2017 Volume 65 Issue 9 Pages 805-821
2017 Volume 65 Issue 9 Pages 805-821
My mission in catalysis research is to develop highly active and reusable supported catalytic systems in terms of fundamental chemistry and industrial application. For this purpose, I developed three types of highly active and reusable supported catalytic systems. The first type involves polymeric base-supported metal catalysts: Novel polymeric imidazole–Pd and Cu complexes were developed that worked at the mol ppm level for a variety of organic transformations. The second involves catalytic membrane-installed microflow reactors: Membranous polymeric palladium and copper complex/nanoparticle catalysts were installed at the center of a microtube to produce novel catalytic membrane-immobilized flow microreactor devices. These catalytic devices mediated a variety of organic transformations to afford the corresponding products in high yield within 1–38 s. The third is a silicon nanowire array-immobilized palladium nanoparticle catalyst. This device promoted a variety of organic transformations as a heterogeneous catalyst. The Mizoroki–Heck reaction proceeded with 280 mol ppb (0.000028 mol%) of the catalyst, affording the corresponding products in high yield.
The development of heterogeneous metal catalytic systems with high catalytic turnover, turnover-frequency, reusability, safety, sustainability, and low metal leaching are important not only to fundamental organic chemistry but also for their pharmaceutical and industrial applications.1–9) I am interested in the nano/micro space-controlled reaction field that should provide novel functionality, including reactivity, stability and selectivity. For this purpose, I have developed three kinds of heterogeneous metal catalytic systems as follows: (1) Highly active polymeric base-metal complex catalysts. A polymeric imidazole was used for the immobilization of palladium and copper salts to form immobilized polymeric metal catalysts. These catalysts with mol ppm were applied to organic transformation to afford the corresponding products in high yield. These catalysts were reused without loss of catalytic activity. (2) Polymeric metal catalyst membrane-installed microflow reactors. Catalytic polymeric palladium and copper were immobilized at the interface of two laminar flows in a microtube of the microreactors, providing catalytic membrane-installed microflow reactors. These microflow devices promoted organic transformation reactions, within a time range of a few seconds to several tens of seconds, to give the corresponding products in high yield. (3) A silicon nanostructure-based palladium nanoforest reactor. A novel silicon nanowire array-immobilized palladium nanoparticle catalyst was readily prepared from a silicon wafer as a support as well as a nano reaction field. By using the catalytic device, a variety of organic transformations efficiently proceeded to give the corresponding products in high yield. Especially, the Mizoroki–Heck reaction proceeded with 280 mol ppb (0.000028 mol%) of the wafer catalyst to afford the corresponding coupling products in high yield, which were applied to the synthesis of ozagrel, a thromboxane A2 (TXA2) synthesis inhibitor. I would like to introduce these results briefly in the following sections.
For the development of polymeric metal catalysts with high catalytic activity,10–17) I paid attention to metalloproteins, supramolecular metal-organic hybrids of polypeptides and metal ions. They efficiently promote enzymatic reactions as highly active catalysts.18) It is known that amphipathic imidazole units in some metalloproteins play a key role in high catalytic activity and stability. Thus, imidazoles in histidine coordinate to metal salts to form their catalytic site, the so-called supramolecular tertiary structure of proteins, and their Lewis basicity. Imidazole units also build the quaternary structure of proteins via ionic interaction and hydrogen bond formation. Therefore, imidazole polymer ligands are used as the building blocks of artificial metal-organic supramolecules.19–32) Especially, I was interested in urease, a polymeric imidazole–nickel complex.33) Since nickel belongs to the group 10 elements, I envisioned that some insoluble self-assembled catalysts of polymeric imidazole amphiphiles and the group 10 element metals should provide high catalytic activity with much higher reusability to produce metalloprotein–inspired polymeric metal catalysts.
Herein, I introduce the development of a novel metalloprotein-inspired polymeric imidazole palladium catalyst, MPPI-Pd.34–36) MPPI-Pd was applied to the allylic arylation/alkenylation/vinylation of allylic esters and carbonates with aryl/alkenylboronic acids, vinylboronic acid esters, and tetraaryl borates. Even amounts as small as 0.8–40 mol ppm Pd (0.00008–0.004 mol% Pd) of MPPI-Pd efficiently promoted the allylic arylation/alkenylation/vinylation in alcohol and/or water with a catalytic turnover number (TON) of 20000–1250000. Moreover, MPPI-Pd efficiently drove the Suzuki–Miyaura reaction of a variety of inactivated aryl chlorides, as well as aryl bromides and iodides in water with a TON of up to 3570000. The catalyst was reused for the allylic arylation and Suzuki–Miyaura reaction of an aryl chloride without loss of catalytic activity.
MPPI-Pd was prepared from poly[(N-vinylimidazole)-co-(N-isopropylacrylamide)5] (1)37) and (NH4)2PdCl4 (2) via molecular convolution. MPPI-Pd was obtained as a precipitated brown powder in 92% yield (Chart 1 and Fig. 1-a). While the polymer (1) was soluble in water, methanol, N,N-dimethylformamide (DMF), tetrahydrofuran (THF) and CH2Cl2, MPPI-Pd was hardly soluble in water, methanol, DMF, EtOAc, CH2Cl2 or hexane.


