Experimental
ChemistryMelting points were determined on a Büchi apparatus and are uncorrected. 1H-NMR spectra and 2D spectra were recorded on a Bruker Avance III 600 or a Bruker Avance DRX 400 instrument, whereas 13C-NMR spectra were recorded on a Bruker Avance III 600 or a Bruker AC 200 spectrometer in deuterated solvents and were referenced to tetramethylsilane (TMS) (δ scale). The signals of 1H and 13C spectra were unambiguously assigned by using 2D-NMR techniques: 1H–1H correlation spectroscopy (COSY), nuclear Overhauser effect spectroscopy (NOESY), hetero-nuclear multiple quantum coherence (HMQC), and heteronuclear multiple bond connectivity (HMBC). Mass spectra were recorded with a LTQ Orbitrap Discovery instrument, possessing an Ionmax ionization source. Flash chromatography was performed on Merck silica gel 60 (0.040–0.063 mm). Analytical TLC was carried out on precoated (0.25 mm) Merck silica gel F-254 plates. The purity of all the synthesized compounds was >95% as ascertained by elemental analysis. Elemental analyses were undertaken using a PerkinElmer, Inc. PE 240C elemental analyzer (Norwalk, CT, U.S.A.) and the measured values for C, H, and N were within±0.4% of the theoretical values.
5-Chloro-3-nitro-1H-pyrazolo[3,4-c]pyridine (7)Chloro derivative 6 (490 mg, 3.19 mmol) was dissolved into concentrated sulfuric acid (9.50 mL) at 0°C and then a mixture of concentrated sulfuric acid (0.49 mL) and nitric acid (0.49 mL) was added dropwise into this solution. This reaction mixture was heated at 95°C for 1h. Upon cooling, it was poured into crashed ice, neutralized with an aqueous solution of NH3 and the product was extracted with ethyl acetate (3×100 mL). The combined organic layers were dried over Na2SO4 and evaporated under reduced pressure to provide 600 mg of pure 7 as a yellow solid. Yield 94%, mp: 195–197°C (EtOAc). 1H-NMR (400 MHz, DMSO-d6) δ: 8.10 (1H, d, H-4, J=1.0 Hz), 9.16 (1H, d, H-7, J=1.0 Hz). 13C-NMR (50 MHz, DMSO-d6) δ: 113.3 (C-4), 122.1 (C-3a), 137.1 (C-7), 137.8 (C-7a), 144.0 (C-3). Anal. Calcd for C6H3ClN4O2: C, 36.29; H, 1.52; N, 28.22. Found: C, 36.43; H, 1.61; N, 28.45.
General Procedure for the Synthesis of 1-Substituted Derivatives 8a, b and 2-Substituted Derivatives 9a, bPotassium carbonate (530 mg, 3.84 mmol) was added into a solution of nitro derivative 7 (540 mg, 2.72 mmol) in anhydrous acetonitrile (20 mL) at 0°C and under argon, and this solution was stirred for 30 min, followed by dropwise addition of iodomethane (0.22 mL, 3.53 mmol) or 4-methoxybenzyl chloride (0.41 mL, 3 mmol). This reaction mixture was heated at 50°C for 5 h. Upon completion of the reaction, the solvent was evaporated and the residue was extracted with ethyl acetate (3×100 mL) and water (100 mL). The combined organic layers were dried over Na2SO4 and evaporated under reduced pressure. The crude product was then purified by column chromatography (silica gel) to provide pure compounds 8a, b and 9a, b.
5-Chloro-1-methyl-3-nitro-1H-pyrazolo[3,4-c]pyridine (8a) and 5-Chloro-2-methyl-3-nitro-2H-pyrazolo[3,4-c]pyridine (9a)These compounds were prepared according to the general procedure described above. Purification was effected using a mixture of cyclohexane–ethyl acetate (7 : 3, v/v) as the eluent.
Data for 8a: Yield 80%. Pale yellow solid, mp: 172°C (Et2O). 1H-NMR (400 MHz, CDCl3) δ: 4.34 (3H, s, CH3), 8.16 (1H, d, H-4, J=1.0 Hz), 8.89 (1H, d, H-7, J=1.0 Hz). 13C-NMR (50 MHz, CDCl3) δ: 38.0 (CH3), 114.4 (C-4), 123.3 (C-3a), 134.2 (C-7), 137.5 (C-7a), 145.9 (C-3). Anal. Calcd for C7H5ClN4O2: C, 39.55; H, 2.37; N, 26.35. Found: C, 39.49; H, 2.47; N, 26.52.
Data for 9a: Yield 14%. Pale yellow solid, mp: 177°C (Et2O). 1H-NMR (400 MHz, CDCl3) δ: 4.67 (3H, s, CH3), 8.03 (1H, d, H-4, J=1.0 Hz), 9.13 (1H, d, H-7, J=1.0 Hz). 13C-NMR (50 MHz, CDCl3) δ: 43.8 (CH3), 112.7 (C-4), 122.6 (C-3a), 136.9 (C-7a), 141.5 (C-5), 144.8 (C-7), 147.3 (C-3). Anal. Calcd for C7H5ClN4O2: C, 39.55; H, 2.37; N, 26.35. Found: C, 39.66; H, 2.42; N, 26.14.
5-Chloro-1-(4-methoxybenzyl)-3-nitro-1H-pyrazolo[3,4-c]pyridine (8b) and 5-Chloro-2-(4-methoxybenzyl)-3-nitro-2H-pyrazolo[3,4-c]pyridine (9b)These compounds were prepared according to the general procedure described above. Purification was effected using a mixture of cyclohexane–ethyl acetate–dichloromethane (9 : 0.5 : 0.5, v/v/v) as the eluent.
Data for 8b: Yield 80%. Yellow solid, mp: 185°C (EtOAc). 1H-NMR (400 MHz, CDCl3) δ: 3.79 (3H, s, OCH3), 5.70 (2H, s, CH2), 6.90 (2H, d, H-3′, H-5′, J=8.7 Hz), 7.30 (2H, d, H-2′, H-6′, J=8.7 Hz), 8.18 (1H, d, H-4, J=1.0 Hz), 8.67 (1H, d, H-7, J=1.0 Hz). 13C-NMR (50 MHz, CDCl3) δ: 55.3 (OCH3), 55.9 (CH2), 114.5 (C-4), 114.8 (C-3′, C-5′), 123.8 (C-3a), 124.7 (C-1′), 129.5 (C-2′, C-6′), 134.8 (C-7), 136.9 (C-7a), 145.7 (C-3), 160.4 (C-4′). Anal. Calcd for C14H11ClN4O3: C, 52.76; H, 3.48; N, 17.58. Found: C, 52.95; H, 3.60; N, 17.33.
Data for 9b: Yield 7%. Yellow solid, mp: 157°C (EtOAc). 1H-NMR (400 MHz, CDCl3) δ: 3.75 (3H, s, OCH3), 6.09 (2H, s, CH2), 6.83 (2H, d, H-3′, H-5′, J=8.7 Hz), 7.36 (2H, d, H-2′, H-6′, J=8.7 Hz), 7.99 (1H, s, H-4), 9.16 (1H, s, H-7). 13C-NMR (50 MHz, CDCl3) δ: 55.3 (OCH3), 58.7 (CH2), 112.9 (C-4), 114.4 (C-3′, C-5′), 122.9 (C-3a), 125.3 (C-1′), 130.1 (C-2′, C-6′), 136.9 (C-7a), 141.6 (C-5), 145.1 (C-7), 147.3 (C-3), 160.2 (C-4′). Anal. Calcd for C14H11ClN4O3: C, 52.76; H, 3.48; N, 17.58. Found: C, 53.01; H, 3.69; N, 17.44.
N-Cyclohexyl-1-methyl-3-nitro-1H-pyrazolo[3,4-c]pyridin-5-amine (10a)Cyclohexylamine (1.14 mL, 10 mmol) was added under argon into a solution of the 5-chloro-pyrazolopyridine 8a (425 mg, 2 mmol) in anhydrous DMSO (3 mL) and this mixture was heated at 140°C for 14 h. Upon completion of the reaction, the mixture was diluted with water (100 mL) and extracted with dichloromethane (3×100 mL). The combined organic layers were washed with water (200 mL), then with a saturated aqueous solution of NaCl (200 mL) and finally were dried over Na2SO4 and evaporated under reduced pressure. The residue was purified by column chromatography (silica gel) using a mixture of cyclohexane–dichloromethane (1 : 9, v/v) as the eluent, to provide pure 10a (275 mg, yield 50%) as an orange colored solid. mp: 148–149°C (EtOAc). 1H-NMR (400 MHz, CDCl3) δ: 1.25–1.34 (3H, m, cyclohexyl H), 1.46 (2H, m, cyclohexyl H), 1.68 (1H, m, cyclohexyl H), 1.80 (2H, m, cyclohexyl H), 2.09 (2H, m, cyclohexyl H), 3.50 (1H, m, cyclohexyl H-1′), 4.22 (3H, s, CH3), 5.24 (1H, br s, D2O exch., NH), 6.96 (1H, s, H-4), 8.69 (1H, s, H-7). 13C-NMR (50 MHz, CDCl3) δ: 24.8 (cyclohexyl C-3′, C-5′), 25.8 (cyclohexyl C-4′), 32.8 (cyclohexyl C-2′, C-6′), 38.0 (CH3), 51.3 (cyclohexyl C-1′), 91.1 (C-4), 125.6 (C-3a), 132.4 (C-7), 133.8 (C-7a), 146.0 (C-3), 155.0 (C-5). Anal. Calcd for C13H17N5O2: C, 56.72; H, 6.22; N, 25.44. Found: C, 56.49; H, 6.02; N, 25.57.
1-Methyl-3-nitro-N-phenyl-1H-pyrazolo[3,4-c]pyridin-5-amine (10b)Aniline (0.99 mL, 10.86 mmol) was added under argon to a solution of the 5-chloro-pyrazolopyridine 8a (900 mg, 4.24 mmol) in dry toluene (70 mL), followed by addition of cesium carbonate (7.3 g, 18.96 mmol), 2-dicyclohexylphosphino-2′,4′,6′-triisopropylbiphenyl (X-phos, 172 mg, 0.36 mmol) and tris(dibenzylideneacetone)dipalladium(0) (207 mg, 0.23 mmol) and the resulting mixture was refluxed for 20 h. Upon completion of the reaction, the mixture was filtered through a celite pad, the filtrate was vacuum-evaporated, extracted with ethyl acetate–water and the organic extracts were dried over Na2SO4 and evaporated to dryness. The crude product was purified by column chromatography (silica gel) using a mixture of dichloromethane–ethyl acetate (97 : 3, v/v) as the eluent, to provide pure compound 10b (670 mg, 75%) as a red solid. mp: 171–172°C (EtOAc). 1H-NMR (400 MHz, DMSO-d6) δ: 4.27 (3H, s, CH3), 6.94 (1H, t, H-4′, J=7.2 Hz), 7.30 (2H, t, H-3′, H-5′, J=7.3 Hz), 7.38 (1H, d, H-4, J=1.0 Hz), 7.55 (2H, d, H-2′, H-6′, J=8 Hz), 9.07 (1H, d, H-7, J=1.0 Hz), 9.29 (1H, s, D2O exch., NH). 13C-NMR (50 MHz, DMSO-d6) δ: 37.8 (CH3), 93.9 (C-4), 118.2 (C-2′, C-6′), 120.9 (C-4′), 123.4 (C-3a), 128.7 (C-3′, C-5′), 134.3 (C-7a), 134.9 (C-7), 141.5 (C-1′), 144.9 (C-3), 152.9 (C-5). Anal. Calcd for C13H11N5O2: C, 57.99; H, 4.12; N, 26.01. Found: C, 58.22; H, 4.16; N, 25.73.
N-Cyclohexyl-1-(4-methoxybenzyl)-3-nitro-1H-pyrazolo[3,4-c]pyridin-5-amine (10c)A mixture of compound 8b (2.50 g, 7.85 mmol) and cyclohexylamine (9 mL, 78.47 mmol) in dimethylsulfoxide (8 mL) was irradiated at 158°C (60 W, Milestone Start E) for 2 h. Upon cooling, the mixture was poured into water (300 mL) and extracted with dichloromethane (3×200 mL). The combined organic layers were washed with water (500 mL), then with a saturated aqueous solution of NaCl (400 mL) and finally were dried over Na2SO4 and evaporated under reduced pressure. The crude product was purified by column chromatography (silica gel) using a mixture of cyclohexane–ethyl acetate (1 : 1, v/v) as the eluent, to provide pure 10c (2 g, 66%) as an orange colored solid. mp: 125–126°C (CHCl3/Et2O). 1H-NMR (400 MHz, CDCl3) δ: 1.26 (3H, m, cyclohexyl H), 1.44 (2H, m, cyclohexyl H), 1.66 (1H, m, cyclohexyl H), 1.78 (2H, m, cyclohexyl H), 2.07 (2H, m, cyclohexyl H), 3.48 (1H, m, cyclohexyl H-1′), 3.79 (3H, s, OCH3), 4.90 (1H, br s, D2O exch., NH), 5.58 (2H, s, CH2), 6.87 (2H, d, H-3″, H-5″, J=8.7 Hz), 6.91 (1H, d, H-4, J=1.0 Hz), 7.28 (2H, d, H-2″, H-6″, J=8.7 Hz), 8.40 (1H, d, H-7, J=1.0 Hz). 13C-NMR (50 MHz, CDCl3) δ: 24.6 (cyclohexyl C-3′, C-5′), 25.7 (cyclohexyl C-4′), 32.7 (cyclohexyl C-2′, C-6′), 51.1 (cyclohexyl C-1′), 55.3 (OCH3), 55.6 (CH2), 91.0 (C-4), 114.7 (C-3″, C-5″), 125.6 (C-1″), 125.8 (C-3a), 129.4 (C-2″, C-6″), 132.8 (C-7a), 133.1 (C-7), 145.4 (C-3), 155.0 (C-5), 160.2 (C-4″). Anal. Calcd for C20H23N5O3: C, 62.98; H, 6.08; N, 18.36. Found: C, 63.22; H, 6.19; N, 18.09.
