, 31.1, 27.7, 27.5; HR-MS (ESI-TOF): Calcd for C28H35N2O9 [(M+H)+] 543.2337. Found 543.2350.
(2S,3S,4S)-1-((Benzyloxy)carbonyl)-3-(2-methoxy-2-oxoethyl)-4-(2-methoxy-6-(methoxycarbonyl)pyridin-3-yl)pyrrolidine-2-carboxylic Acid (27)To a stirred solution of 25 (589 mg, 1.09 mmol) in CH2Cl2 (7.5 mL) was added TFA (2.5 mL) at room temperature. The resulting mixture was stirring at the same temperature for 19 h. Then the reaction mixture was concentrated under reduced pressure. The crude material including carboxylic acid 38 was applied to the following reaction without further purification.
To a suspension of crude material including 38 in Ac2O (5.5 mL) was added NaOAc (894 mg, 10.9 mmol) at room temperature. The resulting mixture was stirred at 110°C for 25 h. Then the mixture was concentrated under reduced pressure and the residue was diluted with water. After being stirred for 1 h, the mixture was extracted with CHCl3. The organic layer was dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The residue was purified by column chromatography (SiO2, CHCl3–MeOH=98 : 2) to afford 27 (370 mg, 70% 2 steps) as a colorless amorphous solid.
27: [α]D25 −55.3 (c=0.87, CHCl3); IR (film, cm−1): 1738, 1591, 1462, 1433, 1362, 1267, 1207, 1171, 1132; 1H-NMR (CDCl3, 500 MHz) δ: 10.08 (br s, 1H), 7.68 (d, J=7.37 Hz, 0.45H), 7.64 (d, J=7.37 Hz, 0.55H), 7.45–7.25 (m, 6H), 5.28–5.13 (m, 2H), 4.26 (d, J=5.10 Hz, 0.55H), 4.22 (d, J=5.10 Hz, 0.45H), 3.80–4.05 (m, 3H), 3.99 (s, 3H), 3.96 (s, 3H), 3.59 (s, 1.35H), 3.58 (s, 1.65H), 3.44–3.36 (m, 1H), 2.35–2.20 (m, 1H), 2.05–1.95 (m, 1H); 13C-NMR (CDCl3, 125 MHz) δ: 176.0, 175.1, 171.6, 171.5, 165.4, 161.4, 155.1, 154.3, 144.1, 136.6, 135.9, 128.5, 128.4, 128.2, 128.0, 127.7, 125.1, 125.0, 118.8, 118.6, 67.7, 67.6, 63.5, 63.0, 53.9, 52.6, 51.8, 49.0, 48.9, 42.8, 41.6, 39.2, 38.4, 33.1; HR-MS (ESI-TOF): Calcd for C24H25N2O9 [(M−H)–] 485.1555. Found 485.1554.
Acromelic Acid A (1)To a stirred solution of 27 (271 mg, 557 µmol) in H2O (1.5 mL) was added 5 M HBr in AcOH (6.0 mL) at room temperature. The resulting mixture was stirred at 100°C for 12 h. Then the reaction mixture was concentrated under reduced pressure. Purification of 1 was carried out according to the reported procedure.4) The residue was charged onto a column containing Dowex-50 WX8 hydrogen form (200–400 mesh). After elution with H2O (25 mL) and 3% aqueous NH3 (25 mL), the collected fractions were concentrated under reduced pressure. The resulting ammonium was charged onto a column containing Amberlite IRC-50 hydrogen form. After elution with H2O, the collected fractions were concentrated under reduced pressure to give free amino acid 1 (172 mg, quant) as a colorless solid.