The precipitates formed a globular-aggregated and self-assembled structure (Fig. 1-b, scanning electron microscope (SEM) image) whose globules ranged from 100 to 1000 nm in diameter, and aggregated to construct a mesoporous suprastructure. Structural elucidation investigations were carried out using XAFS (XANES and EXAFS, Fig. 1-c), X-ray photoelectron spectroscopy (XPS), Raman and far-IR spectroscopy and elemental analysis, as well as density functional theory (DFT) calculation, providing the structure of cis-PdCl2L2 (L=imidazole moiety of the polymer) (Fig. 1-d).
With MPPI-Pd 3 in hand, its catalytic activity and reusability were examined for allylic arylation/alkenylation/vinylation of allylic esters with tetraarylborates, aryl/alkenylboronic acid, and vinylboronic acid esters. While there are numerous reports on aryl–aryl coupling with aryl boron reagents (Suzuki–Miyaura reaction), little attention has been paid to the more challenging allyl–aryl coupling (allylic arylation), which often requires a relatively high reaction temperature with a large amount (1–10 mol%) of catalyst.38–52) Therefore, the newly developed catalyst MPPI-Pd should be applied to the allylic arylation to provide high catalytic activity, reusability and substrate tolerance.
The allylic arylation of cinnamyl acetate (4a) with sodium tetraphenylborate (5a) in the presence of 40 mol ppm Pd (0.004 mol% Pd) of MPPI-Pd 3 was selected for the examination of catalytic activity and reusability (Chart 2). Thus, the reaction of 4a and 5a was performed in i-PrOH–H2O with 40 mol ppm Pd (0.004 mol% Pd) of MPPI-Pd at 50°C for 4 h to give 1,3-diphenylpropene (6a) quantitatively. The turnover number (TON) and turnover frequency (TOF) were 25000 and 6250 h−1, respectively, which are the highest numbers known to date for heterogeneous allylic arylation. MPPI-Pd was reused five times without loss of catalytic activity to afford 6a quantitatively. Inductively coupled plasma atomic emission spectroscopy (ICP-AES) analysis indicated that the coupling reaction with the reused (5th) catalyst provided no leaching of Pd species in the reaction mixture.

A variety of allylic acetates with tetraarylborates were used for allylic arylation with 40 mol ppm Pd of MPPI-Pd 3, as shown in Chart 3, where representative examples are shown. Electron-donating and -withdrawing group-substituted cinnamyl esters with substituted tetraaryl borates were efficiently converted to the corresponding products. For example, the reaction of 4b with 5a gave 6ba at a yield of 98%. The methyl-, ethyl-, and hexyl-vinyl carbinol esters underwent palladium-catalyzed allyl–aryl coupling with substituted tetraaryl borates: The coupling of 4c and 5a proceeded to give 6ca in 97% yield. While the reaction of alkyl vinyl carbinol esters should proceed through the corresponding π-allylpalladium intermediate bearing the β-sp3-hydride, which often suffers from β-hydride elimination to afford undesirable 1,3-dienes, no trace of 1,3-dienes was observed in the reactions. Moreover, the coupling of aliphatic 2-alkenyl acetates is more challenging than that of cinnamyl acetates because of the lower reactivity of the former. However, geranyl acetate, neryl acetate, prenyl acetate, and 2-hexenyl acetate were suitable substrates for this reaction. For instance, the reaction of geranyl acetate 4e with 5a gave the corresponding phenylated compound 6ea in 96% yield, during which isomerization was not observed. The reaction of alicyclic acetates cis-4f proceeded via net inversion to give trans-5-methoxycarbonyl-3-phenyl-1-cyclohexene (6fa) in 96% yield as a single diastereomer.

Since MPPI-Pd efficiently promoted the allylic arylation of allylic acetates, this catalytic system was applied to the allylic arylation of allylic carbonates (Chart 4). Thus, the coupling of methyl cinnamyl carbonate (7a) and 5a was carried out with 40 mol ppm Pd of MPPI-Pd in i-PrOH–H2O at 50°C for 4 h to afford 6a in 97% yield, where the TON and TOF of 3 were 24250 and 6060 h−1, respectively. It is noteworthy that the allylic arylation of ethyl–vinyl carbinol carbonates 7b also proceeded smoothly to quantitatively give the corresponding coupling products 6da in 97% yield, in which no trace of 1,3-dienes was observed. A 2-alkenyl carbonate, methyl neryl carbonate (7c), was converted to the phenylated product 6ga in 98% yield. These results indicated that a variety of allylic carbonates underwent the allylic arylation with 40 mol ppm Pd of MPPI-Pd under mild conditions.