1-(4-Methoxybenzyl)-3-nitro-N-phenyl-1H-pyrazolo[3,4-c]pyridin-5-amine (10d)This compound was prepared following a procedure analogous to that described for the synthesis of 10b, starting from 8b. The product was purified by column chromatography (silica gel) using a mixture of cyclohexane–ethyl acetate (7 : 3, v/v) as the eluent. Yield 68%. Red solid, mp: 179°C (EtOAc). 1H-NMR (400 MHz, DMSO-d6) δ: 3.72 (3H, s, OCH3), 5.79 (2H, s, CH2), 6.92 (3H, m, H-3″, H-5″, H-4′), 7.29 (2H, t, H-3′, H-5′, J=7.5 Hz), 7.40 (3H, m, H-4, H-2″, H-6″), 7.54 (2H, d, H-2′, H-6′, J=7.5 Hz), 9.15 (1H, d, H-7, J=1.0 Hz), 9.31 (1H, s, D2O exch., NH). 13C-NMR (50 MHz, DMSO-d6) δ: 53.8 (CH2), 55.0 (OCH3), 94.3 (C-4), 114.2 (C-3″, C-5″), 118.3 (C-2′, C-6′), 121.0 (C-4′), 123.8 (C-3a), 126.9 (C-1″), 128.7 (C-3′, C-5′), 129.7 (C-2″, C-6″), 133.6 (C-7a), 134.7 (C-7), 141.4 (C-1′), 145.6 (C-3), 152.9 (C-5), 159.2 (C-4″). Anal. Calcd for C20H17N5O3: C, 63.99; H, 4.56; N, 18.66. Found: C, 64.06; H, 4.67; N, 18.58.
N5-Cyclohexyl-1-methyl-1H-pyrazolo[3,4-c]pyridine-3,5-diamine (11a)A solution of nitro derivative 10a (400 mg, 1.45 mmol) in absolute ethanol (40 mL) was hydrogenated in the presence of 10% Pd/C (80 mg) under a pressure of 55 psi at room temperature (r.t.) for 3h. The solution was filtered through a celite pad to remove the catalyst and the filtrate was evaporated to dryness. The residue was purified by column chromatography (silica gel) using a mixture of dichloromethane–methanol (99 : 1, v/v) as the eluent, to provide pure 11a (280 mg, 79%) as a yellow solid. mp: 179–180°C (EtOAc). 1H-NMR (400 MHz, CDCl3) δ: 1.11–1.24 (3H, m, cyclohexyl H), 1.32 (2H, m, cyclohexyl H), 1.60 (1H, m, cyclohexyl H), 1.72 (2H, m, cyclohexyl H), 2.02 (2H, m, cyclohexyl H), 3.29 (1H, m, cyclohexyl H-1′), 3.80 (3H, s, CH3), 4.18 (3H, br s, D2O exch., NH2, NH), 6.33 (1H, d, H-4, J=1.0 Hz), 8.28 (1H, d, H-7, J=1.0 Hz). 13C-NMR (50 MHz, CDCl3) δ: 24.9 (cyclohexyl C-3′, C-5′), 25.8 (cyclohexyl C-4′), 33.1 (cyclohexyl C-2′, C-6′), 35.3 (CH3), 51.7 (cyclohexyl C-1′), 91.5 (C-4), 122.5 (C-3a), 130.3 (C-7), 134.3 (C-7a), 145.4 (C-3), 150.9 (C-5). Anal. Calcd for C13H19N5: C, 63.65; H, 7.81; N, 28.55. Found: C, 63.97; H, 8.08; N, 28.29.
1-Methyl-N5-phenyl-1H-pyrazolo[3,4-c]pyridine-3,5-diamine (11b)This compound was prepared following an analogous procedure to that described for the synthesis of 11a, starting from the nitro derivative 10b. The product was purified by column chromatography (silica gel) using a mixture of cyclohexane–ethyl acetate (from 5 : 5 up to 2 : 8, v/v) as the eluent, to provide pure 11b as a green solid, in 84% yield. mp: 178–179°C (EtOAc). 1H-NMR (400 MHz, DMSO-d6) δ: 3.75 (3H, s, CH3), 5.61 (2H, br s, D2O exch., NH2), 6.78 (1H, t, H-4′, J=7.2 Hz), 7.21 (3H, m, H-3′, H-5′, H-4), 7.37 (2H, dd, H-2′, H-6′, J=8.0 Hz, J=0.9 Hz), 8.56 (2H, m, H-7, NH-aniline). 13C-NMR (50 MHz, DMSO-d6) δ: 35.1 (CH3), 97.9 (C-4), 116.2 (C-2′, C-6′), 118.8 (C-4′), 121.1 (C-3a), 128.6 (C-3′, C-5′), 130.4 (C-7), 134.8 (C-7a), 143.5 (C-1′), 146.4 (C-5), 147.3 (C-3). Anal. Calcd for C13H13N5: C, 65.26; H, 5.48; N, 29.27. Found: C, 65.03; H, 5.74; N, 29.49.
N5-Cyclohexyl-1-(4-methoxybenzyl)-1H-pyrazolo[3,4-c]pyridine-3,5-diamine (11c)This compound was prepared following an analogous procedure to that described for the synthesis of 11a, starting from the nitro derivative 10c. The product was purified by column chromatography (silica gel) using a mixture of dichloromethane–ethyl acetate (8 : 2, v/v) as the eluent, providing 11c as a yellow solid, in 85% yield. mp: 142–143°C (Et2O). 1H-NMR (400 MHz, CDCl3) δ: 1.20 (3H, m, cyclohexyl H), 1.33 (2H, m, cyclohexyl H), 1.60 (1H, m, cyclohexyl H), 1.72 (2H, m, cyclohexyl H), 2.02 (2H, m, cyclohexyl H), 3.29 (1H, m, cyclohexyl H-1′), 3.72 (3H, s, OCH3), 4.14 (2H, br s, D2O exch., NH2), 5.21 (2H, s, CH2), 6.32 (1H, d, H-4, J=1.0 Hz), 6.78 (2H, d, H-3″, H-5″, J=8.7 Hz), 7.13 (2H, d, H-2″, H-6″, J=8.7 Hz), 8.20 (1H, d, H-7, J=1.0 Hz). 13C-NMR (50 MHz, CDCl3) δ: 25.0 (cyclohexyl C-3′, C-5′), 25.9 (cyclohexyl C-4′), 33.2 (cyclohexyl C-2′, C-6′), 51.6 (cyclohexyl C-1′), 52.6 (CH2), 55.3 (OCH3), 91.2 (C-4), 114.1 (C-3″, C-5″), 123.0 (C-3a), 128.8 (C-2″, C-6″), 129.2 (C-1″), 131.4 (C-7), 133.9 (C-7a), 145.8 (C-3), 151.4 (C-5), 159.2 (C-4″). Anal. Calcd for C20H25N5O: C, 68.35; H, 7.17; N, 19.93. Found: C, 68.08; H, 7.04; N, 20.22.
1-(4-Methoxybenzyl)-N5-phenyl-1H-pyrazolo[3,4-c]pyridine-3,5-diamine (11d)Ten percent Pd/C (5 mg) and triethylsilane (0.34 mL, 2.13 mmol) were added into a solution of the nitro derivative 10d (80 mg, 0.21 mmol) in anhydrous methanol (5 mL), under argon, and the resulting solution was stirred at r.t. for 12h. The solution was filtered through a celite pad to remove the catalyst and the filtrate was evaporated to dryness. The residue was purified by column chromatography (silica gel) using a mixture of cyclohexane–ethyl acetate (8 : 2, v/v) as the eluent, to provide pure 11d (60 mg, 82%) as a brown solid. mp: 148°C (Et2O). 1H-NMR (400 MHz, CDCl3) δ: 3.78 (3H, s, OCH3), 4.64 (2H, br s, D2O exch., NH2), 5.27 (2H, s, CH2), 6.84 (2H, d, H-3″, H-5″, J=8.6 Hz), 7.06 (2H, m, H-4′, H-4), 7.18 (2H, d, H-2″, H-6″, J=8.6 Hz), 7.23 (2H, d, H-2′, H-6′, J=7.6 Hz), 7.32 (2H, t, H-3′, H-5′, J=7.6 Hz), 7.58 (1H, br s, D2O exch., NH), 8.20 (1H, s, H-7). 13C-NMR (50 MHz, CDCl3) δ: 53.1 (CH2), 55.3 (OCH3), 96.7 (C-4), 114.3 (C-3″, C-5″), 120.6 (C-2′, C-6′), 123.5 (C-4′), 124.2 (C-3a), 127.0 (C-7), 127.9 (C-1″), 128.9 (C-2″, C-6″), 129.6 (C-3′, C-5′), 133.2 (C-7a), 140.1 (C-1′), 146.2 (C-3), 146.5 (C-5), 159.6 (C-4″). Anal. Calcd for C20H19N5O: C, 69.55; H, 5.54; N, 20.28. Found: C, 69.29; H, 5.37; N, 20.46.
General Procedure for the Synthesis of Derivatives 12a–dChloroacetyl chloride (96 µL, 1.2 mmol) and triethylamine (153 µL, 1.1 mmol) were added into a solution of the amines 11a–d (1 mmol) in anhydrous tetrahydrofuran (THF) (15 mL), under argon, at −40°C for amines 11a, c or at 0°C for amines 11b, d. The resulting solution was allowed to reach r.t. and stirring was continued for one more hour. The solvents were then vacuum-evaporated and the residue was used imediately to the next step, without any further purification.
General Procedure for the Synthesis of Derivatives 13a–lDimethylamine (5.6 M solution in ethanol, 0.89 mL, 5 mmol) or 1-methylpiperazine (0.22 mL, 2 mmol) or aniline (0.36 mL, 4 mmol) was added into a solution of the intermediate chlorides 12a–d (1 mmol) in absolute ethanol (10 mL), under argon, and the resulting solution was heated at 80°C for 4 h. Upon completion of the reaction solvents were evaporated and the residue was purified by column chromatography to provide pure amides 13a–l.
N-(5-(Cyclohexylamino)-1-methyl-1H-pyrazolo[3,4-c]pyridin-3-yl)-2-(dimethylamino)acetamide (13a)This compound was prepared according to the general procedure described above, upon reaction of the chloride 12a with dimethylamine (5.6 M solution in ethanol). The product was purified by column chromatography (silica gel) using a mixture of dichloromethane–methanol (100 : 1, v/v) as the eluent to provide pure 13a as a green oil. Yield: 52%. 1H-NMR (400 MHz, CDCl3) δ: 1.22 (3H, m, cyclohexyl H), 1.40 (2H, m, cyclohexyl H), 1.61 (1H, m, cyclohexyl H), 1.73 (2H, m, cyclohexyl H), 2.05 (2H, m, cyclohexyl H), 2.42 (6H, s, N(CH3)2), 3.17 (2H, s, CH2), 3.44 (1H, m, cyclohexyl H-1′), 3.94 (3H, s, CH3–pyrazole), 6.83 (1H, s, H-4), 8.39 (1H, s, H-7), 9.55 (1H, s, D2O exch., NHCO). 13C-NMR (50 MHz, CDCl3) δ: 24.8 (cyclohexyl C-3′, C-5′), 25.9 (cyclohexyl C-4′), 33.2 (cyclohexyl C-2′, C-6′), 35.7 (CH3–pyrazole), 46.0 (N(CH3)2), 51.2 (cyclohexyl C-1′), 63.0 (CH2), 93.8 (C-4), 123.9 (C-3a), 131.3 (C-7), 134.2 (C-7a), 136.9 (C-3), 152.0 (C-5), 168.6 (CO). HR-MS (electrospray ionization (ESI)) m/z: Calcd for C17H27N6O: [M1+H]+=331.2241. Found 331.2247. Anal. Calcd for C17H26N6O: C, 61.79; H, 7.93; N, 25.43. Found: C, 61.69; H, 7.76; N, 25.55.
N-(5-(Cyclohexylamino)-1-methyl-1H-pyrazolo[3,4-c]pyridin-3-yl)-2-(4-methylpiperazin-1-yl)acetamide (13b)This compound was prepared according to the general procedure described above, upon reaction of the chloride 12a with 1-methylpiperazine. The product was purified by column chromatography (silica gel) using a mixture of dichloromethane–methanol (94 : 6, v/v) as the eluent to provide pure 13b as a yellow solid. Yield: 38%, mp: 154°C (dec.) (Et2O). 1H-NMR (400 MHz, CDCl3) δ: 1.23 (3H, m, cyclohexyl H), 1.40 (2H, m, cyclohexyl H), 1.62 (1H, m, cyclohexyl H), 1.74 (2H, m, cyclohexyl H), 2.06 (2H, m, cyclohexyl H), 2.39 (3H, s, CH3–piperazine), 2.63 (4H, br s, piperazine H-3″, H-5″), 2.77 (4H, br s, piperazine H-2″, H-6″), 3.24 (2H, s, CH2), 3.45 (1H, m, cyclohexyl H-1′), 3.96 (3H, s, CH3–pyrazole), 6.78 (1H, d, H-4, J=1.0 Hz), 8.41 (1H, d, H-7, J=1.0 Hz), 9.40 (1H, s, D2O exch., NHCO). 13C-NMR (50 MHz, CDCl3) δ: 24.9 (cyclohexyl C-3′, C-5′), 26.0 (cyclohexyl C-4′), 33.3 (cyclohexyl C-2′, C-6′), 35.8 (CH3–pyrazole), 45.7 (CH3–piperazine), 51.3 (cyclohexyl C-1′), 53.1 (piperazine C-2″, C-6″), 55.0 (piperazine C-3″, C-5″), 61.5 (CH2), 93.6 (C-4), 124.0 (C-3a), 131.6 (C-7), 134.3 (C-7a), 136.8 (C-3), 152.1 (C-5), 168.2 (CO). HR-MS (ESI) m/z: Calcd for C20H32N7O: [M1+H]+=386.2663. Found 386.2665. Anal. Calcd for C20H31N7O: C, 62.31; H, 8.11; N, 25.43. Found: C, 62.56; H, 8.22; N, 25.29.