1: mp >310°C (decomp.); [α]D25 +30.0 (c=1.11, H2O) [lit. [α]D +27.8 (c=0.35, H2O)]4); IR (film, cm−1): 3422, 1618, 1381, 787; 1H-NMR (D2O, 500 MHz) δ: 7.52 (d, J=7.37 Hz, 1H), 6.94 (d, J=7.37 Hz, 1H), 4.12 (d, J=7.37 Hz, 1H), 3.84–3.68 (m, 3H), 3.20–3.12 (m, 1H), 2.61 (dd, J=16.7, 5.10 Hz, 1H), 2.15 (dd, J=16.7, 10.2 Hz, 1H); 13C-NMR (D2O, 125 MHz) δ: 176.7, 173.6, 166.3, 163.1, 142.7, 139.5, 129.8, 108.9, 65.8, 47.5, 42.5, 42.4, 35.7; HR-MS (ESI-TOF): Calcd for C13H13N2O7 [(M−H)−] 309.0717. Found 309.0715.
tert-Butyl 3-((2S,3S)-5-(tert-Butoxy)-3-(2-((4-methoxybenzyl)oxy)ethyl)-1-nitro-4,5-dioxopentan-2-yl)-6-methoxypicolinate (28)To a stirred solution of 16 (400 mg, 1.43 mmol) in IPA (0.4 mL) were added Ni–diamine complex 20 (36.2 mg, 71.4 µmol), α-ketoester 7a40,43) (880 mg, 2.85 mmol) in IPA (1.1 mL) and Et3N (49 mL, 357 mmol) at −10°C. The resulting mixture was stirred at the same temperature for 14 h. Then the solvent was removed under reduced pressure. The residue was purified by column chromatography (SiO2, n-hexane–EtOAc=7 : 3) to afford 28 (887 mg, quant.) as a colorless oil. The ee value was determined by chiral HPLC analysis.
28: HPLC (DAICEL CHIRALPAK AD-H, n-hexane–IPA=19 : 1, 1.0 mL/min, 254 nm, τminor 14.1 min, tmajor 15.8 min); [α]D25 −33.0 (c=1.01, CHCl3, 91% ee); IR (film, cm−1): 2980, 2938, 2868, 1719, 1601, 1555, 1512, 1481, 1370, 1329, 1283, 1250, 1171, 1148, 1098, 1030, 847, 826; 1H-NMR (CDCl3, 500 MHz) δ: 7.57 (d, J=8.50 Hz, 1H), 7.17 (d, J=8.50 Hz, 2H), 6.85 (d, J=8.50 Hz, 2H), 6.74 (d, J=8.50 Hz, 1H), 4.87 (dd, J=13.0, 5.10 Hz, 1H), 4.81 (dd, J=13.0, 6.80 Hz, 1H), 4.47–4.38 (m, 1H), 4.30 (d, J=11.9 Hz, 1H), 4.26 (d, J=11.9 Hz, 1H), 4.09 (dt, J=9.6, 4.0 Hz, 1H), 3.92 (s, 3H), 3.79 (s, 3H), 3.49–3.41 (m, 2H), 2.18–2.00 (m, 2H), 1.64 (s, 9H), 1.36 (s, 9H); 13C-NMR (CDCl3, 125 MHz) δ: 195.0, 165.3, 162.4, 159.8, 159.2, 147.3, 139.2, 129.7, 129.3, 125.3, 113.7, 113.0, 83.7, 82.7, 77.6, 72.3, 67.3, 55.3, 53.5, 46.0, 39.9, 31.1, 28.1, 27.6; HR-MS (ESI-TOF): Calcd for C30H40N2O10Na [(M+Na)+] 611.2575. Found 611.2570.
1-Benzyl 2-(tert-Butyl) (2S,3S,4S)-4-(2-(tert-Butoxycarbonyl)-6-methoxypyridin-3-yl)-3-(2-((4-methoxybenzyl)oxy)ethyl)pyrrolidine-1,2-dicarboxylate (29a) and 1-Benzyl 2-(tert-Butyl) (3S,4S)-4-(2-(tert-Butoxycarbonyl)-6-methoxypyridin-3-yl)-3-(2-((4-methoxybenzyl)oxy)ethyl)pyrrolidine-1,2-dicarboxylate (29b)To a stirred solution of 28 (7.77 g, 13.2 mmol) in MeOH (65 mL) was added Raney nickel (23 g, purchased from Aldrich, washed with water and MeOH) at room temperature. The resulting mixture was stirred under hydrogen pressure (700 psi) at room temperature for 1.5 h. The mixture was filtered through a pad of Celite and concentrated under reduced pressure. The crude materials including pyrrolidines 39a and b were applied to the following reaction without further purification.