The self-assembled catalyst MPPI-Pd with 40 mol ppm Pd also promoted allylic substitution with arylboronic acids and alkenylboronic acids, which are more versatile and readily available organoboron reagents (Chart 5). Thus, the allylic phenylation of 4a with phenylboronic acid (8a) along with methanolic or aqueous KF solution at 70°C proceeded smoothly to give 6a in quantitative yield. A substituted arylboronic acid 8b readily underwent allylic arylation under similar conditions to give 6ab in 97% yield. The ethyl vinyl carbinol ester 4d was converted to the corresponding alkene 6da in 99% yield. Allylic alkenylation, the allyl–alkenyl coupling reaction of allylic acetates with alkenylboronic acids, is more challenging in terms of both reactivity and the isomerization of olefins compared to allylic arylation.53–56) The reaction of 4a with (E)-1-pentenylboronic acid (8c) proceeded smoothly to give (1E, 4E)-1-phenyloctadiene (6ai) in 98% yield, where no isomerization was observed. Neryl acetate (4g) was also cross-coupled with the alkenylboronic acid 8c to afford the triene 9gc in 98% yield. Furthermore, the allylic vinylation of 4d with vinylboronic acid pinacol ester (8d) afforded the corresponding exo-dienes 6dd in 98% yield without the formation of isomers. The catalytic system was also applied to the reaction of allylic carbonates.

Since MPPI-Pd efficiently promoted allylic arylation and alkenylation without leaching Pd under heterogeneous conditions, 0.8 mol ppm Pd (0.00008 mol% Pd) of MPPI-Pd was used for the reaction of 4a and 5a (Chart 6). The desired coupling product 6a was quantitatively obtained in 12 h at 50°C, in which the TON and TOF were more than one million (1250000) and 104000 h−1, respectively. To the best of our knowledge, this is the highest TON and TOF for allylic arylation using heterogeneous catalysts.

The Suzuki–Miyaura reaction is one of the most important reactions used in synthetic organic chemistry and process chemistry for the preparation of pharmaceutics, solar cells, organic semi-conductors, and a variety of functional materials.57–59) One contemporary topic along these lines is the development of highly active and reusable heterogeneous catalysts for the Suzuki–Miyaura reaction of aryl chlorides, which are versatile and readily available-but inactivated-substrates.60–73) While polymer-supported alkyl phosphine ligands have been used (in the Suzuki–Miyaura reaction[?]), they generally are readily oxidized (decomposed) under atmospheric conditions. Heterogeneous catalysts for this purpose are still under development in terms of producing both high catalytic activity and reusability. Since MPPI-Pd has demonstrated high catalytic activity and reusability for allylic arylation, the big challenge for me was to develop its application to the Suzuki–Miyaura reaction of aryl chlorides, as well as aryl bromides and iodides in water under aerobic conditions with high catalytic activity and reusability.
To exhibit the highest catalytic activity for the heterogeneous catalyst-promoted Suzuki–Miyaura cross-coupling, the reaction of aryl chlorides, bromide and iodide was demonstrated (Chart 7). The reaction of an inactivated, less reactive 4-chlorotoluene (11a) with 8a was carried out with 66 mol ppm Pd of MPPI-Pd to give 4-methylbiphenyl (10aa) in 91% yield, whose TON reached 15000. The reaction of 4-chloroacetophenone (9b) and 4-chlorobenzophenone (9c) with 8a also proceeded to afford the corresponding biaryl products 10ba and 10ca in 92 and 94% yield, respectively. When the reaction of a variety of aryl bromides, 11a–e, was performed with 40 mol ppm Pd of MPPI-Pd, the coupling products 10aa, 10ca, 10da, 10ea were achieved in 97–98% yield. Moreover, the coupling of 4-iodotoluene (12a) with 8a proceeded with 0.28 mol ppm Pd (280 ppb; 0.000028 mol%) of MPPI-Pd to quantitatively give the cross-coupling product 10aa, whereas the same reaction did not proceed without the catalysts. The TON and TOF in this reaction were 3570000 and 119000 h−1, respectively, which are, as far as I know, the highest TON and TOF for heterogeneous catalyst-promoted Suzuki–Miyaura coupling.

