2016 Volume 64 Issue 7 Pages 899-906
2016 Volume 64 Issue 7 Pages 899-906
Enantioselective intramolecular conjugate addition reactions of short-lived C–O axially chiral enolates have been developed. The reactions proceeded with inversion of the configuration and provided dihydrobenzofurans with contiguous tetra- and trisubstituted carbon centers in up to 96% enantiomeric excess (ee).
Asymmetric synthesis had been classified into three categories: 1) optical resolution of the racemate by diastereomer formation, 2) diastereoselective synthesis using chiral auxiliaries, and 3) enantioselective synthesis using chiral catalysts or chiral reagents. On the other hand, we have studied asymmetric reactions that proceed via enolate intermediates with intrinsic axial chirality (MOC: memory of chirality).1–6) This strategy does not belong to any of above three categories. We believe that MOC strategy created the forth category of asymmetric synthesis. The characteristic feature of the asymmetric reactions based on MOC strategy is asymmetric reactions take place at the original stereogenic center of the starting material in the absence of external chiral sources. Accordingly, when the optically active starting materials are abundant and ubiquitous α-amino acids, asymmetric synthesis based on MOC strategy is especially advantageous. MOC strategy also has salient feature in the mechanistic point of view. The asymmetric synthesis proceeds via the transient chiral species with limited half-lives of racemization.1–13) We have developed strategy for asymmetric induction via enolate intermediate A with a chiral C–C axis in 19911) (Chart 1, Eq. 1). The half-life of racemization of the axially chiral enolate A at the reaction temperature (−20°C) was estimated to be ca. 24 d.1) We then developed a method for asymmetric induction via enolate intermediate B with a chiral C–N axis in 20002) (Chart 1, Eq. 2). The half-life of racemization of the axially chiral enolate B was determined to be 22 h at the reaction temperature (−78°C) by the measurement of the time-dependent racemization of enolate B. As logical possibility, the asymmetric induction might take place via configurationally stable carbanion B′ (Chart 1, Eq. 4). However, this possibility was eliminated because di-Boc derivative 2a (>99% enantiomeric excess (ee)) gave the racemic product by the same treatment for 2 (Chart 1, Eq. 5). Since the enolate B″ generated from 2a cannot to be axially chiral along the C–N axis even through rotation of the C–N bond is restricted, racemate formation is a reasonable consequence. We finally succeeded to develop asymmetric synthesis via short-lived C–O axially chiral enolates with the supposed half-life of racemization as short as ca. 1 s at −78°C6) (Chart 1, Eq. 3). The reaction shown in Eq. 3 was the first example of asymmetric reaction that proceeds via an axially chiral enolate with the restricted rotation of the C–O axis as the sole source for asymmetric induction. We report here asymmetric intramolecular conjugated addition reactions via C–O axially chiral enolates as further extension of this novel asymmetric induction (Chart 2). The key to success in this asymmetric induction is to design fast reactions to minimize racemization of the chiral enolate intermediates with extremely short half-lives of racemization. The products obtained by this transformation, chiral 2,2,3-trisubstituted dihydrobenzofurans, have been frequently found in important pharmacophores and biologically active compounds.14–21)

Logical possibility for asymmetric induction via configurationally stable carbanion B′ (Eq. 4) and racemate formation from the di-tert-butoxycarbonyl (Boc) derivative 2a via achiral enolate intermediate B″.
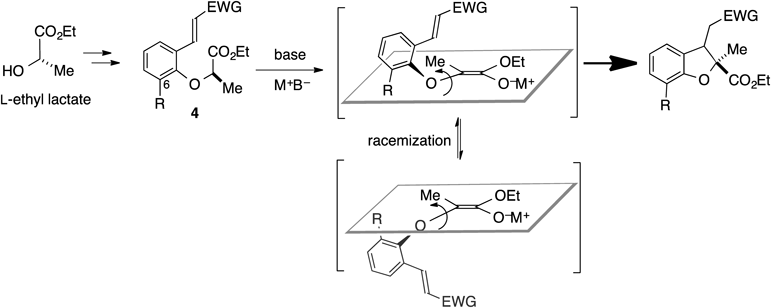
In the asymmetric cyclization shown in Eq. 3, substituent R in 3 played a crucial role for the asymmetric induction. For example, 3 with R=H gave a racemic dihydrobenzofuran, whereas 3 with R=i-Pr gave the corresponding dihydrobenzofuran in 99% ee under the similar reaction conditions.6) Based on these backgrounds, precursors 4 with substituents R (compounds 5, 7, and 9 in Table 1) for the asymmetric intramolecular conjugate addition were prepared starting from L-ethyl lactate via the Mitsunobu reaction with the corresponding phenol derivatives (see Supplementary Materials for the preparation of the substrates 4). We first examined the reaction of 5 (R=H) under the optimized conditions for asymmetric intramolecular alkylation via the C–O axially chiral enolate in Eq 3 in Chrat 1.6) Treatment of 5 with sodium hexamethyldisilazide (NaHMDS) in tetrahydrofuran (THF) at −78°C for 1 h gave a 91 : 9 diastereomeric mixture of 6a/b22) in a combined yield of 82% (Table 1, entry 1). The ee of the major diastereomer was found to be 41% (entry 1). This result was contrary to the asymmetric intermolecular alkylation shown in Chart 1, Eq. 3, where the racemate was obtained from substrate 3 with R=H.6) These results suggest that the intramolecular conjugate addition of the chiral enolate seems to proceed faster than the corresponding intramolecular alkylation. We next investigated the effects of bases on the asymmetric intarmolecular conjugate addition of 5. Use of lithium diisopropylamide (LDA) as a base gave the product with diminished diastereoselectivity and yield (entry 2). The absolute configuration of the major diastereomer (30% ee) was found to be opposite to that obtained in the reaction with NaHMDS.23) On the other hand, the reaction of 5 with lithium hexamethyldisilazide (LiHMDS) gave product 6a with the same absolute configuration as that with NaHMDS (entries 1, 3). Solvent effects in the reactions with NaHMDS and LiHMDS were examined (entries 5–8). Use of neither a less coordinating solvent (toluene) nor a strongly coordinating solvent N,N-dimethylformamide (DMF) improved the efficiency. Since dramatic improvement of the enantioselectivity was attained by employing the substrate 3 with R=Me in asymmetric intramolecular alkylation via the C–O axially chiral enolate6) (Chart 1, Eq. 3), the effects of the substituent R at C(6) were then investigated in the intramolecular conjugate addition of the chiral enolates (entries 9 and 10). Substrate 7 (R=Me) was treated with NaHMDS in THF for 3.5 h at −78°C to give a 90 : 10 diastereomeric mixture of 8a/b in a combined yield of 50% (entry 9). The ee of the major diastereomer 8a was found to be much improved (95% ee) compared with 41% ee of 6a obtained by the reaction of 5 with R=H (entries 9 vs. 1). The reaction of 9 with R=Br gave product 10 in only 9% yield (entry 10).