N-(5-(Cyclohexylamino)-1-methyl-1H-pyrazolo[3,4-c]pyridin-3-yl)-2-(phenylamino)acetamide (13c)This compound was prepared according to the general procedure described above, upon reaction of the chloride 12a with aniline. The product was purified by column chromatography (silica gel) using a mixture of dichloromethane–methanol (100 : 1, v/v) as the eluent to provide pure 13c as a yellow solid. Yield: 35%, mp: 175–176°C (Et2O). 1H-NMR (400 MHz, CDCl3) δ: 1.20–1.31 (3H, m, cyclohexyl H), 1.42 (2H, m, cyclohexyl H), 1.66 (1H, m, cyclohexyl H), 1.78 (2H, m, cyclohexyl H), 2.07 (2H, m, cyclohexyl H), 3.44 (1H, m, cyclohexyl H-1′), 3.91 (3H, s, CH3), 4.00 (2H, s, CH2), 4.57 (1H, br s, D2O exch., NH-cyclohexylamine), 6.71 (2H, d, H-2″, H-6″, J=7.9 Hz), 6.74 (1H, s, H-4), 6.85 (1H, t, H-4″, J=7.3 Hz), 7.24 (2H, t, H-3″, H-5″, J=7.5 Hz), 8.38 (1H, s, H-7), 9.10 (1H, br s, D2O exch., NHCO). 13C-NMR (50 MHz, CDCl3) δ: 24.8 (cyclohexyl C-3′, C-5′), 25.9 (cyclohexyl C-4′), 33.1 (cyclohexyl C-2′, C-6′), 35.7 (CH3), 49.1 (CH2), 51.2 (cyclohexyl C-1′), 93.2 (C-4), 113.4 (C-2″, C-6″), 119.5 (C-4″), 124.4 (C-3a), 129.5 (C-3″, C-5″), 131.1 (C-7), 134.0 (C-7a), 136.4 (C-3), 147.0 (C-1″), 151.9 (C-5), 169.2 (CO). HR-MS (ESI) m/z: Calcd for C21H27N6O: [M1+H]+=379.2241. Found 379.2244. Anal. Calcd for C21H26N6O: C, 66.64; H, 6.92; N, 22.20. Found: C, 66.48; H, 6.69; N, 22.43.
2-(Dimethylamino)-N-(1-methyl-5-(phenylamino)-1H-pyrazolo[3,4-c]pyridin-3-yl)acetamide (13d)This compound was prepared according to the general procedure described above, upon reaction of the chloride 12b with dimethylamine (5.6 M solution in ethanol). The product was purified by column chromatography (silica gel) using a mixture of dichloromethane–methanol (100 : 2, v/v) as the eluent to provide pure 13d as a green solid. Yield: 86%, mp: 166–167°C (EtOH). 1H-NMR (400 MHz, CDCl3) δ: 2.41 (6H, s, N(CH3)2), 3.15 (2H, s, CH2), 4.00 (3H, s, CH3–pyrazole), 6.64 (1H, s, D2O exch., NH–aniline), 6.95 (1H, t, H-4′, J=7.2 Hz), 7.30 (4H, m, H-2′, H-3′, H-5′, H-6′), 7.57 (1H, d, H-4, J=1.2 Hz), 8.54 (1H, d, H-7, J=1.2 Hz), 9.59 (1H, s, D2O exch., NHCO). 13C-NMR (50 MHz, CDCl3) δ: 35.7 (CH3–pyrazole), 46.0 (N(CH3)2), 63.0 (CH2), 98.7 (C-4), 117.8 (C-2′, C-6′), 121.1 (C-4′), 123.2 (C-3a), 129.2 (C-3′, C-5′), 131.5 (C-7), 135.0 (C-7a), 137.5 (C-3), 142.1 (C-1′), 147.9 (C-5), 168.8 (CO). HR-MS (ESI) m/z: Calcd for C17H21N6O: [M1+H]+=325.1771. Found 325.1778. Anal. Calcd for C17H20N6O: C, 62.95; H, 6.21; N, 25.91. Found: C, 63.04; H, 6.26; N, 25.78.
N-(1-Methyl-5-(phenylamino)-1H-pyrazolo[3,4-c]pyridin-3-yl)-2-(4-methylpiperazin-1-yl)acetamide (13e)This compound was prepared according to the general procedure described above, upon reaction of the chloride 12b with 1-methylpiperazine. The product was purified by column chromatography (silica gel) using a mixture of dichloromethane–methanol (9 : 1, v/v) as the eluent to provide pure 13e as a yellow solid. Yield: 93%, mp: 196–197°C (EtOH). 1H-NMR (400 MHz, DMSO-d6) δ: 2.18 (3H, s, CH3–piperazine), 2.38 (4H, br s, piperazine H-3″, H-5″), 2.57 (4H, br s, piperazine H-2″, H-6″), 3.20 (2H, s, CH2), 4.01 (3H, s, CH3–pyrazole), 6.80 (1H, t, H-4′, J=7.2 Hz), 7.21 (3H, m, H-3′, H-5′, H-4), 7.45 (2H, d, H-2′, H-6′, J=8.0 Hz), 8.74 (1H, s, D2O exch., NH–aniline), 8.80 (1H, d, H-7, J=1.0 Hz), 10.14 (1H, br s, D2O exch., NHCO). 13C-NMR (50 MHz, DMSO-d6) δ: 35.5 (CH3–pyrazole), 45.6 (CH3–piperazine), 52.6 (piperazine C-2″, C-6″), 54.5 (piperazine C-3″, C-5″), 60.8 (CH2), 98.4 (C-4), 116.5 (C-2′, C-6′), 119.2 (C-4′), 123.2 (C-3a), 128.6 (C-3′, C-5′), 131.8 (C-7), 134.2 (C-7a), 137.2 (C-3), 143.1 (C-1′), 147.8 (C-5), 168.2 (CO). HR-MS (ESI) m/z: Calcd for C20H26N7O: [M1+H]+=380.2193. Found 380.2200. Anal. Calcd for C20H25N7O: C, 63.30; H, 6.64; N, 25.84. Found: C, 63.41; H, 6.79; N, 25.73.
N-(1-Methyl-5-(phenylamino)-1H-pyrazolo[3,4-c]pyridin-3-yl)-2-(phenylamino)acetamide (13f)This compound was prepared according to the general procedure described above, upon reaction of the chloride 12b with aniline. The product was purified by column chromatography (silica gel) using a mixture of dichloromethane–ethyl acetate (1 : 1, v/v) as the eluent to provide pure 13f as a yellow solid. Yield: 70%, mp: 180–181°C (EtOH). 1H-NMR (400 MHz, DMSO-d6) δ: 3.95 (2H, d, CH2, J=6.0 Hz), 4.01 (3H, s, CH3), 6.04 (1H, t, CH2–NH–, J=6.0 Hz), 6.60 (3H, m, H-2″, H-4″, H-6″), 6.80 (1H, t, H-4′, J=7.2 Hz), 7.11 (2H, t, H-3″, H-5″, J=7.7 Hz), 7.20 (3H, m, H-3′, H-5′, H-4), 7.42 (2H, d, H-2′, H-6′, J=7.8 Hz), 8.72 (1H, s, D2O exch., NH–aniline), 8.79 (1H, d, H-7, J=0.8 Hz), 10.50 (1H, br s, D2O exch., NHCO). 13C-NMR (50 MHz, DMSO-d6) δ: 36.1 (CH3), 47.0 (CH2), 98.9 (C-4), 112.7 (C-2″, C-6″), 116.9 (C-4″), 117.0 (C-2′, C-6′), 119.7 (C-4′), 123.6 (C-3a), 129.1 (C-3′, C-5′), 129.4 (C-3″, C-5″), 132.4 (C-7), 134.7 (C-7a), 137.9 (C-3), 143.7 (C-1′), 148.3 (C-5), 148.7 (C-1″), 169.9 (CO). HR-MS (ESI) m/z: Calcd for C21H21N6O: [M1+H]+=373.1771. Found 373.1772. Anal. Calcd for C21H20N6O: C, 67.73; H, 5.41; N, 22.57. Found: C, 67.94; H, 5.56; N, 22.28.
N-(5-(Cyclohexylamino)-1-(4-methoxybenzyl)-1H-pyrazolo[3,4-c]pyridin-3-yl)-2-(dimethylamino)acetamide (13g)This compound was prepared according to the general procedure described above, upon reaction of the chloride 12c with dimethylamine (5.6 M solution in ethanol). The product was purified by column chromatography (silica gel) using a mixture of dichloromethane–methanol (100 : 1, v/v) as the eluent to provide pure 13g as a yellow oil. Yield: 57%. 1H-NMR (400 MHz, CDCl3) δ: 1.18 (3H, m, cyclohexyl H), 1.36 (2H, m, cyclohexyl H), 1.57 (1H, m, cyclohexyl H), 1.69 (2H, m, cyclohexyl H), 2.01 (2H, m, cyclohexyl H), 2.36 (6H, s, N(CH3)2), 3.11 (2H, s, NHCOCH2), 3.42 (1H, m, cyclohexyl H-1″), 3.70 (3H, s, OCH3), 4.30 (1H, br s, D2O exchang., NH–cyclohexylamine), 5.32 (2H, s, CH2–pyrazole), 6.77 (2H, d, H-3′, H-5′, J=8.6 Hz), 6.84 (1H, s, H-4), 7.11 (2H, d, H-2′, H-6′, J=8.6 Hz), 8.28 (1H, s, H-7), 9.55 (1H, s, D2O exchang., NHCO). 13C-NMR (50 MHz, CDCl3) δ: 24.6 (cyclohexyl C-3″, C-5″), 25.7 (cyclohexyl C-4″), 33.0 (cyclohexyl C-2″, C-6″), 45.9 (N(CH3)2), 50.9 (cyclohexyl C-1″), 52.7 (CH2–pyrazole), 55.0 (OCH3), 62.9 (NHCOCH2), 93.9 (C-4), 114.0 (C-3′, C-5′), 124.2 (C-3a), 128.1 (C-1′), 128.5 (C-2′, C-6′), 131.6 (C-7), 133.5 (C-7a), 137.3 (C-3), 151.8 (C-5), 159.2 (C-4′), 168.5 (CO). HR-MS (ESI) m/z: Calcd for C24H33N6O2: [M1+H]+=437.2660. Found 437.2666. Anal. Calcd for C24H32N6O2: C, 66.03; H, 7.39; N, 19.25. Found: C, 66.21; H, 7.52; N, 19.01.
N-(5-(Cyclohexylamino)-1-(4-methoxybenzyl)-1H-pyrazolo[3,4-c]pyridin-3-yl)-2-(4-methylpiperazin-1-yl)acetamide (13h)This compound was prepared according to the general procedure described above, upon reaction of the chloride 12c with 1-methylpiperazine. The product was purified by column chromatography (silica gel) using a mixture of dichloromethane–methanol (96 : 4, v/v) as the eluent to provide pure 13h as a yellow solid. Yield: 98%, mp: 157–158°C (CHCl3/Et2O). 1H-NMR (400 MHz, CDCl3) δ: 1.21 (3H, m, cyclohexyl H), 1.40 (2H, m, cyclohexyl H), 1.62 (1H, m, cyclohexyl H), 1.74 (2H, m, cyclohexyl H), 2.04 (2H, m, cyclohexyl H), 2.47 (3H, s, CH3–piperazine), 2.74 (4H, br s, piperazine H-3″′, H-5‴), 2.84 (4H, br s, piperazine H-2″′, H-6‴), 3.26 (2H, s, NHCOCH2), 3.44 (1H, m, cyclohexyl H-1″), 3.77 (3H, s, OCH3), 4.29 (1H, br s, D2O exchang., NH–cyclohexylamine), 5.37 (2H, s, CH2–pyrazole), 6.80 (1H, d, H-4, J=1.0 Hz), 6.83 (2H, d, H-3′, H-5′, J=8.7 Hz), 7.16 (2H, d, H-2′, H-6′, J=8.7 Hz), 8.31 (1H, d, H-7, J=1.0 Hz), 9.34 (1H, s, D2O exchang., NHCO). 13C-NMR (50 MHz, CDCl3) δ: 24.9 (cyclohexyl C-3″, C-5″), 26.0 (cyclohexyl C-4″), 33.3 (cyclohexyl C-2″, C-6″), 45.4 (CH3–piperazine), 51.2 (cyclohexyl C-1″), 52.7 (piperazine C-2″′, C-6‴), 53.1 (CH2–pyrazole), 54.8 (piperazine C-3″′, C-5‴), 55.4 (OCH3), 61.5 (NHCOCH2), 94.0 (C-4), 114.4 (C-3′, C-5′), 124.6 (C-3a), 128.4 (C-1′), 128.9 (C-2″, C-6″), 132.1 (C-7), 133.8 (C-7a), 137.3 (C-3), 152.2 (C-5), 159.6 (C-4″), 168.0 (CO). HR-MS (ESI) m/z: Calcd for C27H38N7O2: [M1+H]+=492.3081. Found 492.3085. Anal. Calcd for C27H37N7O2: C, 65.96; H, 7.59; N, 19.94. Found: C, 65.78; H, 7.51; N, 20.20.
N-(5-(Cyclohexylamino)-1-(4-methoxybenzyl)-1H-pyrazolo[3,4-c]pyridin-3-yl)-2-(phenylamino)acetamide (13i)This compound was prepared according to the general procedure described above, upon reaction of the chloride 12c with aniline. The product was purified by column chromatography (silica gel) using a mixture of dichloromethane–methanol (100 : 1, v/v) as the eluent to provide pure 13i as a yellow solid. Yield: 46%, mp: 152°C (CHCl3/Et2O). 1H-NMR (400 MHz, CDCl3) δ: 1.24 (3H, m, cyclohexyl H), 1.40 (2H, m, cyclohexyl H), 1.64 (1H, m, cyclohexyl H), 1.75 (2H, m, cyclohexyl H), 2.05 (2H, m, cyclohexyl H), 3.44 (1H, m, cyclohexyl H-1″), 3.74 (3H, s, OCH3), 3.97 (2H, d, COCH2NH, J=5.2 Hz), 4.32 (1H, br s, D2O exchang., NH–cyclohexylamine), 4.45 (1H, t, D2O exchang., COCH2NH, J=5.2 Hz), 5.31 (2H, s, CH2–pyrazole), 6.70 (2H, d, H-2″′, H-6″′, J=7.7 Hz), 6.76 (1H, s, H-4), 6.78 (2H, d, H-3′, H-5′, J=8.5 Hz), 6.84 (1H, t, H-4″′, J=7.2 Hz), 7.11 (2H, d, H-2′, H-6′, J=8.5 Hz), 7.23 (2H, t, H-3″′, H-5″′, J=7.5 Hz), 8.30 (1H, s, H-7), 9.05 (1H, s, D2O exchang., NHCO). 13C-NMR (50 MHz, CDCl3) δ: 24.8 (cyclohexyl C-3″, C-5″), 25.9 (cyclohexyl C-4″), 33.0 (cyclohexyl C-2″, C-6″), 49.3 (NHCOCH2), 51.2 (cyclohexyl C-1″), 53.2 (CH2–pyrazole), 55.3 (OCH3), 94.1 (C-4), 113.5 (C-2″′, C-6‴), 114.3 (C-3′, C-5′), 119.7 (C-4‴), 125.0 (C-3a), 127.9 (C-1′), 128.8 (C-2′, C-6′), 129.6 (C-3″′, C-5‴), 130.9 (C-7), 133.3 (C-7a), 136.9 (C-3), 146.9 (C-1‴), 151.6 (C-5), 159.5 (C-4′), 169.0 (CO). HR-MS (ESI) m/z: Calcd for C28H33N6O2: [M1+H]+=485.2660. Found 485.2663. Anal. Calcd for C28H32N6O2: C, 69.40; H, 6.66; N, 17.34. Found: C, 69.63; H, 6.80; N, 17.12.