As I described above, to go even further and also to ensure high catalytic activity, stability and reusability, the development of self-assembled polymeric imidazole-supported copper catalysts is one of the most interesting current topics in organic, organometallic, and supramolecular chemistry.74–90) My concept for the preparation of convoluted polymeric imidazole–metal catalysts would offer high catalytic activity with reusability for click reactions: Amphiphilic polymeric imidazole units coordinate to Cu species through self-assembly to provide a supramolecular polymeric metal composite with thermodynamic stability and insolubility. Here I describe the development of a novel self-assembled poly[(acrylamide-imidazole)-copper] catalyst, MPPI-Cu. A catalyst in amounts of 0.00045 mol% Cu (4.5 mol ppm Cu) to 0.25 mol% Cu promoted the Huisgen 1,3-dipolar cycloaddition of organic azides and terminal alkynes (Copper-catalyzed azide–alkyne cycloaddition) efficiently with a TON of up to more than 200000, and was reused without loss of catalytic activity or leaching of copper species.91)
A novel self-assembled polymeric imidazole-copper catalyst MPPI-Cu 13 was prepared from a linear amphiphilic polymer poly(N-isopropylacrylamide-co-N-vinylimidazole) (1) and copper sulfate (12) via a molecular convolution method as I described in Section 2.1. Incorporation of an aqueous solution of 12 into a chloroform solution of 1 drove the self-assembly to provide a polymeric copper composite, 13 (Chart 8). This resulting precipitate 13 was barely soluble in water, tert-butyl alcohol, ethyl acetate, toluene, or ether, whereas the starting polymer 1 was soluble in water, methanol, chloroform, tetrahydrofuran, and N,N-dimethylformamide. For the characterization of MPPI-Cu, SEM, HR-SEM, energy dispersive X-ray spectroscopy (EDX)/SEM, X-ray photoelectron spectroscopy (XPS), UV-Vis, elemental analysis and ICP-AES were utilized.

The amphiphilic polymeric imidazole–copper composite MPPI-Cu 13 was applied to the Huisgen 1,3-dipolar cycloaddition of organic azides and terminal alkynes (Chart 9). When the cyclization of phenylacetylene (14a) and benzyl azide (15a) was carried out with MPPI-Cu (0.25 mol% Cu) in a t-BuOH/H2O solution of sodium ascorbate (10 mol%) at 50°C for 1.5 h, MPPI-Cu drove the reaction smoothly to afford 1-benzyl-4-phenyl-1H-1,2,3-triazole (16aa) in 99% yield. The color of the catalyst changed from blue (Cu(II)) to green (Cu(I)) during the reaction by the reduction with sodium ascorbate. The catalyst was reused without loss of catalytic activity (second use 99%, third use 98%, fourth use 97%, fifth use 96%). There was no leaching of Cu species in the reaction mixture in cycloaddition with the reused catalyst (ICP-AES analysis). A hot filtration test was conducted to prove that the insoluble catalyst promotes the reaction under heterogeneous conditions, and that no copper species were released out into the reaction mixture. The cycloaddition of a variety of alkynes and organic azides (including in situ generation from benzyl bromide and sodium azide) was carried out under similar conditions to afford the corresponding triazoles in high yield. Even 4.5 mol ppm, based on the Cu of MPPI-Cu, drove the cyclization of 14a and 15a to provide 16a in 94% yield. The TON and the TOF of the catalyst reached 209000 and 6740 h−1, respectively; these are, as far as we know, the highest TON and TOF obtained for a heterogeneous-catalyst-promoted Huisgen cycloaddition.

Recently, flow microreactor systems offering many fundamental as well as practical advantages have been developed as innovative devices for rapid organic transformations.92–98) Molecular organic transformations with heterogeneous catalyst-immobilized flow microreactors are representative examples of these systems, where the efficiency of various organic transformations has been found to increase due to the vast interfacial area and the close distance of the molecular diffusion path in the narrow space of the flow microreactor.99–110) If a heterogeneous catalyst was immobilized as a membranous composite at the interface of an organic layer and aqueous layer (the center of the microchannel), two reactants could be oppositely charged into and flow through the divided channel, all the while in contact with the vast interfacial surface of the catalytic membrane from both front and back sides, thereby realizing an instantaneous chemical reaction. This concept is shown schematically in Fig. 2.