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | Substrate | R | Base | Solvent | Time (h) | Products | Yield (%) | dr a : bb,c) | % ee of ad,e,f) |
| 1 | 5 | H | NaHMDS | THF | 1 | 6a, b | 82 | 91 : 9 | 41 |
| 2 | 5 | H | LDA | THF | 1 | 6a, b | 37 | 75 : 25 | −30 |
| 3 | 5 | H | LiHMDS | THF | 2 | 6a, b | 26 | 91 : 9 | 53 |
| 4 | 5 | H | KHMDS | THF | 0.5 | 6a, b | 43 | 89 : 11 | −11 |
| 5 | 5 | H | NaHMDS | Toluene | 2.5 | 6a, b | 74 | 92 : 8 | −24 |
| 6g) | 5 | H | NaHMDS | DMF/THF (6 : 1) | 2.5 | 6a, b | 58 | 86 : 14 | 21 |
| 7 | 5 | H | LiHMDS | Toluene | 3 | 6a, b | —h) | —i) | —i) |
| 8 | 5 | H | LiHMDS | DMF/THF (11 : 1)g) | 2 | 6a, b | 81 | 90 : 10 | 28 |
| 9 | 7 | Me | NaHMDS | THF | 3.5 | 8a, b | 50 | 90 : 10 | 95 |
| 10 | 9 | Br | NaHMDS | THF | 3 | 10a, b | 9 | —i) | —i) |
a) All reactions were run at the substrate concentration of 0.1 M. b) The diastereomeric ratio was determined by 1H-NMR. c) The relative configuration of 6a and b was determined based on the reported 1H-NMR data of 6a and b (ref. 22). The relative configuration of 8 and 10 was tentatively assigned by analogy. d) ee’s were determined by HPLC analysis with a chiral stationary phase. e) Absolute configurations were tentatively assigned. f) ee’s of b were not determined. g) Run at −60°C. h) Recovery of 5. i) Not determined.
According to the strategy shown in Chart 2, minimization of racemization of the intermediary chiral enolate seems to be the key to achieve high asymmetric induction. Therefore, higher asymmetric induction is expected if the relative rate of intramolecular conjugate addition of the enolate increased compared to that of enolate racemization. Introduction of the stronger electron-withdrawing groups in the Michael accepter moiety was expected to be effective in increasing the rate of intramolecular conjugate addition of the enolate. We then examined substrates with malonate-type Michael acceptors (Table 2). The reaction of 11 (R1=R2=H, X=CO2Et) with NaHMDS in THF at −78°C gave an 80 : 20 diastereomeric mixture of 12a/b in a combined yield of 60% (Table 2, entry 1). The ee of the major diastereomer was found to be 66%, which was improved compared to 41% obtained by the reaction of the corresponding mono-ester derivative 5. (Table 2, entry 1 vs. Table 1, entry 1). The reaction of 11 with LiHMDS gave dihydrobenzofuran 12a in 78% ee (12a/b=92 : 8, 67% yield, entry 2). Use of 13 (X=CO2t-Bu) and 15 (X=CO2Bn) instead of 11 (X=CO2Et) in the presence of LiHMDS further improved the diastereoselectivity and the enantioselectivity to give a 96 : 4 diastereomeric mixture of 14a (83% ee) and b in a combined yield of 87% (entry 4) and a 96 : 4 diastereomeric mixture of 16a (86% ee) and b in a combined yield of 77% (entry 6), respectively. The effects of the substituents on the aromatic ring were next investigated. While only trace amount of dihydrobenzofuran derivatives were obtained by treatment of 17 (R1=Me, R2=H) with LiHMDS (entry 8), the desired products 18a and b were obtained by treatment of 17 with NaHMDS as a 67 : 33 diastereomeric mixture in a combined yield in 79% (entry 7). The ee of the major diastereomer 18a was much improved to be 96% compared to that (66%) of the product obtained by the reaction of the corresponding substrate with R1=H, 11 (Table 2, entries 7 vs. 1). We next investigated the effects of the substituent at C(4), because dramatic enhancement of the enantioselectivity was observed by introducing the substituent at C(4) in asymmetric intramolecular six-membered cyclization via the C–O axially chiral enolate.6) However, negligible effects of the C(4)-substituent was observed in the asymmetric intramolercular conjugate addition of 19 with R2=Br. Dihydrobenzofuran derivative 20a was obtained in 66–77% ee by the reaction of 19 (entries 9, 10), which was comparable to the enantioseletivity (66–78% ee) obtained in the intramolecular conjugate addition of 11 with R2=H (entries 1, 2). Even decrease in the enantioselectivity was observed in the reaction of 21 (R2=OMe) compared to that of the reaction of 11 (44–77% ee in entries 11, 12 vs. 66–78% ee in entries 1, 2).