2-(Dimethylamino)-N-(1-(4-methoxybenzyl)-5-phenylamino-1H-pyrazolo[3,4-c]pyridin-3-yl)acetamide (13j)This compound was prepared according to the general procedure described above, upon reaction of the chloride 12d with dimethylamine (5.6 M solution in ethanol). The product was purified by column chromatography (silica gel) using a mixture of dichloromethane–ethyl acetate (6 : 4, v/v) as the eluent to provide pure 13j as a pale green solid. Yield: 70%, mp: 143°C (Et2O). 1H-NMR (400 MHz, CDCl3) δ: 2.36 (6H, s, N(CH3)2), 3.12 (2H, s, NHCOCH2), 3.72 (3H, s, OCH3), 5.36 (2H, s, CH2–pyrazole), 6.82 (2H, d, H-3″, H-5″, J=8.7 Hz), 6.90 (2H, m, H-4′, NH–aniline), 7.17 (2H, d, H-2″, H-6″, J=8.7 Hz), 7.26 (4H, m, H-2′, H-3′, H-5′, H-6′), 7.59 (1H, d, H-4, J=1.0 Hz), 8.45 (1H, d, H-7, J=1.0 Hz), 9.69 (1H, br s, D2O exch., NHCO). 13C-NMR (50 MHz, CDCl3) δ: 46.0 (N(CH3)2), 53.0 (CH2–pyrazole), 55.3 (OCH3), 63.0 (NHCOCH2), 99.1 (C-4), 114.3 (C-3″, C-5″), 117.9 (C-2′, C-6′), 121.1 (C-4′), 123.8 (C-3a), 128.1 (C-1″), 128.9 (C-2″, C-6″), 129.2 (C-3′, C-5′), 131.9 (C-7), 134.5 (C-7a), 138.1 (C-3), 142.2 (C-1′), 148.1 (C-5), 159.5 (C-4″), 168.9 (CO). HR-MS (ESI) m/z: Calcd for C24H27N6O2: [M1+H]+=431.2190. Found 431.2191. Anal. Calcd for C24H26N6O2: C, 66.96; H, 6.09; N, 19.52. Found: C, 67.11; H, 6.24; N, 19.39.
N-(1-(4-Methoxybenzyl)-5-(phenylamino)-1H-pyrazolo[3,4-c]pyridin-3-yl)-2-(4-methylpiperazin-1-yl)acetamide (13k)This compound was prepared according to the general procedure described above, upon reaction of the chloride 12d with 1-methylpiperazine. The product was purified by column chromatography (silica gel) using a mixture of dichloromethane–methanol (98 : 2, v/v) as the eluent to provide pure 13k as a pale green solid. Yield: 70%, mp: 134°C (CH2Cl2/Et2O). 1H-NMR (400 MHz, CDCl3) δ: 2.31 (3H, s, CH3–piperazine), 2.52 (4H, br s, piperazine H-3″′, H-5‴), 2.68 (4H, br s, piperazine H-2″′, H-6‴), 3.18 (2H, s, NHCOCH2), 3.74 (3H, s, OCH3), 5.40 (2H, s, CH2–pyrazole), 6.74 (1H, br s, NH), 6.82 (2H, d, H-3″, H-5″, J=8.7 Hz), 6.92 (1H, m, H-4′), 7.17 (2H, d, H-2″, H-6″, J=8.7 Hz), 7.26 (4H, m, H-2′, H-3′, H-5′, H-6′), 7.51 (1H, d, H-4, J=1.0 Hz), 8.42 (1H, d, H-7, J=1.0 Hz), 9.51 (1H, br s, D2O exch., NHCO). 13C-NMR (50 MHz, CDCl3) δ: 45.8 (CH3–piperazine), 53.1 (CH2–pyrazole), 53.3 (piperazine C-2″′, C-6‴), 54.9 (piperazine C-3″′, C-5‴), 55.3 (OCH3), 61.4 (NHCOCH2), 98.7 (C-4), 114.3 (C-3″, C-5″), 118.0 (C-2′, C-6′), 121.2 (C-4′), 123.9 (C-3a), 128.0 (C-1″), 128.8 (C-2″, C-6″), 129.2 (C-3′, C-5′), 132.0 (C-7), 134.5 (C-7a), 137.8 (C-3), 142.1 (C-1′), 148.1 (C-5), 159.5 (C-4″), 168.4 (CO). HR-MS (ESI) m/z: Calcd for C27H32N7O2: [M1+H]+=486.2612. Found 486.2619. Anal. Calcd for C27H31N7O2: C, 66.78; H, 6.43; N, 20.19. Found: C, 66.94; H, 6.33; N, 20.07.
N-(1-(4-Methoxybenzyl)-5-(phenylamino)-1H-pyrazolo[3,4-c]pyridin-3-yl)-2-(phenylamino)acetamide (13l)This compound was prepared according to the general procedure described above, upon reaction of the chloride 12d with aniline. The product was purified by column chromatography (silica gel) using a mixture of dichloromethane–methanol (100 : 1, v/v) as the eluent to provide pure 13l as a beige solid. Yield: 68%, mp: 208–209°C (CHCl3/Et2O). 1H-NMR (400 MHz, DMSO-d6) δ: 3.70 (3H, s, OCH3), 3.93 (2H, br s, NHCOCH2), 5.50 (2H, s, CH2–pyrazole), 6.00 (1H, br s, CH2NH), 6.58 (1H, t, H-4″′, J=7.2 Hz), 6.61 (2H, d, H-2″′, H-6″′, J=7.8 Hz), 6.79 (1H, t, H-4′, J=7.2 Hz), 6.88 (2H, d, H-3″, H-5″, J=8.5 Hz), 7.10 (2H, t, H-3″′, H-5″′, J=7.5 Hz), 7.19 (3H, m, H-3′, H-5′, H-4), 7.27 (2H, d, H-2″, H-6″, J=8.5 Hz), 7.41 (2H, d, H-2′, H-6′, J=8.2 Hz), 8.73 (1H, s, D2O exch., NH–aniline), 8.86 (1H, s, H-7), 10.57 (1H, s, D2O exch., NHCO). 13C-NMR (50 MHz, DMSO-d6) δ: 46.4 (NHCOCH2), 51.6 (CH2–pyrazole), 55.0 (OCH3), 98.7 (C-4), 112.2 (C-2″′, C-6‴), 113.9 (C-3″, C-5″), 116.3 (C-4‴), 116.6 (C-2′, C-6′), 119.2 (C-4′), 123.5 (C-3a), 128.6 (C-3′, C-5′), 128.9 (C-3″′, C-5‴), 129.0 (C-1″), 129.2 (C-2″, C-6″), 131.9 (C-7), 133.6 (C-7a), 138.0 (C-3), 143.0 (C-1′), 147.9 (C-5), 148.1 (C-1‴), 158.8 (C-4″), 169.4 (CO). HR-MS (ESI) m/z: Calcd for C28H27N6O2: [M1+H]+=479.2190. Found 479.2194. Anal. Calcd for C28H26N6O2: C, 70.28; H, 5.48; N, 17.56. Found: C, 70.03; H, 5.39; N, 17.66.
General Procedure for the Synthesis of Derivatives 14a–fA solution of the compounds 13g–l (0.2 mmol) in trifluoroacetic acid (5 mL) was heated at 70°C for 96 h. Then the solvent was vacuum-evaporated and the residue was neutralized with sodium bicarbonate and extracted with ethyl acetate (3×50 mL). The combined organic extracts were dried (Na2SO4), concentrated under vacuum and the residue was purified by column chromatography to provide pure target compounds 14a–f.
N-(5-(Cyclohexylamino)-1H-pyrazolo[3,4-c]pyridin-3-yl)-2-(dimethylamino)acetamide (14a)This compound was prepared according to the general procedure described above, starting from 13g. The product was purified by column chromatography (silica gel) using a mixture of dichloromethane–methanol (95 : 5, v/v) as the eluent to provide pure 14a as a green solid. Yield: 80%, mp: 132–133°C (Et2O/n-pentane). 1H-NMR (400 MHz, CDCl3) δ: 1.18 (3H, m, cyclohexyl H), 1.32 (2H, m, cyclohexyl H), 1.57 (1H, m, cyclohexyl H), 1.68 (2H, m, cyclohexyl H), 2.01 (2H, m, cyclohexyl H), 2.38 (6H, s, N(CH3)2), 3.17 (2H, s, CH2), 3.40 (1H, m, cyclohexyl H-1′), 4.23 (1H, br s, D2O exchang., NH–cyclohexylamine), 6.80 (1H, s, H-4), 8.37 (1H, s, H-7), 9.62 (1H, br s, D2O exchang., NHCO), 11.62 (1H, br s, D2O exchang., NH–pyrazole). 13C-NMR (50 MHz, CDCl3) δ: 24.7 (cyclohexyl C-3′, C-5′), 25.8 (cyclohexyl C-4′), 33.0 (cyclohexyl C-2′, C-6′), 46.0 (N(CH3)2), 51.2 (cyclohexyl C-1′), 63.0 (CH2), 93.3 (C-4), 123.3 (C-3a), 132.6 (C-7), 134.2 (C-7a), 138.4 (C-3), 151.8 (C-5), 169.2 (CO). HR-MS (ESI) m/z: Calcd for C16H25N6O: [M1+H]+=317.2084. Found 317.2088. Anal. Calcd for C16H24N6O: C, 60.74; H, 7.65; N, 26.56. Found: C, 61.01; H, 7.80; N, 26.33.
N-(5-(Cyclohexylamino)-1H-pyrazolo[3,4-c]pyridin-3-yl)-2-(4-methylpiperazin-1-yl)acetamide (14b)This compound was prepared according to the general procedure described above, starting from 13h. The product was purified by column chromatography (silica gel) using a mixture of dichloromethane–methanol (10 : 1, v/v) as the eluent to provide pure 14b as a pale yellow solid. Yield: 88%, mp: 145°C (Et2O). 1H-NMR (400 MHz, CDCl3) δ: 1.23 (3H, m, cyclohexyl H), 1.40 (2H, m, cyclohexyl H), 1.63 (1H, m, cyclohexyl H), 1.74 (2H, m, cyclohexyl H), 2.06 (2H, m, cyclohexyl H), 2.33 (3H, s, CH3), 2.54 (4H, br s, piperazine H-3″, H-5″), 2.72 (4H, br s, piperazine H-2″, H-6″), 3.24 (2H, s, CH2), 3.44 (1H, m, cyclohexyl H-1′), 4.33 (1H, br s, D2O exchang., NH–cyclohexylamine), 6.80 (1H, s, H-4), 8.44 (1H, s, H-7), 9.55 (1H, s, D2O exchang., NHCO), 10.52 (1H, br s, D2O exchang., NH–pyrazole). 13C-NMR (50 MHz, CDCl3) δ: 24.8 (cyclohexyl C-3′, C-5′), 25.9 (cyclohexyl C-4′), 33.2 (cyclohexyl C-2′, C-6′), 45.9 (CH3), 51.2 (cyclohexyl C-1′), 53.5 (piperazine C-2″, C-6″), 55.1 (piperazine C-3″, C-5″), 61.5 (CH2), 93.3 (C-4), 123.6 (C-3a), 132.4 (C-7), 134.2 (C-7a), 139.0 (C-3), 152.2 (C-5), 168.5 (CO). HR-MS (ESI) m/z: Calcd for C19H30N7O: [M1+H]+=372.2506. Found 372.2511. Anal. Calcd for C19H29N7O: C, 61.43; H, 7.87; N, 26.39. Found: C, 61.19; H, 7.76; N, 26.48.
N-(5-(Cyclohexylamino)-1H-pyrazolo[3,4-c]pyridin-3-yl)-2-(phenylamino)acetamide (14c)This compound was prepared according to the general procedure described above, starting from 13i. The product was purified by column chromatography (silica gel) using a mixture of dichloromethane–methanol (96 : 4, v/v) as the eluent to provide pure 14c as a yellow solid. Yield: 74%, mp: 166°C (CHCl3). 1H-NMR (400 MHz, CDCl3) δ: 1.23 (3H, m, cyclohexyl H), 1.41 (2H, m, cyclohexyl H), 1.64 (1H, m, cyclohexyl H), 1.75 (2H, m, cyclohexyl H), 2.05 (2H, m, cyclohexyl H), 3.41 (1H, m, cyclohexyl H-1′), 4.01 (2H, s, CH2), 4.49 (1H, s, D2O exchang., CH2NH), 6.69 (1H, s, H-4), 6.72 (2H, d, H-2″, H-6″, J=8.0 Hz), 6.85 (1H, t, H-4″, J=7.2 Hz), 7.24 (2H, t, H-3″, H-5″, J=7.7 Hz), 8.42 (1H, s, H-7), 9.07 (1H, s, D2O exchang., NHCO), 10.19 (1H, br s, D2O exchang., NH–pyrazole). 13C-NMR (50 MHz, CDCl3) δ: 24.8 (cyclohexyl C-3′, C-5′), 25.9 (cyclohexyl C-4′), 33.1 (cyclohexyl C-2′, C-6′), 49.2 (CH2), 51.2 (cyclohexyl C-1′), 93.0 (C-4), 113.5 (C-2″, C-6″), 119.7 (C-4″), 123.9 (C-3a), 129.6 (C-3″, C-5″), 132.2 (C-7), 134.0 (C-7a), 138.6 (C-3), 146.8 (C-1″), 152.1 (C-5), 169.4 (CO). HR-MS (ESI) m/z: Calcd for C20H25N6O: [M1+H]+=365.2084. Found 365.2090. Anal. Calcd for C20H24N6O: C, 65.91; H, 6.64; N, 23.06. Found: C, 66.12; H, 6.77; N, 22.89.
