2017 Volume 65 Issue 4 Pages 356-358
2017 Volume 65 Issue 4 Pages 356-358
Red-shifted channelrhodopsins (ChRs) are attractive for optogenetic tools. We developed a new type of red-shifted ChRs that utilized noncovalent incorporation of retinal and 3,4-dehydroretinal-based enamine-type Schiff bases and mutated channelopsin, C1C2-K296G. These ChRs exhibited absorption maxima that were shifted 10–30 nm toward longer wavelengths than that of C1C2-ChR regenerated with all-trans-retinal.
The development of a new method of activating a specific neuron without affecting other cells would greatly benefit neuroscience. Optogenetics—a powerful new technique that enables control of neuronal activity by light—offers a number of advantages over previous electrode-based methods.1–4) In addition, optogenetics appears promising for clarifying neural circuits and understanding the mechanisms of various neurological diseases.5,6)
Channelrhodopsins (ChRs) are members of the rhodopsin family in microbes and consist of the chromophore retinal (1) and a seven-transmembrane helix apoprotein, termed channelopsin (Chop). The amino group of a lysine residue (K296) covalently binds to retinal through a protonated Schiff base (PSB)7) (Fig. 1a). ChRs function as light-gated cation channels by photoisomerization of the retinal from all-trans to 13-cis forms which results in a conformational change of the protein. Two ChRs, Channelrhodopsin-1 (ChR1) and Channelrhodopsin-2 (ChR2), are now widely used in optogenetics, and they are most sensitive to green and blue light, respectively.7,8) However, such short-wavelength light cannot deeply penetrate into the neural tissues, and optical fiber implants are often required to target the tissues. A truly noninvasive method for optogenetics requires the development of ChRs that are sensitive to long-wavelength light, and so red-shifted ChRs are sought-after. Toward this purpose, two types of red-shifted ChRs have been made: (i) mutation of amino acids in the vicinity of the retinal pocket,9–11) and (ii) substitution of retinal with other red-shifted chromophores that covalently bind to ChRs through a PSB.12–14)

In this Communication, we present new types of red-shifted ChRs: red-shifted chromophores 3 and 4, consisting of enamine-conjugated Schiff bases of 1 and 3,4-dehydroretinal (2), that non-covalently binds to chimera ChRs (Fig. 1b). This new red-shifted ChR system would have two benefits: (i) introduction of one or two conjugated double bond(s) into the chromophore would result in absorption of longer wavelength light for a red-shift of the UV-Vis spectrum, and (ii) instead of a covalent bond, formation of the selective chromophore-chimera Chop would occur by action of van der Waals forces.
Herein, we designed chromophores 3 and 4, which we expected to have red-shifted absorption maxima compared to 1 because the presence of the additional double bonds extended the conjugation of the chromophore.15) The most difficult problems for this study were (i) construction of the enamine-conjugated Schiff base unit, and (ii) purification of such compounds that seemed to be labile in acidic conditions. We resolved the former problem by isomerization of imines 5 and 6, which were easily prepared by condensation of allylamine and corresponding aldehydes 1 and 2 in the presence of MgSO4 in Et2O (Chart 1). Among several basic conditions for the isomerization, we found that the use of potassium tert-butoxide (t-BuOK) as a base and 18-crown-6 as an additive in tetrahydrofuran (THF) at 0°C16) effectively isomerized 5 to afford 3 as a mixture (3/5=87 : 13).17) The latter problem was partially cleared up by simply extracting with an Et2O/water system. For this purification, 18-crown-6 and water-soluble contaminants were completely excluded, but the separation of product 3 and unreacted 5 could not be achieved. For example, purification by column chromatography using neutral silica-gel or basic alumina for the stationary phase led to decomposition of 3. Therefore, the mixture of 3 including 5 was used for the binding experiment with chimeric Chops without further purification. Similarly, as above, chromophore 4 was obtained from 2 via imine 6 (4/6=7 : 3).18) The UV-Vis spectrum of the PSB derived from 3, which was measured in HCl–EtOH, exhibited an absorption maximum at 476 nm, which was red-shifted compared with that of the PSB derived from retinal (1) and n-butylamine (444 nm).

