Abstract
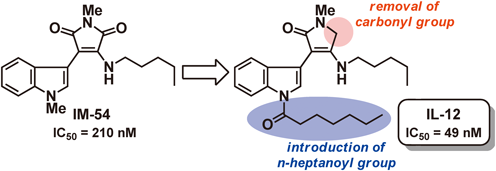
Modification of our previously reported selective inhibitor of oxidative stress-induced necrosis, 2-(1H-indol-3-yl)-3-pentylamino-maleimide (IM-54) by regioselective reduction of the C-4 carbonyl group afforded a 3-amino-2-indolyllactam (IL-1) with more potent activity. To examine the structure–activity relationship of IL derivatives, we developed new synthetic routes with flexibility to incorporate a range of substituents at a late stage. The synthesized IL derivatives were evaluated for activity to inhibit necrotic cell death induced by hydrogen peroxide. Among them, IL-12 showed the most potent activity (IC50=49 nM) among the IL and indolylmaleimide (IM) derivatives examined.