2-(Dimethylamino)-N-(5-(phenylamino)-1H-pyrazolo[3,4-c]pyridin-3-yl)acetamide (14d)This compound was prepared according to the general procedure described above, starting from 13j. The product was purified by column chromatography (silica gel) using a mixture of dichloromethane–methanol (100 : 2, v/v) as the eluent to provide pure 14d as a pale green solid. Yield: 89%, mp: 194°C (EtOAc). 1H-NMR (400 MHz, DMSO-d6) δ: 2.33 (6H, s, N(CH3)2), 3.16 (2H, s, CH2), 6.78 (1H, t, H-4′, J=7.2 Hz), 7.20 (3H, m, H-3′, H-5′, H-4), 7.46 (2H, d, H-2′, H-6′, J=7.8 Hz), 8.66 (1H, d, H-7, J=0.8 Hz), 8.69 (1H, s, D2O exchang., NH–aniline), 10.14 (1H, br s, D2O exchang., NHCO), 12.87 (1H, s, D2O exchang., NH–pyrazole). 13C-NMR (50 MHz, DMSO-d6) δ: 45.2 (N(CH3)2), 62.1 (CH2), 98.5 (C-4), 116.4 (C-2′, C-6′), 119.0 (C-4′), 123.0 (C-3a), 128.6 (C-3′, C-5′), 132.0 (C-7), 134.4 (C-7a), 138.6 (C-3), 143.2 (C-1′), 147.7 (C-5), 168.7 (CO). HR-MS (ESI) m/z: Calcd for C16H19N6O: [M1+H]+=311.1615. Found 311.1619. Anal. Calcd for C16H18N6O: C, 61.92; H, 5.85; N, 27.08. Found: C, 62.11; H, 5.98; N, 27.17.
2-(4-Methylpiperazin-1-yl)-N-(5-(phenylamino)-1H-pyrazolo[3,4-c]pyridin-3-yl)acetamide (14e)This compound was prepared according to the general procedure described above, starting from 13k. The product was purified by column chromatography (silica gel) using a mixture of dichloromethane–methanol (100 : 5, v/v) as the eluent to provide pure 14e as a green solid. Yield: 78%, mp: 186°C (EtOAc). 1H-NMR (400 MHz, DMSO-d6) δ: 2.18 (3H, s, CH3), 2.40 (4H, br s, piperazine H-3″, H-5″), 2.58 (4H, br s, piperazine H-2″, H-6″), 3.20 (2H, s, CH2), 6.78 (1H, t, H-4′, J=7.2 Hz), 7.20 (3H, m, H-3′, H-5′, H-4), 7.45 (2H, d, H-2′, H-6′, J=8.0 Hz), 8.66 (1H, s, H-7), 8.70 (1H, s, D2O exchang., NH–aniline), 10.12 (1H, s, D2O exchang., NHCO), 12.88 (1H, s, D2O exchang., NH–pyrazole). 13C-NMR (50 MHz, DMSO-d6) δ: 45.5 (CH3), 52.5 (piperazine C-2″, C-6″), 54.4 (piperazine C-3″, C-5″), 60.7 (CH2), 98.5 (C-4), 116.5 (C-2′, C-6′), 119.0 (C-4′), 122.8 (C-3a), 128.5 (C-3′, C-5′), 132.0 (C-7), 134.5 (C-7a), 138.6 (C-3), 143.2 (C-1′), 147.7 (C-5), 168.2 (CO). HR-MS (ESI) m/z: Calcd for C19H24N7O: [M1+H]+=366.2037. Found 366.2044. Anal. Calcd for C19H23N7O: C, 62.45; H, 6.34; N, 26.83. Found: C, 62.28; H, 6.19; N, 26.99.
2-(Phenylamino)-N-(5-(phenylamino)-1H-pyrazolo[3,4-c]pyridin-3-yl)acetamide (14f)This compound was prepared according to the general procedure described above, starting from 13l. The product was purified by column chromatography (silica gel) using a mixture of cyclohexane–ethyl acetate (1 : 1, v/v) as the eluent to provide pure 14f as a beige solid. Yield: 84%, mp: 126°C (dec.) (EtOAc). 1H-NMR (400 MHz, CDCl3) δ: 4.00 (2H, s, CH2), 4.40 (1H, br s, D2O exchang., CH2NH), 6.53 (1H, br s, D2O exchang., NH–aniline), 6.71 (2H, d, H-2″, H-6″, J=7.8 Hz), 6.85 (1H, t, H-4″, J=7.2 Hz), 7.00 (1H, t, H-4′, J=7.1 Hz), 7.23 (2H, t, H-3″, H-5″, J=7.7 Hz), 7.28 (2H, d, H-2′, H-6′, J=7.7 Hz), 7.33 (2H, t, H-3′, H-5′, J=7.6 Hz), 7.49 (1H, s, H-4), 8.59 (1H, s, H-7), 9.09 (1H, s, D2O exchang., NHCO), 9.95 (1H, br s, D2O exchang., NH–pyrazole). 13C-NMR (50 MHz, CDCl3) δ: 49.3 (CH2), 97.9 (C-4), 113.5 (C-2″, C-6″), 118.4 (C-2′, C-6′), 119.8 (C-4″), 121.7 (C-4′), 123.4 (C-3a), 129.4 (C-3′, C-5′), 129.6 (C-3″, C-5″), 132.4 (C-7), 135.1 (C-7a), 139.8 (C-3), 141.6 (C-1′), 146.6 (C-1″), 148.4 (C-5), 169.3 (CO). HR-MS (ESI) m/z: Calcd for C20H19N6O: [M1+H]+=359.1615. Found 359.1622. Anal. Calcd for C20H18N6O: C, 67.03; H, 5.06; N, 23.45. Found: C, 66.89; H, 5.01; N, 23.62.
7-Chloro-3-nitro-1H-pyrazolo[3,4-c]pyridine (21)This compound was prepared following an analogous synthetic procedure to that described for the synthesis of the nitroderivative 7, upon nitration of pyrazolopyridine 20, in 94% yield. The nitroderivative 21 was obtained pure, as a yellow solid. mp: 172–173°C (EtOAc). 1H-NMR (600 MHz, DMSO-d6) δ: 8.03 (1H, d, H-4, J=5.6 Hz), 8.27 (1H, d, H-5, J=5.6 Hz). 13C-NMR (151 MHz, DMSO-d6) δ: 113.9 (C-4), 122.1 (C-3a), 136.4 (C-7a), 137.5 (C-3), 141.5 (C-5), 149.6 (C-7). Anal. Calcd for C6H3ClN4O2: C, 36.29; H, 1.52; N, 28.22. Found: C, 36.50; H, 1.72; N, 27.94.
7-Chloro-1-methyl-3-nitro-1H-pyrazolo[3,4-c]pyridine (22a) and 7-Chloro-2-methyl-3-nitro-2H-pyrazolo[3,4-c]pyridine (23)These compounds were prepared following an analogous synthetic procedure to that described for the synthesis of the nitroderivatives 8a and 9a, starting from pyrazolopyridine 21 (540 mg, 2.72 mmol). Purification was effected using a mixture of cyclohexane–ethyl acetate (8 : 2, v/v) as the eluent, to provide the pure isomers 22a (45% yield) and 23 (10% yield).
Data for 22a: Beige solid, mp: 160–161°C (EtOAc). 1H-NMR (600 MHz, CDCl3) δ: 4.57 (3H, s, CH3), 8.08 (1H, d, H-4, J=5.6 Hz), 8.30 (1H, d, H-5, J=5.6 Hz). 13C-NMR (151 MHz, CDCl3) δ: 41.1 (CH3), 114.5 (C-4), 123.6 (C-3a), 135.2 (C-7a), 135.4 (C-3), 142.5 (C-5), 146.8 (C-7). Anal. Calcd for C7H5ClN4O2: C, 39.55; H, 2.37; N, 26.35. Found: C, 39.74; H, 2.43; N, 26.17.
Data for 23: Pale yellow solid, mp: 202–203°C (EtOAc). 1H-NMR (600 MHz, CDCl3) δ: 4.70 (3H, s, CH3), 7.93 (1H, d, H-4, J=5.9 Hz), 8.27 (1H, d, H-5, J=5.9 Hz). 13C-NMR (151 MHz, CDCl3) δ: 43.9 (CH3), 113.2 (C-4), 122.3 (C-3a), 129.0 (C-7a), 140.2 (C-3), 143.5 (C-5), 144.6 (C-7). Anal. Calcd for C7H5ClN4O2: C, 39.55; H, 2.37; N, 26.35. Found: C, 39.82; H, 2.49; N, 26.21.
7-Chloro-1-(4-methoxybenzyl)-3-nitro-1H-pyrazolo[3,4-c]pyridine (22b)This compound was prepared following an analogous synthetic procedure to that described for the synthesis of the derivatives 8b and 9b, starting from pyrazolopyridine 21 (540 mg, 2.72 mmol). Purification was effected using a mixture of cyclohexane–ethyl acetate (8 : 2, v/v) as the eluent, to provide pure 22b as a pale yellow solid, in 65% yield. mp: 142–144°C (CH2Cl2/Et2O). 1H-NMR (600 MHz, CDCl3) δ: 3.75 (3H, s, OCH3), 6.03 (2H, s, CH2), 6.84 (2H, d, H-2″, H-6″, J=8.5 Hz), 7.29 (2H, d, H-3″, H-5″, J=8.5 Hz), 8.10 (1H, d, H-4, J=5.5 Hz), 8.30 (1H, d, H-5, J=5.8 Hz). 13C-NMR (151 MHz, CDCl3) δ: 55.4 (OCH3), 55.8 (CH2), 114.5 (C-2″, C-6″), 114.6 (C-4), 123.9 (C-3a), 126.8 (C-7a), 129.2 (C-3″, C-5″), 134.6 (C-1″), 135.0 (C-3), 142.4 (C-5), 147.4 (C-7), 160.0 (C-4″). Anal. Calcd for C14H11ClN4O3: C, 52.76; H, 3.48; N, 17.58. Found: C, 53.02; H, 3.59; N, 17.26.
N-Cyclohexyl-1-methyl-3-nitro-1H-pyrazolo[3,4-c]pyridin-7-amine (24a)This compound was prepared following an analogous synthetic procedure to that described for the synthesis of the derivative 10a, upon reaction of the chloroderivative 22a with cyclohexylamine for 2 h, in 89% yield. The product was purified by column chromatography using a mixture of cyclohexane–ethyl acetate (from 7 : 3 up to 6 : 4, v/v) as the eluent, to provide 24a as a yellow solid. mp: 171–173°C (EtOAc/Et2O). 1H-NMR (400 MHz, CDCl3) δ: 1.20–1.31 (3H, m, cyclohexyl H), 1.41–1.50 (2H, m, cyclohexyl H), 1.62–1.68 (1H, m, cyclohexyl H), 1.71–1.78 (2H, m, cyclohexyl H), 2.10–2.15 (2H, m, cyclohexyl H), 4.12–4.24 (1H, m, cyclohexyl H-1′), 4.45 (3H, s, CH3), 4.78 (1H, br s, D2O exchang., NH), 7.33 (1H, d, H-4, J=5.8 Hz), 7.95 (1H, d, H-5, J=5.8 Hz). 13C-NMR (50 MHz, CDCl3) δ: 25.0 (cyclohexyl C-3′, C-5′), 25.9 (cyclohexyl C-4′), 33.4 (cyclohexyl C-2′, C-6′), 41.1 (CH3), 49.8 (cyclohexyl C-1′), 104.0 (C-4), 123.0 (C-3a), 128.8 (C-7a), 142.7 (C-5), 145.2 (C-3), 147.0 (C-7). Anal. Calcd for C13H17N5O2: C, 56.72; H, 6.22; N, 25.44. Found: C, 56.93; H, 6.34; N, 25.29.
N-Cyclohexyl-1-(4-methoxybenzyl)-3-nitro-1H-pyrazolo[3,4-c]pyridin-7-amine (24b)This compound was prepared following an analogous synthetic procedure to that described for the synthesis of the derivative 10a, upon reaction of the chloroderivative 22b with cyclohexylamine for 3 h, in 70% yield. The product was purified by column chromatography using a mixture of cyclohexane–ethyl acetate (90 : 10, v/v) as the eluent, to provide 24b as an orange colored solid. mp: 152–154°C (CH2Cl2/Et2O). 1H-NMR (600 MHz, CDCl3) δ: 0.82–0.92 (3H, m, cyclohexyl H), 1.08–1.18 (2H, m, cyclohexyl H), 1.30–1.40 (1H, m, cyclohexyl H), 1.57–1.65 (2H, m, cyclohexyl H), 1.82–1.88 (2H, m, cyclohexyl H), 3.81 (3H, s, OCH3), 3.88–3.96 (1H, m, cyclohexyl H-1′), 4.64 (1H, d, D2O exchang., NH–cyclohexylamine, J=6.4 Hz), 5.82 (2H, s, CH2), 6.94 (2H, d, H-2″, H-6″, J=8.7 Hz), 7.09 (2H, d, H-3″, H-5″, J=8.7 Hz), 7.36 (1H, d, H-4, J=5.8 Hz), 7.94 (1H, d, H-5, J=5.8 Hz). 13C-NMR (151 MHz, CDCl3) δ: 25.1 (cyclohexyl C-3′, C-5′), 25.9 (cyclohexyl C-4′), 33.4 (cyclohexyl C-2′, C-6′), 49.4 (cyclohexyl C-1′), 55.4 (OCH3), 57.7 (CH2), 101.7 (C-4), 114.4 (C-2″, C-6″), 122.4 (C-3a), 126.6 (C-7a), 129.7 (C-3″, C-5″), 134.7 (C-1″), 137.0 (C-3), 145.4 (C-5), 150.2 (C-7), 160.0 (C-4″). Anal. Calcd for C20H23N5O3: C, 62.98; H, 6.08; N, 18.36. Found: C, 62.78; H, 6.01; N, 18.55.