C1C2 chimeric ChR is composed from the helices 1–5 of ChR1 and the helices 6–7 of ChR 2, and it is one of the most widely used ChR in optogenetic applications because of its stability, large conductance, and almost negligible desensitization of photocurrent.19) In addition, the X-ray crystal structure of C1C2 at 2.3 Å resolution is available, and it provides precise information for three-dimensional architecture of C1C2.20) Based on the crystal structure, the cavity of the retinal binding pocket does not seem to be large enough to allow incorporation of our synthetic enamine-type Schiff bases, as the retinal binding lysine residue would interfere with the Schiff base. Thus, we constructed two C1C2 mutants to accommodate the larger chromophore: one is C1C2-K296A, in which the lysine at the retinal binding site, K296, was replaced by alanine, and the other is C1C2-K296G, in which lysine 296 was substituted with glycine.
In order to predict the behavior of the Schiff bases with the two C1C2 mutants, we conducted in silico docking simulations using MOE21) (Fig. 2). The E-values (minimum conformational energy of ligand) were 38.08 kcal/mol for C1C2-K296A and −56.58 kcal/mol for C1C2-K296G. These results indicated that the C1C2-K296G apoprotein was superior to C1C2-K296A for incorporation. The distance between the terminal methyl carbon of the chromophore and the Chop (methyl carbon of alanine in C1C2-K296A and the methylene carbon of glycine in C1C2-K296G) was almost 1 Å shorter for C1C2-K296A (3.34 Å) than that of C1C2-K296G (4.32 Å). This outcome indicated that the holding cavity of C1C2-K296G was larger than that of C1C2-K296A. In fact, in the binding assay of Schiff base 3 with mutated C1C2 Chops, only C1C2-K296G resulted in the formation of a new ChR 7 (see Supplementary materials, Fig. S1). This result was in good accord with the above docking simulation experiments. The steric repulsion of the two methyl groups (terminal methyl of the chromophore and the mutated alanine residue) seemed to interrupt the incorporation of 3 into C1C2-K296A. In the same way as above, Schiff base 4 was successfully incorporated into the C1C2-K296G apoprotein, resulting in ChR 8 characterized by a further red-shifted absorption maximum.
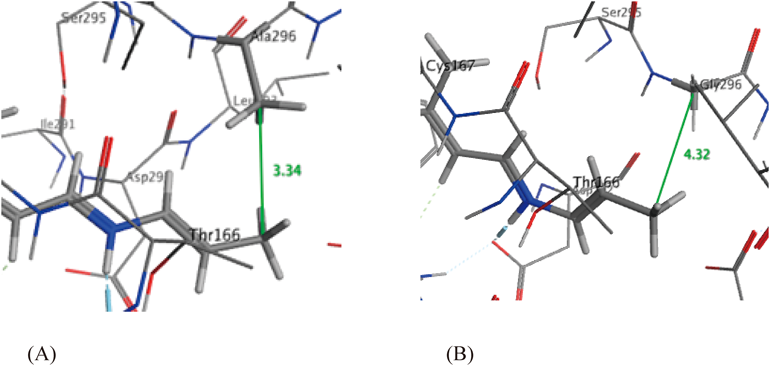
(A) With C1C2-K296A, E=38.08 kcal/mol. (B) With C1C2-K296G, E=−56.58 kcal/mol.
The absorption spectra and data are shown in Fig. 3 and Table 1. The shape of the absorption spectra of the new ChRs (7 and 8) were very characteristic compared to that of the original C1C2. Thus, 7 formed with enamine 3 showed a λmax at 508 nm, having a shoulder at the short-wavelength side (484 nm) and this shape resembled that of the original C1C2 (λmax at 476 nm, shoulder at 452 nm). On the other hand, 8 derived from enamine 4 exhibited its λmax at 488 nm and contained a shoulder at the long-wavelength side (520 nm). The λmax values were red-shifted 32 nm for 7 and 12 nm for 8, while the shoulder peak of 8 was shifted 46 nm toward longer wavelengths. Remarkably, the band widths of the absorption spectra were broadened compared to that of original C1C2, and the red-shift values of the half λmax value became greater than those of λmax. Thus, the red-shift values of half λmax were 38 nm for 7 and 47 nm for 8 in comparison with that of original C1C2. While the interaction between the chromophore and amino acid residues of C1C2-K296G probably underlie this phenomenon, the specific reason for it remains unclear.

0.02% DDM: n-dodecyl-β-D-maltoside, PM: 50 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), 140 mM NaCl, 3 mM MgCl2, pH 6.0.
| Chromophore | λmax (nm) | 1/2 λmax (nm) | |
|---|---|---|---|
| Schiff basea) | C1C2-K296Gb) | C1C2-K296Gb) | |
| Retinal (1) | 360c) | 452sh, 476d) | 507d) |
| 3 | 390 | 484sh, 508 | 545 |
| 4 | 399 | 488, 520sh | 554 |
a) In ethanol. b) In DDM/PM buffer. 0.02% DDM: n-dodecyl-β-D-maltoside, PM: 50 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), 140 mM NaCl, 3 mM MgCl2, pH 6.0. c) Prepared from n-butylamine. d) C1C2.
In summary, we prepared novel compounds with a conjugated double bond in the enamine side of the Schiff base in retinal and 3,4-dehydroretinal, and these chromophores were then incorporated into C1C2-K296G resulting in new ChRs, in which the UV-Vis absorption maxima exhibited a 10–30 nm red shift compared with the original C1C2. These results provide valuable insights toward the generation of a new type of ChR model, which is capable of longer wavelength absorption of light. The photochemical characterization of these new ChRs is now in progress.
We thank to Mr. Naoyuki Futamura and Ms. Yuka Niimi for their assistance in the chemical experiments. This work was supported in part by JSPS KAKENHI Grant No. 26450146 (to AW), JSPS Core-to-Core Program, A (Advanced Research Networks), The Science Research Promotion Fund and The Kobe Pharmaceutical Collaboration Fund.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.