In this section, I describe the formation of a variety of membranous polymeric palladium catalysts inside a microchannel reactor at the laminar flow interface of the channel, in order to develop catalyst-immobilized microflow chemical reaction devices. These were applied to the palladium-catalyzed carbon–carbon bond forming reactions of allylic arylation and the Suzuki–Miyaura coupling under microflow conditions, where instantaneous production was achieved quantitatively within 5 s of residence time in the defined channel region.111–113)
Whitesides and co-authors reported polymer deposition at the laminar interface in the salt precipitation of a polymeric sulfonate salt and a polymeric ammonium salt.114–117) However, to the best of my knowledge, nothing has appeared in research to date on the interfacial deposition of transition metal complexes with the view of using them as catalytic membranes.
The formation of catalytic membranes was performed with a glass microchannel reactor118) having a Y-junction and a channel pattern of 100 µm wide, 40 µm deep, and 40 or 140 mm long (Fig. 3). The coordinative convolution of a poly(acrylamide-triarylphosphine) and palladium species was performed by the installation of an EtOAc solution of poly[(N-isopropylacrylamide)5-co-(4-diphenylstyrylphosphine)] and an aqueous solution of (NH4)2PdCl4, oppositely, into the microchannel (Chart 10). The two-phase parallel laminar flow was readily formed under flowing conditions, and a polymer membrane119–122) was precipitated at the interface of the laminar flow to give the microchannel device-1 (the μ-device 1) (Chart 10, 17). The palladium complex membrane of poly(4-vinylpyridine) was also installed into the Y-junction microchannel under similar conditions via coordinative convolution, affording the μ-device 2 (Chart 10, 18). The ionic convolution of cationic polyviologen, poly[4,4′-bipyridyl-co-1,4-bis(bromomethyl)benzene] and anionic PdCl42− was carried out under similar microflow conditions to give the μ-device 3 (Chart 10, 19). Optical microscopic observation showed that a sheet of polymeric Pd membrane was continuously formed at the interface of the laminar flow from the confluent position to the outlet.


Figure 3 shows the SEM and high resolution SEM images of the μ-device 1. SEM observation indicated that the polymeric Pd membrane was 1.3 µm thick and 40 µm high, and that the membrane was stuck to the glassware of the microchannel. A high resolution SEM image showed the membrane having a rough nap in the size range of several tens of nanometers.
In order to explore the utility of the microchannel devices in catalytic organic transformations, the synthetic ability of the three types of microchannel devices prepared above (the μ-devices 1–3) was examined for Suzuki–Miyaura coupling (Chart 11).123–125) A solution of iodobenzene (12b) in EtOAc/i-PrOH and an aqueous solution of 4-methoxyphenylboronic acid (8b) with Na2CO3 were oppositely introduced into the membrane-divided channels, the μ-devices 1–3, at 50°C, and two parallel laminar layers flowed through the channel in 4 s.126) The μ-device 1 had previously been successfully applied to the catalytic reaction to give a quantitative yield of the desired biaryl product. Thus, the μ-device 1 promoted the Suzuki–Miyaura reaction of 12b with 8b at 50°C for 4 s of residence time to afford 4-methoxybiphenyl (10bb) in 99% yield. In contrast, the μ-devices 2 and 3 gave 10bb in 0 and 15% yield, respectively. In the reaction with the μ-device 1, it was found that no palladium or phosphorus species were detected in the collected samples (ICP-AES).

With the instantaneous Suzuki–Miyaura coupling system in hand, I examined the coupling of diverse aryl/heteroaryl/alkenyl halides and arylboronic acids using the μ-device 1 where all reactions were completed within 4–5 s (Chart 12). The coupling of 3-ethoxycarbonyl-1-iodobenzene (12c) was carried out with 8b to afford the biaryl compound 10cb in 99% yield. The reaction of 2-iodothiophene (12d) with 8a was performed with the μ-device 1 to afford 2-phenylthiophene (10da) in 99% yield. The coupling of 3-iodopyridine (12e) was carried out with o-methylphenylboronic acid (8c) to give the phenylpyridine 10ec in 99% yield. The μ-device 1 efficiently promoted the cross-coupling of 5-iodo-2-furaldehyde (12f) with 3-thiopheneboronic acid 8d to give the 5-(3-thiophenyl)-2-furaldehydes (10fd) in 99% yield. The reaction of the alkenyl bromide 12g proceeded smoothly to afford ethyl cis-3-cinnamate (10ga) in 92% yield. Moreover, as a synthetic application to pharmaceutical compounds, the ethyl ester 10 ha of fenbufen, a cyclooxygenase inhibitor and a non-steroidal anti-inflammatory drug used to treat inflammation in osteoarthritis, ankylosis spondylitis, and tendinitis, was obtained quantitatively via the Suzuki–Miyaura reaction of 12h with 8a inside the μ-device 1 in 5 s.

The allyl–aryl coupling reaction (allylic arylation) was examined with cinnamyl acetate (4a) and sodium tetraphenylborate (5a) using μ-devices 1 (Chart 13). Thus, a solution of 4a in i-PrOH and an aqueous solution of 5a were oppositely introduced into the membrane-divided channels, the μ-device 1 (40 mm long), at 70°C. Two parallel laminar layers flowed through the channel in 1 s, and the resulting organic/aqueous micro stream was collected from the outlet to afford (E)-1,3-diphenylpropene (6aa). The catalytic membrane of the μ-device 1 remained intact during the reaction, and its morphology, as well as its catalytic activity, was similar to that before the catalytic reaction and after the 120 min flow of 4a and 5a, continuously affording 6aa in 99% yield.