N7-Cyclohexyl-1-methyl-1H-pyrazolo[3,4-c]pyridine-3,7-diamine (25a)This compound was prepared following an analogous synthetic procedure to that described for the synthesis of the aminoderivative 11a, starting from the nitroderivative 24a (600 mg, 2.18 mmol), in 82% yield. The product was purified by column chromatography using a mixture of cyclohexane–ethyl acetate (from 6 : 4 up to 3 : 7, v/v) as the eluent, to provide pure 25a as a beige solid. mp: 144–146°C (CH2Cl2/petroleum ether). 1H-NMR (600 MHz, DMSO-d6) δ: 1.15–1.25 (3H, m, cyclohexyl H), 1.30–1.40 (2H, m, cyclohexyl H), 1.58–1.65 (1H, m, cyclohexyl H), 1.70–1.78 (2H, m, cyclohexyl H), 1.96–2.10 (2H, m, cyclohexyl H), 3.92 (3H, s, CH3), 3.93–3.98 (1H, m, cyclohexyl H-1′), 5.27 (2H, br s, D2O exchang., NH2), 5.62 (1H, d, D2O exchang., NH, J=7.3 Hz), 6.82 (1H, d, H-4, J=5.7 Hz), 7.44 (1H, d, H-5, J=5.7 Hz). 13C-NMR (151 MHz, DMSO-d6) δ: 24.9 (cyclohexyl C-3′, C-5′), 25.7 (cyclohexyl C-4′), 32.3 (cyclohexyl C-2′, C-6′), 37.8 (CH3), 49.0 (cyclohexyl C-1′), 104.0 (C-4), 118.2 (C-3a), 128.3 (C-7a), 134.5 (C-5), 144.9 (C-3), 148.0 (C-7). HR-MS (ESI) m/z: Calcd for C13H20N5: [M1+H]+=246.1713. Found 246.1715. Anal. Calcd for C13H19N5: C, 63.65; H, 7.81; N, 28.55. Found: C, 63.90; H, 8.01; N, 28.09.
N7-Cyclohexyl-1-(4-methoxybenzyl)-1H-pyrazolo[3,4-c]pyridine-3,7-diamine (26a)This compound was prepared following an analogous synthetic procedure to that described for the synthesis of the aminoderivative 11a, starting from the nitroderivative 24b (720 mg, 1.89 mmol), in 80% yield. The product was purified by column chromatography using a mixture of cyclohexane–ethyl acetate (from 1 : 1 up to 3 : 7, v/v) as the eluent, to provide pure 26a as a grey solid. mp: 130–132°C (CH2Cl2/n-hexane). 1H-NMR (400 MHz, DMSO-d6) δ: 1.10–1.25 (3H, m, cyclohexyl H), 1.30–1.40 (2H, m, cyclohexyl H), 1.52–1.65 (1H, m, cyclohexyl H), 1.66–1.74 (2H, m, cyclohexyl H), 1.86–1.94 (2H, m, cyclohexyl H), 3.70 (3H, s, OCH3), 3.94 (1H, br s, cyclohexyl H-1′), 5.31 (2H, s, CH2), 6.14 (2H, br s, D2O exchang., NH2), 6.31 (1H, br s, D2O exchang., NH), 6.69 (1H, d, H-4, J=6.1 Hz), 6.88 (2H, d, H-2″, H-6″, J=8.5 Hz), 7.06–7.16 (3H, m, H-5, H-3″, H-5″). 13C-NMR (50 MHz, DMSO-d6) δ: 25.0 (cyclohexyl C-3′, C-5′), 25.3 (cyclohexyl C-4′), 32.5 (cyclohexyl C-2′, C-6′), 48.6 (cyclohexyl C-1′), 50.3 (CH2), 55.1 (OCH3), 103.0 (C-4), 108.4 (C-3a), 113.8 (C-2″, C-6″), 128.6 (C-3″, C-5″), 128.9 (C-7a, C-1″), 135.2 (C-5), 141.0 (C-3), 147.8 (C-7), 158.6 (C-4″). Anal. Calcd for C20H25N5O: C, 68.35; H, 7.17; N, 19.93. Found: C, 68.21; H, 7.10; N, 20.12.
2-Chloro-N-(7-(cyclohexylamino)-1-methyl-1H-pyrazolo[3,4-c]pyridin-3-yl)acetamide (25b) and 2-Chloro-N-(7-(cyclohexylamino)-1-(4-methoxybenzyl)-1H-pyrazolo[3,4-c]pyridin-3-yl)acetamide (26b)These compounds were prepared following an analogous synthetic procedure to that described for the synthesis of the compounds 12a and c, starting from the amines 25a and 26a, respectively. These intermediates were used imediately to the next step, with no further purification.
N-(7-(Cyclohexylamino)-1-methyl-1H-pyrazolo[3,4-c]pyridin-3-yl)-2-(dimethylamino)acetamide (27a)This compound was prepared following an analogous synthetic procedure to that described for the synthesis of the compounds 13a–l, upon reaction of the chloride 25b with dimethylamine (5.6 M solution in ethanol). The product was purified by column chromatography (silica gel) using a mixture of dichloromethane–methanol (from 100 : 5 up to 100 : 15, v/v) as the eluent to provide pure 27a as a beige solid. Yield: 97%, mp: 145–147°C (Et2O). 1H-NMR (600 MHz, CDCl3) δ: 1.21–1.29 (3H, m, cyclohexyl H), 1.43–1.51 (2H, m, cyclohexyl H), 1.61–1.67 (1H, m, cyclohexyl H), 1.70–1.76 (2H, m, cyclohexyl H), 2.09–2.15 (2H, m, cyclohexyl H), 2.40 (6H, s, N(CH3)2), 3.15 (2H, s, CH2), 4.06–4.14 (1H, m, cyclohexyl H-1′), 4.22 (3H, s, CH3– pyrazole), 4.64 (1H, d, D2O exchang., NH–cyclohexylamine, J=6.1 Hz), 7.00 (1H, d, H-4, J=5.9 Hz), 7.63 (1H, d, H-5, J=5.9 Hz), 9.43 (1H, br s, D2O exchang., NHCO). 13C-NMR (151 MHz, CDCl3) δ: 24.9 (cyclohexyl C-3′, C-5′), 25.9 (cyclohexyl C-4′), 33.4 (cyclohexyl C-2′, C-6′), 39.2 (CH3–pyrazole), 46.1 (N(CH3)2), 49.4 (cyclohexyl C-1′), 63.1 (CH2), 105.6 (C-4), 121.7 (C-3a), 128.4 (C-7a), 136.7 (C-5), 138.0 (C-3), 145.1 (C-7), 168.9 (CO). HR-MS (ESI) m/z: Calcd for C17H27N6O: [M1+H]+=331.2241. Found 331.2247. Anal. Calcd for C17H26N6O: C, 61.79; H, 7.93; N, 25.43. Found: C, 61.96; H, 7.99; N, 25.31.
N-(7-(Cyclohexylamino)-1-methyl-1H-pyrazolo[3,4-c]pyridin-3-yl)-2-(4-methylpiperazin-1-yl)acetamide (27b)This compound was prepared following an analogous synthetic procedure to that described for the synthesis of the compounds 13a–l, upon reaction of the chloride 25b with 1-methylpiperazine. The product was purified by column chromatography (silica gel) using a mixture of dichloromethane–methanol (from 100 : 5 up to 100 : 20, v/v) as the eluent to provide pure 27b as a yellow oil. Yield: 96%. 1H-NMR (600 MHz, CDCl3) δ: 1.20–1.30 (3H, m, cyclohexyl H), 1.40–1.50 (2H, m, cyclohexyl H), 1.60–1.68 (1H, m, cyclohexyl H), 1.69–1.77 (2H, m, cyclohexyl H), 2.05–2.15 (2H, m, cyclohexyl H), 2.32 (3H, s, CH3–piperazine), 2.50–2.60 (4H, br s, piperazine H-3″, H-5″), 2.65–2.80 (4H, br s, piperazine H-2″, H-6″), 3.19 (2H, s, CH2), 4.06–4.14 (1H, m, cyclohexyl H-1′), 4.24 (3H, s, CH3–pyrazole), 4.60 (1H, d, D2O exchang., NH–cyclohexylamine, J=7.0 Hz), 6.94 (1H, d, H-4, J=5.9 Hz), 7.65 (1H, d, H-5, J=5.9 Hz), 9.31 (1H, br s, D2O exchang., NHCO). 13C-NMR (151 MHz, CDCl3) δ: 24.9 (cyclohexyl C-3′, C-5′), 26.0 (cyclohexyl C-4′), 33.5 (cyclohexyl C-2′, C-6′), 39.2 (CH3–pyrazole), 45.9 (CH3–piperazine), 49.3 (cyclohexyl C-1′), 53.4 (piperazine C-2″, C-6″), 55.1 (piperazine C-3″, C-5″), 61.5 (CH2), 105.4 (C-4), 121.7 (C-3a), 128.4 (C-7a), 137.1 (C-5), 137.8 (C-3), 145.2 (C-7), 168.5 (CO). HR-MS (ESI) m/z: Calcd for C20H32N7O: [M1+H]+=386.2663. Found 386.2665. Anal. Calcd for C20H31N7O: C, 62.31; H, 8.11; N, 25.43. Found: C, 62.63; H, 8.29; N, 25.21.
N-(7-(Cyclohexylamino)-1-methyl-1H-pyrazolo[3,4-c]pyridin-3-yl)-2-(phenylamino)acetamide (27c)This compound was prepared following an analogous synthetic procedure to that described for the synthesis of the compounds 13a–l, upon reaction of the chloride 25b with aniline. The product was purified by column chromatography (silica gel) using a mixture of chloroform–methanol (100 : 2, v/v) as the eluent to provide pure 27c as a pale yellow solid. Yield: 51%. mp: >300°C (EtOAc). 1H-NMR (600 MHz, CDCl3) δ: 1.20–1.30 (3H, m, cyclohexyl H), 1.42–1.52 (2H, m, cyclohexyl H), 1.60–1.68 (1H, m, cyclohexyl H), 1.69–1.78 (2H, m, cyclohexyl H), 2.06–2.14 (2H, m, cyclohexyl H), 3.94 (2H, s, CH2), 4.06–4.14 (1H, m, cyclohexyl H-1′), 4.16 (3H, s, CH3), 4.62 (1H, br s, D2O exchang., NH–cyclohexylamine), 4.83 (1H, br s, D2O exchang., NH–aniline), 6.67 (2H, d, H-2″, H-6″, J=7.9 Hz), 6.81 (1H, t, H-4″, J=7.3 Hz), 6.91 (1H, d, H-4, J=5.9 Hz), 7.20 (2H, t, H-3″, H-5″, J=7.8 Hz), 7.58 (1H, d, H-5, J=5.9 Hz), 9.06 (1H, br s, D2O exchang., NHCO). 13C-NMR (151 MHz, CDCl3) δ: 24.9 (cyclohexyl C-3′, C-5′), 25.9 (cyclohexyl C-4′), 33.3 (cyclohexyl C-2′, C-6′), 39.3 (CH3), 49.2 (CH2), 49.8 (cyclohexyl C-1′), 105.4 (C-4), 113.5 (C-2″, C-6″), 119.4 (C-4″), 122.0 (C-3a), 128.1 (C-7a), 129.6 (C-3″, C-5″), 135.7 (C-5), 137.6 (C-3), 144.8 (C-1″), 147.1 (C-7), 169.7 (CO). HR-MS (ESI) m/z: Calcd for C21H27N6O: [M1+H]+=379.2241. Found 379.2245. Anal. Calcd for C21H26N6O: C, 66.64; H, 6.92; N, 22.20. Found: C, 66.87; H, 7.00; N, 22.11.
N-(7-(Cyclohexylamino)-1-(4-methoxybenzyl)-1H-pyrazolo[3,4-c]pyridin-3-yl)-2-(dimethylamino)acetamide (28)This compound was prepared following an analogous synthetic procedure to that described for the synthesis of the compounds 13a–l, upon reaction of the chloride 26b with dimethylamine (5.6 M solution in ethanol). The product was purified by column chromatography (silica gel) using a mixture of ethyl acetate–methanol (from 100 : 5 up to 100 : 10, v/v) as the eluent to provide pure 28 as a white solid. Yield: 76%, mp: 138–139°C (Et2O). 1H-NMR (600 MHz, CDCl3) δ: 0.78–0.88 (3H, m, cyclohexyl H), 1.00–1.10 (2H, m, cyclohexyl H), 1.26–1.35 (1H, m, cyclohexyl H), 1.47–1.58 (2H, m, cyclohexyl H), 1.77–1.85 (2H, m, cyclohexyl H), 2.39 (6H, s, N(CH3)2), 3.14 (2H, s, NHCOCH2), 3.75 (3H, s, OCH3), 3.82–3.90 (1H, m, cyclohexyl H-1″), 4.41 (1H, d, D2O exchang., NH–cyclohexylamine, J=7.2 Hz), 5.61 (2H, s, CH2–pyrazole), 6.85 (2H, d, H-2′, H-6′, J=8.7 Hz), 7.01–7.06 (3H, m, H-4, H-3′, H-5′), 7.64 (1H, d, H-5, J=5.9 Hz), 9.51 (1H, br s, D2O exchang., NHCO). 13C-NMR (151 MHz, CDCl3) δ: 24.7 (cyclohexyl C-3″, C-5″), 25.86 (cyclohexyl C-4″), 33.0 (cyclohexyl C-2″, C-6″), 46.1 (N(CH3)2), 49.1 (cyclohexyl C-1″), 55.1 (CH2–pyrazole), 55.4 (OCH3), 63.2 (NHCOCH2), 105.5 (C-4), 114.8 (C-2′, C-6′), 122.2 (C-3a), 127.5 (C-3′, C-5′), 128.2 (C-7a), 128.9 (C-1′), 137.2 (C-5), 138.4 (C-3), 145.0 (C-7), 159.8 (C-4′), 168.9 (CO). HR-MS (ESI) m/z: Calcd for C24H33N6O2: [M1+H]+=437.2660. Found 437.2668. Anal. Calcd for C24H32N6O2: C, 66.03; H, 7.39; N, 19.25. Found: C, 66.33; H, 7.54; N, 18.98.
N-(7-(Cyclohexylamino)-1H-pyrazolo[3,4-c]pyridin-3-yl)-2-(dimethylamino)acetamide (29)This compound was prepared following an analogous synthetic procedure to that described for the synthesis of the compounds 14a–f, starting from the derivative 28. The product was purified by column chromatography (silica gel) using a mixture of dichloromethane–methanol (9 : 1, v/v) as the eluent to provide pure 29 as a colorless oil. Yield: 99%. 1H-NMR (400 MHz, DMSO-d6) δ: 1.15–1.18 (3H, m, cyclohexyl H), 1.21–1.42 (2H, m, cyclohexyl H), 1.58–1.64 (1H, m, cyclohexyl H), 1.71–1.79 (2H, m, cyclohexyl H), 1.97–2.05 (2H, m, cyclohexyl H), 2.35 (6H, s, N(CH3)2), 3.21 (2H, s, CH2), 3.93–4.05 (1H, m, cyclohexyl H-1′), 6.55 (1H, br s, D2O exchang., NH–cyclohexylamine), 6.80 (1H, d, H-4, J=5.9 Hz), 7.49 (1H, d, H-5, J=5.9 Hz), 10.11 (1H, br s, D2O exchang., NHCO), 12.77 (1H, br s, D2O exchang., NH–pyrazole). 13C-NMR (50 MHz, DMSO-d6) δ: 24.6 (cyclohexyl C-3′, C-5′), 25.5 (cyclohexyl C-4′), 32.7 (cyclohexyl C-2′, C-6′), 45.1 (N(CH3)2), 48.4 (cyclohexyl C-1′), 61.9 (CH2), 104.0 (C-4), 119.0 (C-3a), 127.8 (C-7a), 136.0 (C-5), 139.8 (C-3), 144.4 (C-7), 168.2 (CO). HR-MS (ESI) m/z: Calcd for C16H25N6O: [M1+H]+=317.2084. Found 317.2087. Anal. Calcd for C16H24N6O: C, 60.74; H, 7.65; N, 26.56. Found: C, 60.43; H, 7.52; N, 26.75.