To a solution of the crude materials including 39a and b in CH2Cl2 (70 mL) were added Et3N (3.66 mL, 26.4 mmol) and CbzCl (2.81 mL, 19.8 mmol) at 0°C. The resulting mixture was stirred at the same temperature for 30 min. Then the reaction mixture was quenched with saturated aqueous NaHCO3 and extracted with CH2Cl2. The organic layer was dried over anhydrous MgSO4, filtered and concentrated under reduced pressure. The residue was purified by column chromatography (SiO2, n-hexane–EtOAc=3 : 1) to afford 29a (2.30 g, 26% 2steps) as a colorless oil and 29b (4.19 g, 47% 2 steps) as a colorless oil. These compounds exist as a mixture of rotamers in CDCl3 at 25°C.
29a: [α]D20 −17.2 (c=1.03, CHCl3); IR (film, cm−1): 2978, 2938, 2870, 1736, 1709, 1599, 1512, 1481, 1414, 1368, 1356, 1331, 1281, 1248, 1157, 1032, 824, 698; 1H-NMR (CDCl3, 500 MHz) δ: 7.44–7.28 (m, 6H), 7.21–7.13 (m, 2H), 6.86–6.70 (m, 3H), 5.22–5.07 (m, 2H), 4.36–4.33 (m, 2H), 4.26–4.21 (m, 1H), 4.11–4.04 (m, 1H), 3.96–3.85 (m, 4H), 3.80–3.62 (m, 4H), 3.48–3.19 (m, 2H), 2.80–2.70 (m, 1H), 1.70–1.33 (m, 20H); 13C-NMR (CDCl3, 125 MHz) δ: 171.2, 165.9, 162.0, 159.1, 154.8, 154.5, 148.2, 148.1, 138.5, 136.6, 136.4, 130.3, 129.2, 129.1, 128.5, 128.4, 128.0, 127.9, 127.8, 127.7, 124.9, 113.7, 112.4, 82.5, 82.4, 81.7, 81.6, 72.7, 68.3, 68.0, 67.1, 64.8, 64.4, 55.2, 53.5, 50.0, 45.0, 43.6, 40.4, 39.5, 29.1, 29.0, 28.1, 28.0, 27.8; HR-MS (ESI-TOF) Calcd for C38H48N2O9Na [(M+Na)+] 699.3252. Found 699.3263.
29b: [α]D20 −31.0 (c=1.18, CHCl3); IR (film, cm−1): 3002, 2977, 2941, 2904, 1740, 1721, 1709, 1698, 1601, 1513, 1480, 1412, 1368, 1329, 1281, 1248, 1169, 1144, 1032, 847, 824, 755, 698; 1H-NMR (CDCl3, 500 MHz) δ: 7.89 (t, J=8.50 Hz, 1H), 7.40–7.27 (m, 5H), 7.21–7.08 (m, 2H), 6.85–6.72 (m, 3H), 5.22–5.08 (m, 2H), 4.49 (d, J=9.1 Hz, 0.45H), 4.44 (d, J=9.1 Hz, 0.55H), 4.36–4.20 (m, 2H), 4.14–3.89 (m, 2H), 3.93 (s, 3H), 3.83–3.70 (m, 1H), 3.78 (s, 3H), 3.40–3.00 (m, 3H), 1.66–1.54 (m, 11H), 1.44 (s, 4H), 1.29 (s, 5H); 13C-NMR (CDCl3, 125 MHz) δ: 170.5, 170.4, 166.2, 162.0, 159.1, 154.8, 154.4, 148.1, 147.9, 140.2, 140.1, 137.9, 136.5, 136.3, 130.5, 129.2, 128.9, 128.5, 128.4, 128.0, 127.9, 126.4, 126.1, 113.7, 113.6, 112.6, 112.5, 82.6, 82.5, 82.2, 82.1, 72.5, 72.4, 68.3, 68.2, 67.2, 67.1, 63.4, 62.9, 55.2, 53.5, 52.4, 51.8, 41.9, 40.9, 40.8, 39.9, 28.2, 28.0, 27.8, 27.7, 27.6; HR-MS (ESI-TOF): Calcd for C38H48N2O9Na [(M+Na)+] 699.3252. Found 699.3246.