I also envisaged the possibility of reducing the polymeric Pd complexes inside a microflow reactor to create the first polymeric Pd nanoparticle membrane-installed microflow devices. Such devices should promote some organic transformations that cannot proceed with their metal complex counterparts, thus providing instantaneous completion of the reactions (Fig. 4). Here, I describe the first development of a polymeric palladium nanoparticle membrane-immobilized microchannel device and its application to instantaneous, mild, safe and non-explosive hydrodehalogenation. The microflow hydrodehalogenation of aryl chlorides, bromides, iodides and triflates was carried out within a residence time of 2–8 s at 50–90°C using aqueous sodium formate as a reducing agent, and without the use of explosive hydrogen gas or toxic organic solvents. Hydrodehalogenation proceeded instantaneously to quantitatively afford the corresponding dehalogenated products.127)

A novel polymeric palladium nanoparticle membrane-immobilized microchannel device was prepared from linear polymer ligands and palladium species through molecular convolution followed by reduction. Thus, a yellow-colored palladium complex pre-catalytic membrane of poly(4-vinylpyridine) and Na2PdCl4 was prepared inside a microchannel under similar conditions to those mentioned above. Saturated aqueous sodium formate was streamed into the microchannel at 50°C for 30 min to yield the black-colored polymeric Pd nanoparticle membrane-installed μ-device 4 (Fig. 4). SEM images of the membrane of the μ-device 4 prepared in a batch reveals that the composite is a mesoporous material (ϕ=tens of nanometers) with homogeneity (Fig. 5). A transmission electron microscope (TEM) image of the μ-device 4 showed that Pd nanoparticles, with a diameter of 6.0±1.4 nm, were dispersed in a polymer matrix.

The hydrodehalogenation of diverse aryl chlorides, bromides, iodides, and triflates using the μ-device 4 with a residence time of 2–8 s was investigated (Chart 14). The reaction of electron-rich aryl halides, 4-methoxyphenyl chloride (9d), bromide (11d) and triflate (20d), proceeded at 50°C in 8 s to give 21a in quantitative yield. Surprisingly, a more reactive aryl iodide (4-methoxyphenyl iodide, 12d) did not react at 50°C (21a: 9% yield), although the general ease of the reductive hydrodehalogenation of organic halides followed the order of I>Br≈OTf>Cl.128) The decomposition of polychlorinated biphenyl (PCB) and polybrominated biphenyl (PBB) was also investigated. The hydrodehalogenation of 1000 ppm of chlorobiphenyl (22b, one of the PCBs) and bromobiphenyl (22c, one of the PBBs) was definitely transformed at 50°C with a residence time of 8 s to quantitatively give a fungicide biphenyl (23a). Furthermore, 10 ppm and 100 ppm of 22b were perfectly decomposed to provide 23a in quantitative yield; no 22b was detected by GC/FID analysis.

As described above, I have developed a highly active, insoluble, amphiphilic polymeric imidazole Cu catalyst, which, with 4.5–45 mol ppm Cu, drove the Huisgen 1,3-dipolar cycloaddition of alkynes and organic azides. I envisioned that if highly active heterogeneous catalysts could be immobilized inside a microchannel, as shown in the previous section, the Huisgen cycloaddition should be competed instantaneously.129–136) Herein, I describe the development of the first polymeric membranous copper catalyst-installed microflow reactor, and its applicability to the Huisgen cycloaddition of alkenes and organic azides. A variety of triazoles were quantitatively produced within a residence time of a few seconds by using the membranous copper catalyst-installed microflow reactor.137)
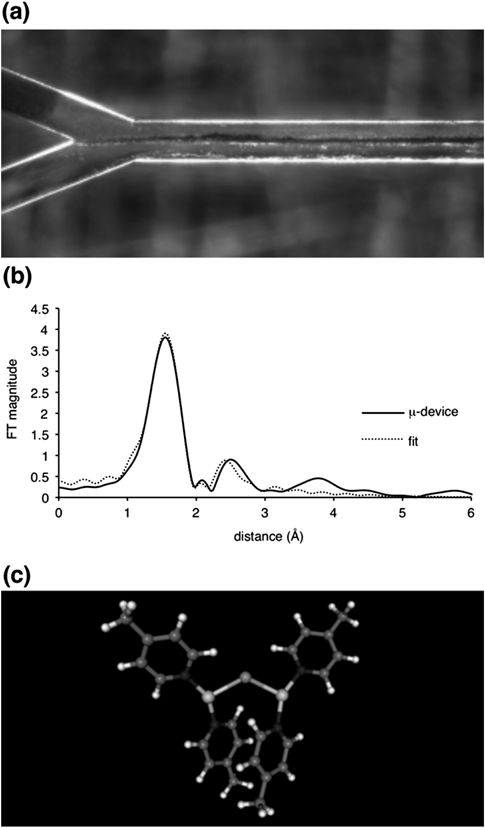
A novel polymeric membranous copper catalyst–installed microflow device, μ-device 5, was prepared (Chart 15 and Fig. 6). A poly(4-vinylpyridine) (PVPy) in ethyl acetate/methanol (3 : 1), aqueous copper(II) sulfate, and an aqueous solution of sodium ascorbate (NaAsc) and sodium chloride (NaCl) were installed in the microchannel at 25°C for 60 s, then 2% methanolic ethyl acetate and an aqueous solution of NaAsc and NaCl were installed at 50°C for 10 min. Instantaneously, two-phase parallel laminar flow formed under flow conditions, and a polymeric copper membrane of approximately 10 µm thickness precipitated out at the interface of the laminar flow. EDX/SEM analysis showed that Cu and Cl species were dispersed uniformly in the membrane, and S species were not detected (Fig. 6). XANES, EXAFS and elemental analyses, as well as second-order Møller–Plesset perturbation theory (MP2) calculations and DFT calculations, suggested that the local structure near the Cu complex is a μ-chloro dinuclear copper complex [CuI2(μ-Cl)(py)4]+ (py=pyridine unit in PVPy).