 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Entry | Substrate | R1 | R2 | X | Base | Time (h) | Products | Yield (%) | dr a : bb,c,d) | % ee of ae,f,g) |
| 1 | 11 | H | H | CO2Et | NaHMDS | 1 | 12a, b | 60 | 80 : 20 | 66 |
| 2 | 11 | H | H | CO2Et | LiHMDS | 3 | 12a, b | 67 | 92 : 8 | 78 |
| 3 | 13 | H | H | CO2t-Bu | NaHMDS | 1 | 14a, b | 75 | 90 : 10 | 74 |
| 4 | 13 | H | H | CO2t-Bu | LiHMDS | 3 | 14a, b | 87 | 96 : 4 | 83 |
| 5 | 15 | H | H | CO2Bn | NaHMDS | 3 | 16a, b | 90 | 83 : 17 | 59 |
| 6 | 15 | H | H | CO2Bn | LiHMDS | 3 | 16a, b | 77 | 96 : 4 | 86 |
| 7 | 17 | Me | H | CO2Et | NaHMDS | 3 | 18a, b | 79 | 67 : 33 | 96 |
| 8 | 17 | Me | H | CO2Et | LiHMDS | 3 | 18a, b | traceh) | —i) | —i) |
| 9 | 19 | H | Br | CO2Et | NaHMDS | 1 | 20a, b | 86 | 75 : 25 | 67 |
| 10 | 19 | H | Br | CO2Et | LiHMDS | 3 | 20a, b | 82 | 92 : 8 | 77 |
| 11 | 21 | H | OMe | CO2Et | NaHMDS | 1 | 22a, b | 94 | 86 : 14 | 44 |
| 12 | 21 | H | OMe | CO2Et | LiHMDS | 3 | 22a, b | 60 | 90 : 10 | 77 |
a) All reactions were run at the substrate concentration of 0.1 M. b) Diastereomeric ratio was determined by 1H-NMR. c) The relative configuration of 18a was estimated by its NOE spectra. The relative configuration of 18b was determined by NOESY spectra after its conversion into the corresponding bicyclic analogue: For detail, see Supplementary Materials. d) Relative configuration of 12, 14, 16, 20, and 22 was tentatively assigned by analogy. e) ee’s were determined by HPLC analysis with a chiral stationary phase. f) The absolute configurations of 18a and b was determined by chemical correlation with (R)-24, see Chart 3. The absolute configurations of 12, 14, 16, 20, and 22 was tentatively assigned by analogy. g) ee’s of b were not determined. h) Recovery of 17. i) Not determined.
The absolute configurations of 18a/b were determined by chemical correlation with the known compound 24 (Chart 3). Introduction of a phenylthio group at the α-position of the ester carbonyl group,24) oxidation of the sulfur atom, followed by thermal β-elimination of resulting sulfoxide gave the unsaturated ester 23. Oxidative cleavage of the double bond25) of 23 followed by the reduction of the benzylic carbonyl group gave known (R)-24.6) Accordingly, the absolute configuration of the tertrasubstituted carbon center of 18a and b was determined to be R, and the intermolecular conjugate addition of 17 was disclosed to proceed with inversion of the configuration.

A hypothetical model for the mechanism of intramolecular conjugate addition of 4 is shown in Chart 4. Deprotonation of conformer 4a would give enantiomerically enriched enolate D with a chiral C–O axis, which would give product with inversion of configuration. On the other hand, deprotonation of conformer 4b would give the product with retention of configuration via enolate ent-D. Due to the better steric accessibility of the α-H in 4a than that in 4b on deprotonation, formation of the chiral enolate D seems to be preferable, and the overall reaction would proceed in inversion of the configuration. Both enolates D and ent-D are expected to readily undergo racemization in the reaction medium. The half-life of racemization of the C–O axially chiral enolates might be supposed to be roughly 1 s at −78°C based on our previous results in asymmetric intramolecular alkylation reactions.6) Because the enolate racemization and asymmetric intramolecular conjugate addition of the chiral enolate are competing to each other, the relative rate between them is critically involved in the enantioselectivity of the reaction. The rate of racemization of the chiral enolate generated from the substrates with R=Me is assumed to be relatively smaller compared to that from the substrates with R=H, because the C–O bond rotation responsible for the enolate racemization is expected to be affected by the steric bulkiness of the R group. This would explain the substituent effects at C(6) observed among 5 and 7 (Table 1, entries 1 vs. 9) and those among 11 and 17 (Table 2, entries 1 vs. 7).

In conclusion, we have developed a novel method for asymmetric intramolecular conjugate addition via short-lived axially chiral enolates with a chiral C–O axis. This method provides a unique entry to chiral dihydrobenzofuran derivatives with contiguous tetra- and trisubstituted chiral centers. While the chiral dihydrobenzofuran skeleton has been constructed via asymmetric C–O bond formation,25–28) asymmetric C–C bond formation at the original chiral center was employed in the present method. Readily available and abundant L-ethyl lactate is used not only as a functionalized carbon resource, but also as a chiral source for asymmetric induction.
1H-NMR were measured in CDCl3 and referenced from TMS (0.00 ppm) using JEOL ECX-400 (400 MHz) spectrophotometer, unless otherwise noted. 13C-NMR were measured in CDCl3 solution and referenced to CDCl3 (77.0 ppm) using JEOL ECX-400 (100 MHz) spectrophotometer, unless otherwise noted. Chemical shifts are reported in ppm. When peak multiplicities are reported, the following abbreviations are used: s, singlet; d, doublet; t, triplet; q, quartet; quint, quintet; sept, septet; m, multiplet; br, broadened. IR spectra were recorded on JASCO FT/IR-4200 spectrometer. Mass spectra were obtained on JEOL JMS-700 electron ionization (EI) or Bruker impact-HD electrospray ionization (ESI) mass spectrometers. Elemental analyses were performed with CHN J-science-lab. Microcoder JM10 analyzer. Optical rotations were determined on HORIBA SEPA-200 or JASCO P-2200 polarimeters. Flash column chromatography was performed on Silica Gel (SiliaFlash® F60 or 60N (KANTO)). TLC was performed on precoated plates (0.25 mm, silica gel Merck Kieselgel 60F245), and compounds were visualized with UV light followed by p-anisaldehyde stain or phosphomolybdic acid stain. All reactions were performed in oven-dried glassware under positive pressure of argon, unless otherwise noted. Anhydrous THF was purchased form Kanto Kagaku and pre-treated with activated MS4Å for 1 d or longer. Anhydrous toluene purchased from Wako Pure Chemical Industries, Ltd. was distilled over CaH2 and kept with MS4Å. Anhydrous DMF, which was purchased from Wako Pure Chemical Industries, Ltd. and pre-treated with MS4Å for 1 d, was distilled over P2O5 and kept with MS4Å. NaHMDS was purchased from TCI. LDA and LiHMDS were freshly prepared from the corresponding amine (1 eq) with n-BuLi (1 eq) in THF or toluene at 0°C for 30 min. KHMDS was prepared from HMDS with KH in THF or toluene.
General Procedure 1. Asymmetric Conjugate Addition of Acrylates (5, 7, 9) with Bases (Table 1)A solution of the substrate in the solvent indicated in Table 1 was added dropwise to a solution of base (2.0 eq) in the solvent indicated in Table 1 at −78°C (for THF or toluene solutions) or −60°C (for DMF solution) (the final concentration of the substrate: 0.1 M). After being stirred at the same temperature, the mixture was poured into aqueous sat. NH4Cl and vigorously stirred for about 1 min. The aqueous layer was extracted with AcOEt and the extracts were washed with brine, dried over MgSO4, filtered, and concentrated. The residue was purified through silica gel column chromatography.