Epimerization of 29b to aTo a stirred solution of 29b (60 mg, 0.884 µmol) in the 9 : 1 mixture of t-BuOH and benzene (total 2.0 mL) was added t-BuOK (14.9 mg, 0.133 µmol) at 0°C. The resulting mixture was stirred at room temperature for 6 h. Then the reaction mixture was quenched with saturated aqueous NH4Cl and extracted with EtOAc. The organic layer was dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The residue was purified by column chromatography (SiO2, n-hexane–EtOAc=3 : 1) to afford 29a (37 mg, 62%) as a colorless oil.
1-Benzyl 2-(tert-Butyl) (2S,3S,4S)-4-(2-(tert-Butoxycarbonyl)-6-methoxypyridin-3-yl)-3-(2-hydroxyethyl)pyrrolidine-1,2-dicarboxylate (40)To a stirred solution of 29a (100 mg, 147 mmol) in the 20 : 1 mixture of CH2Cl2 and H2O (0.735 mL) was added 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ) (50 mg, 0.221 mmol) at room temperature. The resulting mixture was stirred at the same temperature for 1 h. Then the reaction mixture was quenched with saturated aqueous NaHCO3 and extracted with CH2Cl2. The organic layer was dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The residue was purified by column chromatography (SiO2, n-hexane–EtOAc=1 : 1 and n-hexane–Et2O=1 : 2) to afford alcohol 40 (89.9 mg, 99%) as a colorless oil. This compound exists as a mixture of rotamers in CDCl3 at 25°C.
40: [α]D25 −34.0 (c=1.45, CHCl3); IR (film, cm−1): 2978, 2936, 1740, 1719, 1701, 1690, 1655, 1597, 1560, 1481, 1458, 1420, 1368, 1331, 1283, 1157, 1028, 847, 698; 1H-NMR (CDCl3, 500 MHz) δ: 7.43–7.28 (m, 6H), 6.77 (d, J=8.50 Hz, 0.55H), 6.74 (d, J=8.50 Hz, 0.45H), 5.21–5.11 (m, 2H), 4.18 (d, J=5.10 Hz, 0.45H), 4.16 (d, J=5.10 Hz, 0.55H), 4.09–4.01 (m, 1H), 3.93 (s, 3H), 3.92–3.86 (m, 1H), 3.80–3.67 (m, 1H), 3.64–3.54 (m, 2H), 2.81–2.72 (m, 1H), 1.70–1.35 (m, 2H), 1.59 (s, 9H), 1.49 (s, 4H), 1.39 (s, 5H); 13C-NMR (CDCl3, 125 MHz) δ: 171.5, 171.4, 166.2, 162.1, 154.7, 154.4, 148.1, 148.0, 138.3, 136.5, 136.3, 128.5, 128.4, 128.0, 127.9, 125.5, 125.4, 112.7, 82.8, 82.7, 82.0, 67.3, 67.2, 64.8, 64.4, 60.9, 60.8, 53.5, 50.6, 44.9, 43.8, 40.5, 39.6, 32.1, 28.1, 27.9, 27.8; HR-MS (ESI-TOF) Calcd for C30H41N2O8 [(M+H)+] 557.2857. Found 557.2858.
2-((2R,3S,4S)-1-((Benzyloxy)carbonyl)-2-(tert-butoxycarbonyl)-4-(2-(tert-butoxycarbonyl)-6-methoxypyridin-3-yl)pyrrolidin-3-yl)acetic Acid (30)To a stirred solution of 40 (250 mg, 449 mmol) in the 1 : 1 mixture of CH2Cl2–phosphate buffer (pH 7.6) (total 2.7 mL) were added AZADO (13.7 mg, 89.8 mmol) and PhI(OAc)2 (434 mg, 1.34 mmol) at 0°C. The resulting mixture was stirred at the same temperature for 8 h. Then the mixture was added to saturated aqueous Na2S2O3 at 0°C. After 1 h, the resulting mixture was extracted with EtOAc. The organic layer was dried over anhydrous MgSO4, filtered and concentrated under reduced pressure. The residue was purified by column chromatography (SiO2, n-hexane–EtOAc=1 : 1) to afford 30 (222 mg, 87%) as a colorless amorphous solid. This compound exists as a mixture of rotamers in CDCl3 at 25°C.