The reaction of a variety of substituted benzylic azides 3 and alkynes 4 was carried out using μ-device 5 (Chart 16). When an acetone/water solution of 15a, 14a and NaAsc was injected from one inlet, and an acetone/water solution was installed from the other inlet at 50°C, the reaction with the microflow device A proceeded smoothly in 8 s to give 1-benzyl-4-phenyl-1H-1,2,3-triazole (16aa) in 99% yield, and leaching of the Cu species was found to be less than 50 ppb (by ICP-MS analysis). A fluoro compound, 4-fluorobenzyl azide (15d), was readily converted under similar conditions to afford the triazole 16ad in 99% yield, in 13 s. The reaction of a naphthyl substrate, 2-naphthylmethyl azide (15e), for 13 s gave the triazole 16ae in 92% yield. An alkynol, 5-hexyn-1-ol (14c), underwent the cycloaddition with 15a in 38 s to afford 16ca in 99% yield. When the N-acetyl-D-glucosamine (GlcNAc) derivative 15f, bearing amide and acetal moieties, was connected with the alkyne 14e bearing a terminal alcohol, a plausible connector to a variety of proteins and sugars, at 70°C in 38s, the desired triazole carbohydrate 16ef was readily obtained in 85% yield.

Innovative nanodevices for catalytic organic transformations are expected to realize instantaneous, selective catalytic reaction systems.138,139) I envisioned that the development of hybrid catalysts of Pd nanoparticles140–143) and a silicon nanowire array as a macroscopic and nanoscopic hybrid catalyst would be promising for this purpose (Chart 17). The silicon nanowire array, applied to silicon-based optoelectronics, fuel cells, solar cells and photoelectrodes, was obtained by the metal-assisted chemical etching of silicon wafers.144–153) Copious nanospaces can be provided on the surface of a silicon wafer whose area is a square centimeters wide. The hybrid catalysts should be equipped with confined nano-size reaction fields surrounded by a lot of Pd nanoparticles with a square centimeter wide silicon wafer.

I describe a novel platform for the catalytic organic transformations: a silicon nanowire array-stabilized palladium nanoparticle catalyst, SiNA-Pd. SiNA-Pd was applied to the Mizoroki-Heck reaction, where the quantitative production of coupling compounds was achieved with 490 mol ppb (0.000049 mol%) Pd, which is also described. SiNA-Pd was reused without loss of catalytic activity. Moreover, SiNA-Pd promoted the hydrogenation of an alkene, the hydrogenolysis of nitrobenzene, the hydrosilylation of an α,β-unsaturated ketone, and the C-H arylation of thiophenes and indoles.154)
The silicon nanowire array-stabilized Pd nanoparticle catalyst, SiNA-Pd, was prepared as shown in Chart 17.155–164) Thus, a p-type silicon wafer was treated with H2SO4/H2O2 and aqueous HF for cleaning and installation of the Si-H surface (H-termination), respectively.165 AgNO3 reacted with the H-terminated wafer to give an Ag nanoparticle-coated wafer that was treated with aqueous HF/H2O2, affording the Ag nanoparticle-deposited silicon nanowire array. The removal of Ag nanoparticles and the regeneration of the Si-H surface were carried out with HNO3 and aqueous HF, respectively. Immobilization of Pd nanoparticles was performed with K2PdCl4 on the silicon nanowire array to obtain the silicon nanowire array-stabilized Pd nanoparticle catalyst (SiNA-Pd).
An SEM image of the section of SiNA-Pd revealed that the length and width of its nanospace were 5 µm and <800 nm, respectively, where the aspect ratio was ca. 60 (Fig. 7). BET analysis indicated that the specific surface area of SiNA-Pd was 30 times larger than the original flat silicon wafer (SBET 117 cm2/cm2 vs. 4 cm2/cm2). A TEM image of SiNA-Pd showed the dispersion of Pd nanoparticles whose diameter ranged from approximately 5–10 nm. Pd 3d XPS of Pd nanoparticles suggested the formation of a zerovalent Pd species.