(2R,3R)-2-Ethoxycarboyl-3-(ethoxycarbonyl)methyl-2-methyl-2,3-dihydrobenzofuran (6a)Colorless oil; HPLC conditions: Daicel Chiralcel OD-H, hexane/2-propanol=99/1, flow=1.0 mL/min, λ=284 nm, retention time (tR)=15 (minor enantiomer), 25 (major enantiomer) min; [α]D20−37.5 (c 0.60, CHCl3, 49% ee); IR (neat) cm−1: 3447, 2983, 1736, 1597, 1479, 1462, 1377, 1244, 1110, 1081, 1021, 753; 1H-NMR (400 MHz, CDCl3) δ: 1.27 (3H, t, J=6.8 Hz), 1.29 (3H, t, J=6.8 Hz), 1.57 (3H, s), 2.59 (1H, dd, J=8.8, 16.0 Hz), 2.77 (1H, dd, J=6.8, 16.0 Hz), 4.17–4.26 (5H, m), 6.86–6.90 (2H, m), 7.12 (1H, d, J=7.6 Hz), 7.17 (1H, br t, J=7.6 Hz); 13C-NMR (100 MHz, CDCl3) δ: 13.97 (q), 14.06 (q), 18.71 (q), 35.56 (t), 44.33 (d), 60.77 (t), 61.70 (t), 88.91 (s), 109.89 (d), 121.15 (d), 124.40 (d), 128.46 (s), 128.80 (d), 157.65 (s), 171.34 (s), 172.88 (s); MS (ESI) m/z 315 ((M+Na)+, base peak); high resolution (HR)-MS (ESI) m/z Calcd for C16H20NaO5 ((M+Na)+): 315.1203. Found: 315.1202.
(2R,3S)-2-Ethoxycarboyl-3-(ethoxycarbonyl)methyl-2-methyl-2,3-dihydrobenzofuran (6b)Colorless oil; IR (neat) cm−1: 3444, 2982, 1735, 1598, 1481, 1376, 1251, 1166, 1125, 1088, 1021, 752; 1H-NMR (400 MHz, CDCl3) δ: 1.28 (3H, t, J=7.2 Hz), 1.29 (3H, t, J=7.2 Hz), 1.71 (3H, s), 2.56 (1H, dd, J=8.0, 16.4 Hz), 2.64 (1H, dd, J=6.8, 16.4 Hz), 3.86 (1H, br t, J=7.2 Hz), 4.15–4.32 (4H, m), 6.86–6.89 (2H, m), 7.10–7.12 (1H, m), 7.16–7.20 (1H, m); 13C-NMR (100 MHz, CDCl3) δ: 14.14 (q), 24.66 (q), 36.68 (t), 47.87 (d), 60.83 (t), 61.64 (t), 89.90 (s), 110.07 (d), 121.06 (d), 124.41 (d), 128.17 (s), 128.99 (d), 158.06 (s), 171.15 (s), 171.23 (s); MS (ESI) m/z 315 ((M+Na)+, base peak); HR-MS (ESI) m/z Calcd for C16H20NaO5 ((M+Na)+): 315.1203. Found: 315.1198.
(2R,3R)-2,7-Dimethyl-2-ethoxycarboyl-3-(ethoxycarbonyl)methyl-2,3-dihydrobenzofuran (8a)Colorless oil; HPLC conditions: Daicel Chiralcel OD-H, hexane/2-propanol=99/1, flow=0.5 mL/min, λ=284, tR=25 (minor enantiomer), 46 (major enantiomer) min; [α]D22−57.4 (c 1.05, CHCl3, 94% ee); IR (neat) cm−1: 3438, 2982, 1736, 1597, 1467, 1377, 1255, 1098, 1028; 1H-NMR (400 MHz, CDCl3) δ: 1.26 (3H, t, J=7.2 Hz), 1.28 (3H, t, J=7.2 Hz), 1.58 (3H, s), 2.24 (3H, s), 2.57 (1H, dd, J=8.4, 16.0 Hz), 2.74 (1H, dd, J=6.8, 16.0 Hz), 4.15–4.24 (5H, m), 6.77 (1H, t, J=7.2 Hz), 6.93 (1H, d, J=7.2 Hz), 6.98 (1H, d, J=7.2 Hz); 13C-NMR (100 MHz, CDCl3) δ: 14.01 (q), 14.13 (q), 15.14 (q), 18.75 (q), 35.77 (t), 44.72 (d), 60.79 (t), 61.63 (t), 88.64 (t), 120.12 (s), 121.00 (d), 121.71 (d), 127.83 (s), 130.04 (d), 156.33 (s), 171.48 (s), 173.19 (s); MS (ESI) m/z 329 ((M+Na)+, base peak); HR-MS (ESI) m/z Calcd for C17H22NaO5 ((M+Na)+): 329.1359. Found: 329.1353.
(2R,3S)-2,7-Dimethyl-2-ethoxycarboyl-3-(ethoxycarbonyl)methyl-2,3-dihydrobenzofuran (8b)Colorless oil; IR (neat) cm−1: 3448, 2982, 2920, 1737, 1597, 1467, 1376, 1264, 1028, 760; 1H-NMR (400 MHz, CDCl3) δ: 1.276 (3H, t, J=7.2 Hz), 1.283 (3H, t, J=7.2 Hz), 1.72 (3H, s), 2.24 (3H, s), 2.56 (1H, dd, J=7.6, 16.4 Hz), 2.62 (1H, dd, J=7.6, 16.4 Hz), 3.86 (1H, t, J=7.6 Hz), 4.13–4.27 (4H, m), 6.78 (1H, t, J=7.6 Hz), 6.91 (1H, d, J=7.6 Hz), 6.99 (1H, d, J=7.6 Hz); 13C-NMR (100 MHz, CDCl3) δ: 14.09 (q), 14.17 (q), 15.20 (q), 24.72 (q), 36.70 (t), 48.26 (d), 60.80 (t), 61.48 (t), 89.59 (s), 120.14 (s), 120.84 (d), 121.51 (d), 127.42 (s), 130.17 (d), 156.70 (s), 171.35 (s); MS (ESI) m/z 329 ((M+Na)+, base peak); HR-MS (ESI) m/z Calcd for C17H22NaO5 ((M+Na)+): 329.1359. Found: 329.1354.
