Abstract
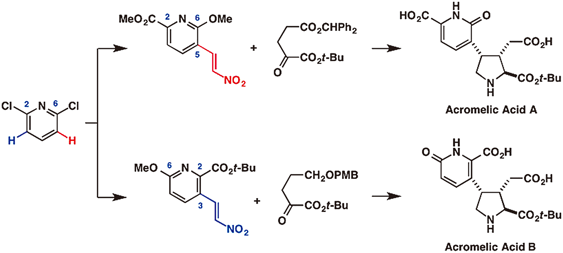
Practical total syntheses of acromelic acids A (1) and B (2), which were scarce natural products isolated from toxic mushroom by Shirahama and Matsumoto, were accomplished in 13 (36% total yield) and 17 steps (6.9% total yield), respectively, from 2,6-dichloropyridine (8). Beginning with regioselective transformation of symmetric 8 by either ortho-lithiation or bromination, nitroalkenes 15 and 16 were provided. Stereoselective construction of the vicinal stereocenters at the C-3, 4 positions of 1 and 2 was performed by a Ni-catalyzed asymmetric conjugate addition of α-ketoesters to the nitroalkenes. Construction of the pyrrolidine ring was accomplished in a single operation via a sequence consisting of reduction of the nitro group, intramolecular condensation with the ketone, and reduction of the resulting ketimine.