1-Methyl-3-nitro-N-phenyl-1H-pyrazolo[3,4-c]pyridin-7-amine (30)Aniline (0.17 mL, 1.87 mmol) was added into a solution of the chlorocompound 22a (80 mg, 0.37 mmol) in 2-ethoxyethanol (2 mL), under argon, and the resulting solution was heated at 130°C for 3h. The solvent was then vacuum-evaporated and the residue was purified by column chromatography (silica gel) using dichloromethane as the eluent, to give pure 30 (60 mg, 59%) as a yellow solid. mp: 197–198°C (EtOAc). 1H-NMR (600 MHz, CDCl3) δ: 4.34 (3H, s, CH3), 7.14 (1H, t, H-4′, J=7.3 Hz), 7.28 (2H, d, H-2′, H-6′, J=7.6 Hz), 7.36 (2H, t, H-3′, H-5′, J=7.5 Hz), 7.57 (1H, br s, H-4), 7.88 (1H, br s, H-5). 13C-NMR (151 MHz, CDCl3) δ: 41.4 (CH3), 107.7 (C-4), 119.6 (C-3a), 120.7 (C-2′, C-6′), 124.6 (C-4′), 129.9 (C-3′, C-5′), 130.6 (C-7a), 134.9 (C-5), 140.9 (C-3), 142.6 (C-1′), 147.3 (C-7). Anal. Calcd for C13H11N5O2: C, 57.99; H, 4.12; N, 26.01. Found: C, 58.14; H, 4.22; N, 25.84.
1-Methyl-N7-phenyl-1H-pyrazolo[3,4-c]pyridine-3,7-diamine (31)This compound was prepared following an analogous synthetic procedure to that described for the synthesis of the compound 11a, upon hydrogenation of the nitroderivative 30. The product was purified by column chromatography (silica gel) using a mixture of cyclohexane–ethyl acetate (from 5 : 5 up to 2 : 8, v/v) as the eluent to provide pure 31 as an orange colored solid. Yield: 75%, mp: 120–122°C (CH2Cl2/n-hexane). 1H-NMR (400 MHz, DMSO-d6) δ: 3.93 (3H, s, CH3), 5.52 (2H, br s, D2O exchang., NH2), 6.90 (1H, t, H-4′, J=7.3 Hz), 7.20 (1H, d, H-4, J=5.6 Hz), 7.25 (2H, t, H-3′, H-5′, J=7.8 Hz), 7.44 (2H, d, H-2′, H-6′, J=7.8 Hz), 7.60 (1H, d, H-5, J=5.6 Hz), 8.37 (1H, br s, D2O exchang., NH). 13C-NMR (50 MHz, DMSO-d6) δ: 37.6 (CH3), 108.3 (C-4), 118.8 (C-2′, C-6′), 119.7 (C-3a), 120.6 (C-4′), 128.4 (C-3′, C-5′), 129.8 (C-7a), 134.0 (C-5), 141.6 (C-3), 142.2 (C-1′), 148.4 (C-7). Anal. Calcd for C13H13N5: C, 65.25; H, 5.48; N, 29.27. Found: C, 65.37; H, 5.60; N, 29.03.
2-Chloro-N-(1-methyl-7-(phenylamino)-1H-pyrazolo[3,4-c]pyridin-3-yl)acetamide (32)This compound was prepared following an analogous synthetic procedure to that described for the synthesis of the compounds 12b and d, starting from the amine 31. This intermediate was used imediately to the next step, with no further purification.
2-(Dimethylamino)-N-(1-methyl-7-(phenylamino)-1H-pyrazolo[3,4-c]pyridin-3-yl)acetamide (33a)This compound was prepared following an analogous synthetic procedure to that described for the synthesis of the compounds 13a–l, upon reaction of the chloride 32 with dimethylamine (5.6 M solution in ethanol). The product was purified by column chromatography (silica gel) using a mixture of dichloromethane–methanol (from 100 : 0.5 up to 100 : 5, v/v) as the eluent to provide pure 33a as a beige solid, in 97% yield. mp: 140–142°C (EtOH/Et2O). 1H-NMR (600 MHz, CDCl3) δ: 2.44 (6H, s, N(CH3)2), 3.19 (2H, s, CH2), 4.09 (3H, s, CH3–pyrazole), 6.77 (1H, br s, D2O exchang., NH–aniline), 7.02 (1H, t, H-4′, J=7.3 Hz), 7.21 (2H, d, H-2′, H-6′, J=7.6 Hz), 7.29 (2H, t, H-3′, H-5′, J=7.7 Hz), 7.45 (1H, br s, H-4), 7.79 (1H, br s, H-5), 9.62 (1H, br s, D2O exchang., NHCO). 13C-NMR (151 MHz, CDCl3) δ: 38.9 (CH3–pyrazole), 46.1 (N(CH3)2), 63.1 (CH2), 111.0 (C-4), 119.2 (C-2′, C-6′), 122.7 (C-3a, C-4′), 129.5 (C-3′, C-5′), 130.6 (C-7a), 136.6 (C-5), 138.7 (C-3), 141.9 (C-1′), 142.3 (C-7), 168.9 (CO). HR-MS (ESI) m/z: Calcd for C17H21N6O: [M1+H]+=325.1771. Found 325.1773. Anal. Calcd for C17H20N6O: C, 62.95; H, 6.21; N, 25.91. Found: C, 63.11; H, 6.28; N, 25.72.
N-(1-Methyl-7-(phenylamino)-1H-pyrazolo[3,4-c]pyridin-3-yl)-2-(4-methylpiperazin-1-yl)acetamide (33b)This compound was prepared following an analogous synthetic procedure to that described for the synthesis of the compounds 13a–l, upon reaction of the chloride 32 with 1-methylpiperazine. The product was purified by column chromatography (silica gel) using a mixture of dichloromethane–methanol (from 100 : 2 up to 100 : 10, v/v) as the eluent to provide pure 33b as a white solid, in 99% yield. mp: 202–204°C (EtOH/Et2O). 1H-NMR (600 MHz, CDCl3) δ: 2.34 (3H, s, CH3–piperazine), 2.50–2.60 (4H, br s, piperazine H-3″, H-5″), 2.65–2.80 (4H, br s, piperazine H-2″, H-6″), 3.23 (2H, s, CH2), 4.10 (3H, s, CH3–pyrazole), 6.58 (1H, br s, D2O exchang., NH–aniline), 7.03 (1H, t, H-4′, J=7.3 Hz), 7.22 (2H, d, H-2′, H-6′, J=7.8 Hz), 7.30 (2H, t, H-3′, H-5′, J=7.9 Hz), 7.46 (1H, d, H-4, J=5.8 Hz), 7.85 (1H, d, H-5, J=5.8 Hz), 9.49 (1H, br s, D2O exchang., NHCO). 13C-NMR (151 MHz, CDCl3) δ: 38.9 (CH3–pyrazole), 46.1 (CH3–piperazine), 53.7 (piperazine C-2″, C-6″), 55.3 (piperazine C-3″, C-5″), 61.7 (CH2), 111.1 (C-4), 119.1 (C-2′, C-6′), 122.7 (C-4′), 122.8 (C-3a), 129.5 (C-3′, C-5′), 130.6 (C-7a), 136.9 (C-5), 138.7 (C-3), 141.8 (C-7), 142.1 (C-1′), 168.7 (CO). HR-MS (ESI) m/z: Calcd for C20H26N7O: [M1+H]+=380.2193. Found 380.2198. Anal. Calcd for C20H25N7O: C, 63.30; H, 6.64; N, 25.84. Found: C, 63.55; H, 6.84; N, 25.59.
N-(1-Methyl-7-(phenylamino)-1H-pyrazolo[3,4-c]pyridin-3-yl)-2-(phenylamino)acetamide (33c)This compound was prepared following an analogous synthetic procedure to that described for the synthesis of the compounds 13a–l, upon reaction of the chloride 32 with aniline. The product was purified by column chromatography (silica gel) using a mixture of dichloromethane–ethyl acetate (1 : 1, v/v) as the eluent to provide pure 33c as a beige solid, in 55% yield. mp: 180–181°C (EtOH/Et2O). 1H-NMR (400 MHz, DMSO-d6) δ: 3.94 (2H, s, CH2), 4.22 (3H, s, CH3), 6.05 (1H, m, D2O exchang., NHCH2), 6.56–6.65 (3H, m, H-2″, H-4″, H-6″), 6.94 (1H, t, H-4′, J=7.4 Hz), 7.09–7.15 (3H, m, H-4, H-3′, H-5′), 7.28 (2H, t, H-3″, H-5″, J=7.9 Hz), 7.48 (2H, d, H-2′, H-6′, J=8.1 Hz), 7.65 (1H, d, H-5, J=5.7 Hz), 8.48 (1H, br s, D2O exchang., NH–aniline), 10.45 (1H, br s, D2O exchang., NHCO). 13C-NMR (50 MHz, DMSO-d6) δ: 38.8 (CH3), 46.6 (CH2), 109.4 (C-4), 112.3 (C-2″, C-6″), 116.5 (C-4″), 119.4 (C-2′, C-6′), 121.2 (C-4′), 122.5 (C-3a), 128.5 (C-3″, C-5″), 128.9 (C-3′, C-5′), 129.4 (C-7a), 135.4 (C-5), 138.4 (C-3), 141.7 (C-1″), 142.3 (C-7), 148.3 (C-1′), 169.7 (CO). HR-MS (ESI) m/z: Calcd for C21H21N6O: [M1+H]+=373.1771. Found 373.1774. Anal. Calcd for C21H20N6O: C, 67.73; H, 5.41; N, 22.57. Found: C, 67.81; H, 5.44; N, 22.47.
3-Nitro-N-phenyl-1H-pyrazolo[3,4-c]pyridin-7-amine (34)This compound was prepared following an analogous synthetic procedure to that described for the synthesis of 30, upon treatment of 21 with aniline in 1,4-dioxane, at 100°C for 12h. The product was purified by column chromatography (silica gel) using a mixture of dichloromethane–methanol (98 : 2, v/v) as the eluent, to provide pure 34, in 95% yield as a yellow solid. mp: 260–262°C (EtOAc). 1H-NMR (400 MHz, DMSO-d6) δ: 7.11 (1H, t, H-4′, J=7.1 Hz), 7.38–7.44 (3H, m, H-4, H-3′, H-5′), 7.82 (2H, d, H-2′, H-6′, J=7.8 Hz), 7.95 (1H, br s, H-5), 9.30 (1H, br s, D2O exchang., NH–aniline). 13C-NMR (50 MHz, DMSO-d6) δ: 104.6 (C-4), 120.0 (C-2′, C-6′), 120.5 (C-3a), 123.2 (C-4′), 129.1 (C-3′, C-5′), 130.1 (C-7a), 139.2 (C-3, C-1′), 142.8 (C-5), 148.8 (C-7). Anal. Calcd for C12H9N5O2: C, 56.47; H, 3.55; N, 27.44. Found: C, 56.66; H, 3.61; N, 27.31.
2-(4-Methoxybenzyl)-3-nitro-N-phenyl-2H-pyrazolo[3,4-c]pyridin-7-amine (35)This compound was prepared following an analogous synthetic procedure to that described for the synthesis of the compound 22b. The product was purified by column chromatography (silica gel) using a mixture of cyclohexane–ethyl acetate–dichloromethane (from 90 : 10 : 3 up to 85 : 15 : 4, v/v/v) as the eluent, to provide pure 34 as an orange colored solid, in 45% yield. mp: 175–177°C (MeOH). 1H-NMR (600 MHz, CDCl3) δ: 3.78 (3H, s, OCH3), 6.04 (2H, s, CH2), 6.87 (2H, d, H-2″, H-6″, J=8.6 Hz), 7.12 (1H, t, H-4′, J=7.3 Hz), 7.29 (1H, d, H-4, J=6.0 Hz), 7.35 (2H, d, H-3″, H-5″, J=8.6 Hz), 7.41 (2H, t, H-3′, H-5′, J=7.8 Hz), 7.70 (1H, br s, D2O exchang., NH), 7.89 (2H, d, H-2′, H-6′, J=7.8 Hz), 8.05 (1H, d, H-5, J=6.0 Hz). 13C-NMR (151 MHz, CDCl3) δ: 55.5 (OCH3), 58.0 (CH2), 104.5 (C-4), 114.5 (C-2″, C-6″), 119.9 (C-2′, C-6′), 122.2 (C-3a), 123.4 (C-4′), 126.4 (C-7a), 129.2 (C-3′, C-5′), 129.9 (C-3″, C-5″), 134.7 (C-1″), 137.3 (C-3), 139.1 (C-1′), 144.7 (C-5), 147.8 (C-7), 160.2 (C-4″). Anal. Calcd for C20H17N5O3: C, 63.99; H, 4.56; N, 18.66. Found: C, 64.34; H, 4.87; N, 18.33.