7-Bromo-2-ethoxycarbonyl-3-(ethoxycarbonyl)methyl-2-methyl-2,3-dihydrobenzofuran (10)Colorless oil; IR (neat) cm−1: 3440, 2983, 1733, 1604, 1584, 1449, 1377, 1255, 1022, 772; 1H-NMR (400 MHz, CDCl3) δ: 1.26 (3H, t, J=7.2 Hz), 1.28 (3H, t, J=7.2 Hz), 1.62 (3H, s), 2.57 (1H, dd, J=8.8, 16.0 Hz), 2.77 (1H, dd, J=6.4, 16.0 Hz), 4.17–4.28 (5H, m), 6.76 (1H, t, J=8.0 Hz), 7.05–7.07 (1H, m), 7.32 (1H, d, J=8.0 Hz); 13C-NMR (100 MHz, CDCl3) δ: 14.00 (q), 14.15 (q), 18.58 (q), 35.72 (t), 45.33 (d), 60.99 (t), 61.97 (t), 89.59 (s), 102.96 (s), 122.55 (d), 123.56 (d), 130.19 (s), 132.02 (d), 155.45 (s), 171.13 (s), 172.42 (s); MS (ESI) m/z 393 ((M+Na)+, base peak); HR-MS (ESI) m/z Calcd for C16H19BrNaO5 ((M+Na)+): 393.0308. Found: 393.0302.
General Procedure for Asymmetric Conjugate Addition of Benzylidenemalonate (11, 13, 15, 17, 19, 21) with Bases (Table 2)A solution of the substrate (1.0 eq) in THF was added dropwise to a solution of the base (2.0 eq or 1.5 eq) in THF at −78°C (the final substrate concentration: 0.1 M). After being stirred for the time indicated in Table 2, the mixture was poured into aqueous sat. NH4Cl, and the resulting mixture was extracted with AcOEt. The extracts were washed with brine, dried over Na2SO4, filtered, and concentrated. The residual oil was purified through silica gel column chromatography to give dihydrobenzofuran derivatives.
(2R,3R)-3-Bis(ethoxycarbonyl)methyl-2-ethoxycarbonyl-2-methyl-2,3-dihydrobenzofuran (12a)Colorless oil; HPLC conditions: Daicel Chiralcel OD-H, hexane/2-propanol=99/1, flow=1 mL/min, λ=284, tR=12.6 (minor enantiomer), 15.4 (major enantiomer) min; [α]D22−86.9 (c 1.09, CHCl3, 72% ee); IR (neat) cm−1: 3441, 2983, 1732, 1478, 1462, 1370, 1250, 1109, 1027, 755; 1H-NMR (400 MHz, CDCl3) δ: 1.12 (3H, t, J=7.2 Hz), 1.24 (3H, t, J=7.2 Hz), 1.30 (3H, t, J=7.2 Hz), 1.65 (3H, s), 3.74 (1H, d, J=8.4 Hz), 4.07 (2H, q, J=7.2 Hz), 4.16–4.22 (2H, m), 4.27 (2H, q, J=7.2 Hz), 4.51 (2H, d, J=8.4 Hz), 6.83–6.88 (2H, m), 7.12 (1H, d, J=7.2 Hz), 7.15–7.19 (1H, m); 13C-NMR (100 MHz, CDCl3) δ: 13.73 (q), 13.96 (q), 14.02 (q), 18.65 (q), 46.93 (d), 53.27 (d), 46.93 (d), 53.27 (d), 61.70 (t), 61.93 (t), 61.99 (t), 89.14 (s), 109.94 (d), 121.13 (d), 125.66 (d), 125.76 (s), 129.39 (d), 158.44 (s), 167.37 (s), 167.82 (s), 172.89 (s); MS (ESI) m/z 387 ((M+Na)+, base peak); HR-MS (ESI) m/z Calcd for C19H24NaO7 ((M+Na)+): 387.1414. Found: 387.1423.
(2R,3S)-3-Bis(ethoxycarbonyl)methyl-2-ethoxycarbonyl-2-methyl-2,3-dihydrobenzofuran (12b)Colorless oil; IR (neat) cm−1: 3440, 2983, 1739, 1486, 1463, 1371, 1303, 1254, 1177, 1153, 1022, 755; 1H-NMR (400 MHz, CDCl3) δ: 1.14 (3H, t, J=7.2 Hz), 1.29 (3H, t, J=7.2 Hz), 1.34 (3H, t, J=7.2 Hz), 1.70 (3H, s), 4.01 (1H, d, J=7.6 Hz), 4.05–4.31 (7H, m), 6.85–6.88 (2H, m), 7.13–7.23 (2H, m); 13C-NMR (100 MHz, CDCl3) δ: 13.70 (q), 13.92 (q), 13.97 (q), 25.69 (q), 50.37 (d), 53.74 (d), 61.57 (t), 61.81 (t), 61.85 (t), 88.99 (s), 110.23 (d), 120.94 (d), 125.11 (s), 125.67 (d), 129.40 (d), 157.96 (s), 167.44 (s), 167.82 (s), 170.85 (s); MS (ESI) m/z 387 ((M+Na)+, base peak); HR-MS (ESI) m/z Calcd for C19H24NaO7 ((M+Na)+): 387.1414. Found: 387.1420.
(2R,3R)-3-Bis(tert-butoxycarbonyl)methyl-2-ethoxycarbonyl-2-methyl-2,3-dihydrobenzofuran (14a)Colorless oil; HPLC conditions: Daicel Chiralcel OD-H, hexane/2-propanol=99.5/0.5, flow=0.5 mL/min, λ=284 nm, tR=29 (major), 32 (minor) min; [α]D21−81.6 (c 1.05, CHCl3, 82% ee); IR (neat) cm−1: 3437, 2980, 1746 1595, 1478. 1462, 1254, 1160, 754; 1H-NMR (400 MHz, CDCl3) δ: 1.23 (3H, t, J=7.2 Hz), 1.29 (9H, s), 1.49 (9H, s); 1.71 (3H, s); 3.60 (1H, d, J=6.8 Hz), 4.18 (2H, q, J=7.2 Hz), 4.41 (1H, d, J=6.8 Hz), 6.83–6.86 (2H, m); 7.16 (1H, dd, J=1.2, 8.0 Hz), 7.26 (1H, d, J=6.8 Hz); 13C-NMR (100 MHz, CDCl3) δ: 13.93 (q); 18.80 (q), 27.42 (q); 27.73 (q); 46.67 (d), 55.05 (d), 61.72 (t), 82.04 (s), 82.11 (s), 89.11 (s), 109.67 (d), 120.89 (d), 126.24 (s), 126.39 (d), 128.98 (d), 158.46 (s), 166.64 (s), 167.13 (s), 173.23 (s); MS (ESI) m/z 443 ((M+Na)+, base peak); HR-MS (ESI) m/z Calcd for C23H32NaO7 ((M+Na)+): 443.2040. Found: 443.2033.