30: [α]D25 −39.6 (c=1.05, CHCl3); IR (film, cm−1): 2980, 2941, 2906, 1710, 1599, 1560, 1481, 1413, 1367, 1332, 1282, 1253, 1228, 1161, 1093, 1030, 844, 736, 698; 1H-NMR (CDCl3, 500 MHz) δ: 7.42–7.28 (m, 6H), 6.77 (d, J=8.50 Hz, 0.55H), 6.74 (d, J=8.50 Hz, 0.45H), 5.22–5.08 (m, 2H), 4.20–4.11 (m, 2H), 3.96–3.89 (m, 1H), 3.92 (s, 3H), 3.80–3.67 (m, 1H), 3.21–3.11 (m, 1H), 2.27–2.16 (m, 2H), 1.57 (s, 9H), 1.47 (s, 4H), 1.37 (s, 5H); 13C-NMR (CDCl3, 125 MHz) δ: 176.4, 176.3, 170.4, 165.6, 162.2, 154.6, 154.4, 148.1, 138.0, 136.4, 136.2, 128.4, 128.3, 128.0, 127.9, 127.8, 124.6, 124.5, 112.8, 82.7, 82.6, 82.0, 81.9, 67.3, 64.9, 64.6, 53.5, 50.1, 49.9, 43.6, 42.6, 39.9, 39.0, 33.8, 28.0, 27.9, 27.7; HR-MS (ESI-TOF): Calcd for C30H38N2O9Na [(M+Na)+] 593.2470. Found 593.2486.
Acromelic Acid B (2)To a stirred solution of 30 (195 mg, 342 µmol) in H2O (1.7 mL) was added 30% HBr in AcOH (3.4 mL) at room temperature. The resulting mixture was stirred at 100°C for 36 h. Then the reaction mixture was concentrated under reduced pressure. The residue was charged onto a column containing Dowex-50 WX8 hydrogen form (200–400 mesh). After elution with H2O and 3% aqueous NH3, the collected fractions were concentrated under reduced pressure. The resulting ammonium salt was charged onto a column containing Amberlite IRC-50 hydrogen form. After elution with H2O, the collected fractions were concentrated under reduced pressure to give free amino acid 2 (105 mg, 99%) as a colorless amorphous solid.
2: [α]D20 −68.8 (c=0.98, H2O) [lit. [α]D27 −74.0 (c=0.1, H2O)]37); IR (film, cm−1) 3300–2700, 1655, 1597, 1419, 1363, 1251, 1167, 1060, 842, 801, 673. 1H-NMR (D2O, 500 MHz) δ: 7.68 (d, J=9.16 Hz, 1H), 6.70 (d, J=9.16 Hz, 1H), 4.65 (dt, J=11.5, 8.0 Hz, 1H), 4.08 (d, J=5.7 Hz, 1H), 3.80 (dd, J=11.5, 8.0 Hz, 1H), 3.65 (t, J=11.5 Hz, 1H), 3.27–3.20 (m, 1H), 2.52 (dd, J=16.6, 6.3 Hz, 1H), 2.35 (dd, J=16.6, 8.6 Hz, 1H); 13C-NMR (D2O, 125 MHz) δ: 175.7, 173.0, 166.5, 163.1, 143.3, 141.2, 120.4, 115.3, 65.5, 47.3, 42.3, 38.6, 34.7; HR-MS (ESI-TOF): Calcd for C13H15N2O7 [(M+H)+] 311.0874. Found 311.0877.
Dedicated to the memory of Dr. Takeshi Matsumoto, a close colleague of Dr. Ōmura.
This work was financially supported by the Uehara Memorial Foundation (Y.H.), MEXT/JSPS KAKENHI Grant Numbers 23390007, Grants-in-Aid for Scientific Research on Priority Areas 26102736 and 26105751 from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, and a Grant for Platform for Drug Discovery, Informatics, and Structural Life Science from MEXT of Japan.
The authors declare no conflict of interest.