SiNA-Pd was applied to a variety of organic transformations (Chart 18). The hydrogenation of an alkene, stilbene (24), proceeded in the presence of SiNA-Pd (0.3 mol% Pd) in EtOH under hydrogen (1 atm) to give 1,2-diphenylethane (25) in 99% yield. The catalyst was readily recovered and reused to afford 6 in 99% (2nd use) and 99% (3rd use) yield. The hydrogenation of nitrobenzene (26) was performed to give aniline (27) in 99% yield. The hydrosilylation of an α,β-unsaturated aldehyde 28 and Et3SiH was carried out to give the enolsilyl ether 29 in 82% yield.166)

Moreover, since the development of C-H arylation is an important topic, SiNA-Pd was applied to these C-H bond functionalization reactions of thiophenes and indoles (Chart 19).167–173) The reaction of 12b with 2-methylthiophene (30) was carried out with SiNA-Pd (0.3 mol%) and CsOAc in DMF, then the reaction proceeded to give 2-methyl-5-phenylthiophene (31) in 80% yield.174–177) The coupling of 12b with an indole (32) also proceeded under similar conditions to give the corresponding indole 33 in 63% yield.178)

To attain the highest catalytic activity for the heterogeneous catalyst-promoted Mizoroki–Heck reaction, SiNA-Pd with 490 mol ppb Pd (0.000049 mol% Pd) was used for the reaction of the 10-g scale substrate (Chart 20). When the reaction of 12b (10.2 g) and 34 was carried out with 490 mol ppb Pd, the desired product 35 was obtained in 95% yield. The TON and TOF were 2000000 and 40000 h−1, respectively. As far as I know, this is the highest TON for the Mizoroki–Heck reaction with heterogeneous catalysts.

Ozagrel 37, an important antiasthmatic agent (thromboxane A2 synthesis inhibitor),179) was synthesized via the 490 mol ppb Pd SiNA-Pd-catalyzed Mizoroki–Heck reaction. The reaction of 4-iodobenzylalcohol (12i) (11.7 g) and 34 was carried out with 490 mol ppb Pd of SiNA-Pd under similar conditions to give the cinnamate 36 in 71% yield. Installation of an imidazole unit, alkaline hydrolysis, and acidification provided ozagrel hydrochloride 37.
I have presented the development of three types of heterogeneous metal catalytic systems. The first involves the development of highly active polymeric base-metal complex catalysts. A polymeric imidazole was used for the immobilization of palladium and copper salts to form immobilized polymeric metal catalysts. These immobilized catalysts with mol ppm were applied to organic transformations to afford the corresponding products in high yield. These catalysts were reused without loss of catalytic activity. The second involves the development of polymeric metal catalyst membrane-installed microflow reactors. Catalytic polymeric palladium and copper were immobilized at the interface of two laminar flows in a microtube of the microreactors, providing catalytic membrane-installed microflow reactors. These microflow devices promoted organic transformation reactions within a few seconds to several ten seconds to give the corresponding products in high yield. The third one is the development of a novel silcon nanostructure-based palladium nanoforest reactor. A novel silicon nanowire array-immobilized palladium nanoparticle catalyst was prepared from a silicon wafer to act as both support and as a nano reaction field. By using these catalytic devices, a variety of organic transformations efficiently proceeded to give the corresponding products in high yield.
Still, my mission for the development of much more highly active and much more reusable supported catalytic systems in terms of fundamental chemistry and industrial application is ongoing. I believe ultimate heterogeneous catalytic systems should be created in the near future, and I would like to contribute to developing these.
This review of the author’s work was written by the author upon receiving the 2016 Pharmaceutical Society of Japan Award for Divisional Scientific Promotion.
I would like to sincerely express my respect and gratitude to Professor Dr. Yasuhiro Uozumi, Institute for Molecular Science and RIKEN, for his kind, powerful and valuable suggestions and advice regarding this research. I also thank all my co-workers in these studies for their contributions, including their excellent creativity, interest and enthusiasm. We gratefully acknowledge financial support from Japan Science and Technology Agency (JST) ACT-C (JPMJCR12ZC), JST CREST, the Japan Society for the Promotion of Science (JSPS) (15K05510, 24550126, 20655035, 16790025), the Takeda Science Foundation, the Naito Foundation, and RIKEN.
The author declares no conflict of interest.