(2R,3S)-3-Bis(tert-butoxycarbonyl)methyl-2-ethoxycarbonyl-2-methyl-2,3-dihydrobenzofuran (14b)Colorless oil; IR (neat) cm−1: 3237, 2979, 2932, 1743, 1480, 1369, 1255, 1147, 752, 734; 1H-NMR (400 MHz, CDCl3) δ: 1.30 (9H, s), 1.34 (3H, t, J=7.2 Hz), 1.48 (9H, s), 1.69 (3H, s), 3.88 (1H, d, J=7.2 Hz), 4.00 (1H, d, J=7.2 Hz); 4.29–4.31 (2H, m), 6.84–6.88 (2H, m), 7.15–7.19 (1H, m), 7.25–7.27 (1H, m); 13C-NMR (100 MHz, CDCl3) δ: 14.03 (q), 25.89 (q), 27.54 (q), 27.86 (q), 50.17 (d), 55.40 (d), 61.75 (t), 81.94 (s), 82.21 (s), 88.93 (s), 110.11 (d), 120.79 (d), 125.81 (s), 126.49 (d), 129.09 (d), 158.02 (s), 166.82 (s), 167.41 (s), 170.87 (s); MS (ESI) m/z 443 ((M+Na)+, base peak); HR-MS (ESI) m/z Calcd for C23H32NaO7 ((M+Na)+): 443.2040. Found: 443.2036.
(2R,3R)-3-Bis(benzyloxycarbonyl)methyl-2-ethoxycarbonyl-2-methyl-2,3-dihydrobenzofuran (16a)Colorless oil; HPLC conditions: Daicel Chiralpak AD, hexane/2-propanol=99/1, flow=1.0 mL/min, λ=284 nm, tR=18 (major), 23 (minor) min; [α]D22−74.5 (c 1.01, CHCl3, 78% ee); IR (neat) cm−1: 3439, 2982, 1738, 1594, 1478, 1461, 1382, 1159, 1129, 1109, 1018, 753; 1H-NMR (400 MHz, CDCl3) δ: 1.21 (3H, t, J=7.2 Hz), 1.56 (3H, s), 3.84 (1H, d, J=8.8 Hz), 4.15 (2H, q, J=7.2 Hz), 4.54 (1H, d, J=8.8 Hz), 4.96 (1H, d, J=12.4 Hz), 5.01 (1H, d, J=12.4 Hz), 5.17 (1H, d, J=12.4 Hz), 5.22 (1H, d, J=12.4 Hz), 6.74 (1H, dt, J=0.8, 7.6 Hz), 6.85 (1H, d, J=8.0 Hz), 6.98 (1H, d, J=7.6 Hz), 7.12–7.17 (3H, m), 7.26–7.37 (8H, m); 13C-NMR (100 MHz, CDCl3) δ: 13.93 (q), 18.54 (q), 46.95 (d), 53.25 (d), 61.87 (t), 67.47 (t), 67.66 (t), 89.01 (s), 109.94 (d), 125.51 (s), 125.54 (d), 128.32 (d), 128.37 (d), 128.40 (d), 128.42 (d), 128.45 (d), 128.51 (d), 129.37 (d), 134.57 (s), 134.82 (s), 158.32 (s), 166.98 (s), 167.43 (s), 172.70 (s); MS (ESI) m/z 511 ((M+Na)+, base peak); HR-MS (ESI) m/z Calcd for C29H28NaO7 ((M+Na)+): 511.1727. Found: 511.1737.
(2R,3S)-3-Bis(benzyloxycarbonyl)methyl-2-ethoxycarbonyl-2-methyl-2,3-dihydrobenzofuran (16b)Colorless oil; IR (neat) cm−1: 3392, 2981, 2918, 1737, 1481, 1459, 1376, 1255, 1153, 751; 1H-NMR (400 MHz, CDCl3) δ: 1.23 (3H, t, J=7.6 Hz), 1.67 (3H, s), 4.06–4.15 (4H, m), 4.98 (1H, d, J=12.4 Hz), 5.03 (1H, d, J=12.4 Hz), 5.15 (2H, s), 6.75 (1H, br t, J=7.2 Hz), 6.82 (1H, d, J=8.0 Hz), 6.97 (1H, d, J=7.2 Hz), 7.13–7.37 (11H, m); 13C-NMR (100 MHz, CDCl3) δ: 13.94 (q), 25.61 (q), 50.42 (d), 53.88 (d), 61.87 (t), 67.42 (t), 67.51 (t), 88.96 (s), 110.30 (d), 121.02 (d), 124.93 (s), 125.58 (d), 128.28 (d), 128.36 (d), 128.38 (d), 128.43 (d), 128.50 (d), 128.52 (d), 129.45 (d), 157.91 (s), 167.15 (s), 167.53 (s), 170.84 (s); MS (ESI) m/z 511 ((M+Na)+), 999 ((2M+Na)+, base peak); HR-MS (ESI) m/z Calcd for C29H28NaO7 ((M+Na)+): 511.1727. Found: 511.1739.