2-(4-Methoxybenzyl)-N7-phenyl-2H-pyrazolo[3,4-c]pyridine-3,7-diamine (36)This compound was prepared following an analogous synthetic procedure to that described for the synthesis of the compound 11a, upon hydrogenation of the nitroderivative 34. The product was purified by column chromatography (silica gel) using a mixture of cyclohexane–ethyl acetate (from 7 : 3 up to 5 : 5, v/v) as the eluent to provide pure 35 as a brown solid, in 96% yield. mp: 54–56°C (CH2Cl2/n-hexane). 1H-NMR (600 MHz, DMSO-d6) δ: 3.71 (3H, s, OCH3), 5.40 (2H, s, CH2), 6.23 (2H, br s, D2O exchang., NH2), 6.87–6.91 (3H, m, H-4′, H-2″, H-6″), 6.95 (1H, d, H-4, J=5.9 Hz), 7.17 (2H, d, H-3″, H-5″, J=8.5 Hz), 7.25 (2H, t, H-3′, H-5′, J=7.8 Hz), 7.31 (1H, d, H-5, J=5.9 Hz), 8.04 (2H, d, H-2′, H-6′, J=7.7 Hz), 8.57 (1H, br s, D2O exchang., NH). 13C-NMR (151 MHz, DMSO-d6) δ: 50.4 (CH2), 55.1 (OCH3), 106.0 (C-4), 109.3 (C-3a), 113.8 (C-2″, C-6″), 118.9 (C-2′, C-6′), 120.7 (C-4′), 128.2 (C-3′, C-5′, C-7a), 128.6 (C-3″, C-5″), 128.9 (C-1″), 131.0 (C-5), 135.8 (C-3), 141.3 (C-1′), 145.8 (C-7), 158.6 (C-4″). Anal. Calcd for C20H19N5O: C, 69.55; H, 5.54; N, 20.28. Found: C, 69.23; H, 5.31; N, 20.49.
2-Chloro-N-(2-(4-methoxybenzyl)-7-(phenylamino)-2H-pyrazolo[3,4-c]pyridin-3-yl)acetamide (37)This compound was prepared following an analogous synthetic procedure to that described for the synthesis of the compounds 12b and d, starting from the amine 36. This intermediate was used imediately to the next step, with no further purification.
2-(Dimethylamino)-N-(2-(4-methoxybenzyl)-7-(phenylamino)-2H-pyrazolo[3,4-c]pyridin-3-yl)acetamide (38a) and N-(2-(4-Methoxybenzyl)-7-(phenylamino)-2H-pyrazolo[3,4-c]pyridin-3-yl)-2-(4-methylpiperazin-1-yl)acetamide (38b)These compounds were prepared following an analogous synthetic procedure to that described for the synthesis of the compounds 13a–l, upon reaction of the chloride 37 with dimethylamine (5.6 M solution in ethanol) or 1-methylpiperazine, respectively. The product was purified by column chromatography using a mixture of dichloromethane–methanol (95 : 5, v/v) as the eluent to provide pure 38a or b.
Data for 38a: Yield 98%. Beige solid, mp: 183–185°C (CH2Cl2/Et2O). 1H-NMR (600 MHz, CDCl3) δ: 2.32 (6H, s, N(CH3)2), 3.11 (2H, s, NHCOCH2), 3.76 (3H, s, OCH3), 5.45 (2H, s, CH2–pyrazole), 6.83–6.87 (3H, m, H-4, H-2″, H-6″), 7.02 (1H, t, H-4′, J=7.3 Hz), 7.12 (2H, d, H-3″, H-5″, J=8.5 Hz), 7.35 (2H, t, H-3′, H-5′, J=7.8 Hz), 7.68 (1H, d, H-5, J=6.1 Hz), 7.87 (2H, d, H-2′, H-6′, J=7.9 Hz), 8.70–9.40 (1H, br s, D2O exchang., NHCO). 13C-NMR (151 MHz, CDCl3) δ: 46.1 (N(CH3)2), 54.3 (CH2–pyrazole), 55.4 (OCH3), 62.8 (NHCOCH2), 104.7 (C-4), 114.4 (C-2″, C-6″), 118.1 (C-3a), 119.4 (C-2′, C-6′), 122.2 (C-4′), 127.1 (C-7a), 128.0 (C-1″), 128.7 (C-3″, C-5″), 129.0 (C-3′, C-5′), 136.1 (C-3), 136.6 (C-5), 140.1 (C-1′), 147.3 (C-7), 159.7 (C-4″), 169.5 (CO). HR-MS (ESI) m/z: Calcd for C24H27N6O2: [M1+H]+=431.2190. Found 431.2198. Anal. Calcd for C24H26N6O2: C, 66.96; H, 6.09; N, 19.52. Found: C, 67.17; H, 6.18; N, 19.44.
Data for 38b: Yield 97%. White solid, mp: 159–160°C (EtOAc/Et2O). 1H-NMR (600 MHz, CDCl3) δ: 2.27 (3H, s, CH3), 2.30–2.40 (4H, br s, piperazine H-3″′, H-5‴), 2.55–2.59 (4H, br s, piperazine H-2″′, H-6‴), 3.17 (2H, s, NHCOCH2), 3.77 (3H, s, OCH3), 5.49 (2H, s, CH2–pyrazole), 6.79 (1H, d, H-4, J=6.1 Hz), 6.86 (2H, d, H-2″, H-6″, J=8.7 Hz), 7.02 (1H, t, H-4′, J=7.4 Hz), 7.07 (2H, d, H-3″, H-5″, J=8.7 Hz), 7.35 (2H, t, H-3′, H-5′, J=8.0 Hz), 7.58 (1H, br s, D2O exchang., NH–aniline), 7.70 (1H, d, H-5, J=6.1 Hz), 7.88 (2H, d, H-2′, H-6′, J=7.6 Hz), 9.00 (1H, br s, D2O exchang., NHCO). 13C-NMR (151 MHz, CDCl3) δ: 46.0 (CH3), 53.6 (piperazine C-2″′, C-6‴), 54.3 (CH2–pyrazole), 55.0 (piperazine C-3″′, C-5‴), 55.4 (OCH3), 61.3 (NHCOCH2), 104.4 (C-4), 114.6 (C-2″, C-6″), 118.2 (C-3a), 119.3 (C-2′, C-6′), 122.3 (C-4′), 127.3 (C-7a), 127.9 (C-1″), 128.3 (C-3″, C-5″), 129.0 (C-3′, C-5′), 136.2 (C-3), 137.0 (C-5), 140.1 (C-1′), 147.3 (C-7), 159.7 (C-4″), 169.0 (CO). HR-MS (ESI) m/z: Calcd for C27H32N7O2: [M1+H]+=486.2612. Found 486.2616. Anal. Calcd for C27H31N7O2: C, 66.78; H, 6.43; N, 20.19. Found: C, 67.05; H, 6.54; N, 19.98.
2-(Dimethylamino)-N-(7-(phenylamino)-1H-pyrazolo[3,4-c]pyridin-3-yl)acetamide (39a) and 2-(4-Methylpiperazin-1-yl)-N-(7-(phenylamino)-1H-pyrazolo[3,4-c]pyridin-3-yl)acetamide (39b)These compounds were prepared following an analogous synthetic procedure to that described for the synthesis of the compounds 14a–f, upon reaction of the derivatives 38a and b with trifluoroacetic acid, respectively. The product was purified by column chromatography using a mixture of dichloromethane–methanol (9 : 1, v/v) as the eluent to provide pure 39a or b.
-
Data for 39a: Yield 92%. Beige gum. 1H-NMR (400 MHz, DMSO-d6) δ: 2.32 (6H, s, N(CH3)2), 3.16 (2H, s, CH2), 6.97 (1H, t, H-4′, J=7.3 Hz), 7.12 (1H, d, H-4, J=5.8 Hz), 7.34 (2H, t, H-3′, H-5′, J=8.0 Hz), 7.69 (1H, d, H-5, J=5.8 Hz), 7.89 (2H, d, H-2′, H-6′, J=8.0 Hz), 8.95 (1H, br s, D2O exchang., NH–aniline), 10.17 (1H, br s, D2O exchang., NHCO), 12.90 (1H, br s, D2O exchang., NH–pyrazole). 13C-NMR (50 MHz, DMSO-d6) δ: 45.3 (N(CH3)2), 62.2 (CH2), 107.4 (C-4), 116.3 (C-3a), 118.4 (C-2′, C-6′), 119.9 (C-7a), 121.3 (C-4′), 128.7 (C-3′, C-5′), 135.3 (C-5), 140.8 (C-3), 141.7 (C-7), 157.9 (C-1′), 168.7 (CO). HR-MS (ESI) m/z: Calcd for C16H19N6O: [M1+H]+=311.1615. Found 311.1617. Anal. Calcd for C16H18N6O: C, 61.92; H, 5.85; N, 27.08. Found: C, 61.68; H, 5.71; N, 27.37.
-
Data for 39b: Yield 95%. Beige foam. 1H-NMR (400 MHz, DMSO-d6) δ: 2.31 (3H, s, CH3), 2.52–2.70 (8H, br s, piperazine H-2″, H-3″, H-5″, H-6″), 3.25 (2H, s, CH2), 6.97 (1H, t, H-4′, J=7.3 Hz), 7.12 (1H, d, H-4, J=5.8 Hz), 7.33 (2H, t, H-3′, H-5′, J=7.8 Hz), 7.69 (1H, d, H-5, J=5.8 Hz), 7.93 (2H, d, H-2′, H-6′, J=7.8 Hz), 9.13 (1H, br s, D2O exchang., NH–aniline), 10.20 (1H, br s, D2O exchang., NHCO), 13.11 (1H, br s, D2O exchang., NH–pyrazole). 13C-NMR (50 MHz, DMSO-d6) δ: 45.0 (CH3), 51.9 (piperazine C-2″, C-6″), 54.2 (piperazine C-3″, C-5″), 60.5 (CH2), 107.4 (C-4), 116.3 (C-3a), 118.4 (C-2′, C-6′), 119.7 (C-7a), 121.3 (C-4′), 128.7 (C-3′, C-5′), 135.3 (C-5), 140.9 (C-3), 141.9 (C-7), 158.1 (C-1′), 168.2 (CO). HR-MS (ESI) m/z: Calcd for C19H24N7O: [M1+H]+=366.2037. Found 366.2044. Anal. Calcd for C19H23N7O: C, 62.45; H, 6.34; N, 26.83. Found: C, 62.71; H, 6.48; N, 26.59.
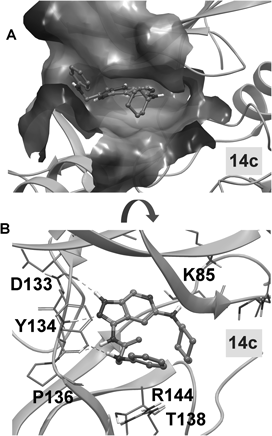
The crystal structure of human GSK3β (pdb id: 1UV5) was utilized for docking calculations using the modules of Schrodinger Small-Molecule Drug Discovery Suite 2016.21,26) Protein preparation was performed by the corresponding routine as implemented in Maestro. Prior to calculations, the studied compounds were prepared in terms of correct protonation states, tautomerism and stereoisomerism using the LigPrep routine of Maestro. Rigid docking was performed using the Glide-SP algorithm. The Van der Waals atom radii scaling was set to 0.8 for both the protein and docked ligands. The best poses were redocked by utilizing a flexible representation of the active site residues in a 5 Å sphere around the ligand using MacroModel. The OPLS2005 force-field, an implicit GB/SA water solvent model and the TNCG minimization algorithm were used.
Kinase Activity EvaluationKinase activities were assayed for each protein kinase in the appropriate buffers (listed below), with either a protein or a peptide as substrate in the presence of 15 µM [γ-33P] ATP (3000 Ci/mmol; 10 mCi/mL) in a final volume of 30 µL following an assay previously described.27) Controls were performed with appropriate dilutions of dimethylsulfoxide. Full-length kinases are used unless specified. Peptide substrates were obtained from Proteogenix (Oberhausbergen, France).
-
Buffers:
-
(A) 10 mM MgCl2, 1 mM ethylene glycol bis(2-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 1 mM dithiothreitol (DTT), 25 mM Tris–HCl pH 7.5, 50 µg/mL heparin, 0.15 mg/mL of BSA +0.23 mg/mL of DTT
-
(B) 60 mM β-glycerophosphate, 30 mM p-nitrophenylphosphate, 25 mM MOPS (pH 7), 5 mM EGTA, 15 mM MgCl2, 1 mM DTT, 0.1 mM sodium orthovanadate
-
(D) 25 mM MOPS, pH 7.2, 12.5 mM β-glycerolphosphate, 25 mM MgCl2, 5 mM EGTA, 2 mM ethylenediaminetetraacetic acid (EDTA), 0.25 mM DTT
-
(H) MOPS 25 mM pH 7.5, 10 mM MgCl2
-
(K) Tris 50 mM pH 7.5, 20 mM MgCl2, 2 mM MnCl2
-
(R) 5 mM MOPS pH 7.2, 2.5 mM β-glycerophosphate, 4 mM MgCl2, 2.5 mM MnCl2, 1 mM EGTA, 0.4 mM EDTA, 50 µg/mL BSA, 0.05 mM DTT
-
Each protein kinase was tested as follow:
-
HsRIPK3 (human, recombinant, expressed in Sf9 insect cells infected with 6xHIS : HsRIPK3 specific baculoviruses) was assayed in buffer R with 0.1 µg/µL of MBP (Myelin Basic Protein) as substrate.
-
HsHaspin-kd (human, kinase domain, amino acids 470 to 798, recombinant, expressed in bacteria) was assayed in buffer H with 0.007 µg/µL of Histone H3 (1–21) peptide (ARTKQTARKSTGGKAPRKQLA) as substrate.
-
HsCDK5/p25 (human, recombinant, expressed in bacteria) was assayed in buffer B, with 0.8 µg/µL of histone H1 as substrate.
-
HsAuroraB (human, recombinant, expressed by baculovirus in Sf9 insect cells, SignalChem, product #A31-10G) was assayed in buffer D with 0.2 µg/µL of MBP as substrate.
-
SscGSK3α/β (glycogen synthase kinase-3, porcine brain, native, affinity purified) was assayed in buffer A with 0.010 µg/µL of GS-1 peptide, a GSK-3-selective substrate (YRRAAVPPSPSLSRHSSPHQSpEDEEE, “Sp” stands for phosphorylated serine).
-
SscCK1δ/ε (casein kinase 1δ/ε, porcine brain, native, affinity purified) was assayed in buffer B, with 0.022 µg/µL of the following peptide: RRKHAAIGSpAYSITA as CK1-specific substrate.
-
RnDYRK1A-kd (Rattus norvegicus, amino acids 1 to 499 including the kinase domain, recombinant, expressed in bacteria, DNA vector kindly provided by Dr. W. Becker, Aachen, Germany) was assayed in buffer A with 0.033 µg/µL of the following peptide: KKISGRLSPIMTEQ as substrate.
-
MmCLK1 (from Mus musculus, recombinant, expressed in bacteria) was assayed in buffer A with 0.027 µg/µL of the following peptide: GRSRSRSRSRSR.