(2R,3R)-3-Bis(ethoxycarbonyl)methyl-2-ethoxycarbonyl-2,7-dimethyl-2,3-dihydrobenzofuran (18a)Colorless oil; HPLC conditions: Daicel Chiralpak ID, hexane/2-propanol=99/1, flow=1.0 mL/min, λ=284 nm, tR=17.7 (minor), 22.4 (major) min; [α]D22−107.4 (c 0.98, CHCl3, 96% ee); IR (neat) cm−1: 3446, 2983, 1737, 1596, 1467, 1370, 1251, 1176, 1097, 1027, 759; 1H-NMR (400 MHz, CDCl3) δ: 1.13 (3H, t, J=7.2 Hz), 1.23 (3H, t, J=7.2 Hz), 1.30 (3H, t, J=7.2 Hz), 1.64 (3H, s), 2.24 (3H, s), 3.71 (1H, d, J=8.8 Hz), 4.05–4.11 (2H, m), 4.13–4.21 (2H, m), 4.26 (2H, q, J=7.2 Hz), 4.49 (1H, d, J=8.8 Hz), 6.74 (1H, t, J=7.6 Hz), 6.92 (1H, d, J=7.6 Hz), 6.98 (1H, d, J=7.6 Hz); 13C-NMR (100 MHz, CDCl3) δ: 13.69 (q), 13.92 (q), 13.94 (q), 15.10 (q), 18.54 (q), 47.20 (d), 53.35 (d), 61.60 (t), 61.73 (t), 61.90 (t), 88.81 (s), 120.07 (s), 120.89 (d), 122.81 (d), 125.00 (s), 130.46 (d), 156.99 (s), 167.38 (s), 167.80 (s), 173.00 (s); MS (ESI) m/z 401 ((M+Na)+, base peak); HR-MS (ESI) m/z Calcd for C20H26NaO7 ((M+Na)+): 401.1571. Found: 401.1570.
(2R,3S)-3-Bis(ethoxycarbonyl)methyl-2-ethoxycarbonyl-2,7-dimethyl-2,3-dihydrobenzofuran (18b)Colorless oil; IR (neat) cm−1: 3448, 2983, 1737, 1594, 1468, 1371, 1301, 1267, 1177, 1156, 1028, 766; 1H-NMR (400 MHz, CDCl3) δ: 1.16 (3H, t, J=7.2 Hz), 1.28 (3H, t, J=7.2 Hz), 1.31 (3H, t, J=7.2 Hz), 1.69 (3H, s), 2.22 (3H, s), 3.96 (1H, d, J=8.0 Hz), 4.08–4.28 (7H, m), 6.75 (1H, t, J=8.0 Hz), 6.89 (1H, d, J=8.0 Hz), 7.00 (1H, d, J=8.0 Hz); 13C-NMR (100 MHz, CDCl3) δ: 13.75 (q), 13.93 (q) 15.21 (q), 25.53 (q), 50.88 (d), 54.09 (d), 61.57 (t), 61.69 (t), 61.79 (t), 88.72 (s), 120.28 (s), 120.68 (d), 122.70 (d), 124.39 (s), 130.55 (d), 156.61 (s), 167.60 (s), 167.81 (s), 170.94 (s); MS (ESI) m/z 401 ((M+Na)+, base peak); HR-MS (ESI) m/z Calcd for C20H26NaO7 ((M+Na)+): 401.1571. Found: 401.1569.
(2R,3R)-3-Bis(ethoxycarbonyl)methyl-5-bromo-2-ethoxycarbonyl-2-methyl-2,3-dihydrobenzofuran (20a)Colorless oil; HPLC conditions: Daicel Chiralcel OD-H, hexane/2-propanol=99/1, flow=1.0 mL/min, λ=284 nm, tR=12 (minor), 18 (major) min; [α]D22−49.7 (c 1.10, CHCl3, 77% ee); IR (neat) cm−1: 3450, 2983, 1733, 1471, 1370, 1305, 1255, 1027, 758; 1H-NMR (400 MHz, CDCl3) δ: 1.14 (3H, t, J=7.2 Hz), 1.25 (3H, t, J=7.2 Hz), 1.31 (3H, t, J=7.2 Hz), 1.66 (3H, s), 3.73 (1H, d, J=8.0 Hz), 4.04–4.13 (2H, m), 4.20 (2H, q, J=7.2 Hz), 4.27 (2H, q, J=7.2 Hz), 4.46 (1H, d, J=8.0 Hz), 6.75 (1H, d, J=8.4 Hz), 7.26–7.29 (2H, m); 13C-NMR (100 MHz, CDCl3) δ: 13.72 (q), 13.90 (q), 13.96 (q), 18.54 (q), 46.85 (d), 52.94 (d), 61.85 (t), 62.06 (t), 62.10 (t), 89.70 (s), 111.44 (d), 112.96 (s), 128.23 (s), 128.79 (d), 132.16 (d), 157.65 (s), 167.08 (s), 167.53 (s), 172.41 (s); MS (ESI) m/z 465 ((M+Na)+, base peak); HR-MS (ESI) m/z Calcd for C19H23BrNaO7 ((M+Na)+): 465.0519. Found: 465.0507.
(2R,3S)-3-Bis(ethoxycarbonyl)methyl-5-bromo-2-ethoxycarbonyl-2-methyl-2,3-dihydrobenzofuran (20b)Colorless oil; IR (neat) cm−1: 3448, 2982, 1737, 1473, 1371, 1308, 1256, 1177, 1024; 1H-NMR (400 MHz, CDCl3) δ: 1.16 (3H, t, J=7.2 Hz), 1.29 (3H, t, J=7.2 Hz), 1.33 (3H, t, J=7.2 Hz), 1.68 (3H, s), 4.00 (1H, d, J=7.6 Hz), 4.06–4.14 (3H, m), 4.16–4.30 (4H, m), 6.74 (1H, d, J=8.4 Hz), 7.26–7.30 (2H, m); 13C-NMR (100 MHz, CDCl3) δ: 13.75 (q), 13.91 (q), 13.94 (q), 25.61 (q), 50.25 (d), 53.36 (d), 61.75 (t), 61.96 (t), 61.98 (t), 89.60 (s), 111.77 (d), 112.73 (s), 127.63 (s), 128.77 (d), 132.20 (d), 157.17 (s), 167.20 (s), 167.58 (s), 170.34 (s); MS (ESI) m/z 465 ((M+Na)+, base peak); HR-MS (ESI) m/z Calcd for C19H23BrNaO7 ((M+Na)+): 465.0519. Found: 465.0510.
(2R,3R)-3-Bis(ethoxycarbonyl)methyl-2-ethoxycarbonyl-2-methyl-5-methoxy-2,3-dihydrobenzofuran (22a)Colorless oil; HPLC conditions: Daicel Chiralcel OD-H, hexane/2-propanol=99/1, flow=1.0 mL/min, λ=284 nm, tR=25 (minor), 30 (major) min; [α]D21−60.9 (c 1.21, CHCl3, 61% ee); IR (neat) cm−1: 3437, 2983, 1733, 1488, 1370, 1227, 1175, 1149, 1106, 1031; 1H-NMR (400 MHz, CDCl3) δ: 1.13 (3H, t, J=7.2 Hz), 1.24 (3H, t, J=7.2 Hz), 1.30 (3H, t, J=7.2 Hz), 1.64 (3H, s), 3.71 (3H, s), 3.73 (1H, d, J=8.4 Hz), 4.05–4.10 (2H, m), 4.19 (2H, dq, J=0.8, 7.2 Hz), 4.28 (2H, q, J=7.2 Hz), 4.47 (1H, d, J=8.4 Hz), 6.68–6.74 (2H, m), 6.77–6.79 (1H, m); 13C-NMR (100 MHz, CDCl3) δ: 13.66 (q), 13.87 (q), 13.94 (q), 18.58 (q), 47.19 (d), 53.09 (d), 55.74 (s), 61.61 (t), 61.81 (t), 61.91 (t), 109.88 (d), 111.60 (d), 114.56 (d), 126.64 (s), 152.49 (s), 154.31 (s), 167.25 (s), 167.68 (s), 173.02 (s); MS (ESI) m/z 417 ((M+Na)+, base peak); HR-MS (ESI) m/z Calcd for C20H26NaO8 ((M+Na)+): 417.1520. Found: 417.1521.
(2R,3S)-3-Bis(ethoxycarbonyl)methyl-2-ethoxycarbonyl-2-methyl-5-methoxy-2,3-dihydrobenzofuran (22b)Colorless oil; IR (neat) cm−1: 3393, 2983, 1737, 1480, 1307, 1227, 1148, 1032; 1H-NMR (400 MHz, CDCl3) δ: 1.14 (3H, t, J=7.2 Hz), 1.28 (3H, t, J=7.2 Hz), 1.33 (3H, t, J=7.2 Hz), 1.67 (3H, s), 3.72 (3H, s), 4.01 (1H, d, J=7.6 Hz), 4.03–4.12 (3H, m), 4.17–4.30 (4H, m), 6.71–6.77 (3H, m); 13C-NMR (100 MHz, CDCl3) δ: 13.77 (q), 13.96 (q), 14.02 (q), 25.71 (q), 50.73 (d), 53.61 (d), 55.92 (q), 61.62 (t), 61.86 (t), 89.21 (s), 110.29 (d), 111.75 (d), 114.72 (d), 126.06 (s), 152.08 (s), 154.31 (s), 167.44 (s), 167.86 (s), 170.97 (s); MS (ESI) m/z 417 ((M+Na)+, base peak); HR-MS (ESI) m/z Calcd for C20H26NaO8 ((M+Na)+): 417.1520. Found: 417.1520.
Determination of Absolute Configuration of 18a/bK2CO3 (0.59 g, 4.23 mmol) was added to a solution of a diastereomeric mixture of 18a/b (1.07 g, 2.82 mmol) in MeCN (28 mL) at r.t. After being stirred for 30 min at r.t., phenyl succinimidyl sulfide (1.17 g, 5.67 mmol)24) was added. The resulting mixture was stirred for 30 min and diluted with CH2Cl2. The mixture was washed with water and brine, and dried over MgSO4, filtered, and concentrated. The residue was purified through flash silica gel column chromatography to give phenylthio malonate (1.25 g, 91% yield). m-Chloroperbenzoic acid (mCPBA) (0.74 g, 3.09 mmol) was portionwise added to a solution of phenylthio malonate (1.25 g, 2.57 mmol) in CH2Cl2 (30 mL) at 0°C. The mixture was gradually warmed to r.t., and stirred for 30 min. The resulting mixture was diluted with CH2Cl2 and quenched by addition of aqueous sat. NaHCO3. The aqueous layer was extracted with CH2Cl2. The extracts were washed with water and brine, and dried over MgSO4, filtered, and concentrated to give an oil, which was dissolved in toluene (30 mL). The stirring mixture was refluxed for 12 h. After removal of volatiles, the residue was purified through flash silica gel column chromatography to give 23 (0.62 g, 64% yield) as a colorless oil. To a solution of 23 (0.62 g, 1.64 mmol) and MgSO4 (0.43 g, 3.61 mmol) in water–acetone (20 mL, 3 : 2, v/v) was added KMnO4 (0.52 g, 9.84 mmol) portion-wise at r.t. After being stirred for 13 h, the mixture was diluted with water and extracted with AcOEt. The extracts were dried over MgSO4, filtered, and concentrated. The residue was purified through silica gel column chromatography to give ketone (69.1 mg, 18% yield). A mixture of ketone (23.7 mg, 0.10 mmol) and Pd(OH)2/C (6.8 mg, 0.01 mmol) was stirred under H2 atmosphere (4 atm) for 54 h at r.t. The mixture was filtered through a pad of Celite and the filtrate was concentrated. The residue was purified through silica gel column chromatography to give 24 (13.1 mg, 55% yield) as a colorless oil; [α]D20+5.4 (c 1.0, CHCl3) [lit.1) [α]D19−8.6 (c 0.9, CHCl3, 86% ee) for (S)-24]. The proton and carbon NMR spectra were identified with reported ones.1)
(R)-Diethyl 2-(2-(Ethoxycarbonyl)-2,7-dimethylbenzofuran-3(2H)-ylidene) Malonate (23)Colorless oil; 1H-NMR (400 MHz, CDCl3) δ: 1.22 (3H, t, J=7.3 Hz), 1.28 (3H, t, J=7.3 Hz), 1.38 (3H, t, J=6.9 Hz), 1.83 (3H, s), 2.23 (3H, s), 4.10–4.29 (4H, m), 4.38–4.47 (2H, m), 6.88 (1H, dd, J=0.5, 7.8 Hz), 7.19–7.23 (2H, m); 13C-NMR (100 MHz, CDCl3) δ: 13.87 (q), 13.94 (q), 14.02 (q), 20.93 (q), 61.37 (t), 61.63 (t), 61.77 (t), 91.67 (s), 115.62 (s), 120.78 (s), 121.76 (d), 122.07 (s), 122.30 (d), 135.33 (d), 156.05 (s), 161.90 (s), 163.46 (s), 166.35 (s), 167.25 (s).
This work was also supported by a Grant-in-Aid for JSPS Fellows to K.T. and K.K.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.