2018 Volume 66 Issue 3 Pages 191-206
2018 Volume 66 Issue 3 Pages 191-206
The global occurrence of viral infectious diseases poses a significant threat to human health. Dengue virus (DENV) infection is one of the most noteworthy of these infections. According to a WHO survey, approximately 400 million people are infected annually; symptoms deteriorate in approximately one percent of cases. Numerous foundational and clinical investigations on viral epidemiology, structure and function analysis, infection source and route, therapeutic targets, vaccines, and therapeutic drugs have been conducted by both academic and industrial researchers. At present, CYD-TDV or Dengvaxia® is the only approved vaccine, but potent inhibitors are currently under development. In this review, an overview of the viral life circle and the history of DENVs is presented, and the most recently reported antiviral candidates and newly discovered promising targets are focused and summarized. We believe that these successes and failures have enabled progress in anti-DENV drug discovery and hope that our review will stimulate further innovation in this area.
Approximately 390 million cases of dengue virus (DENV) infection occur worldwide annually,1) including 96 million that result in severe symptoms. As a mosquito-borne viral disease, high infection and incidence rates are found in tropical and sub-tropical countries. Climate variation2) and human behaviors, such as urbanization and international travelling, have also accelerated DENV transmission. According to the WHO, the Western Pacific region reported more than 375000 suspected incidents in 2016.3) In the Asia-Pacific region, the infected population accounts for almost three-quarters of the total number of global infections.4)
Although most subjects can recover from the mild flu-like dengue fever (DF), the deterioration to dengue hemorrhagic fever (DHF) or dengue shock syndrome (DSS) should never be neglected. In 2006, in order to improve the management of dengue cases, the WHO revised dengue symptoms from “DF, DHF, DSS” to “DF with warning signs, DF without warning signs, and severe dengue (SD).5) Each year, an estimated 12500 SD deaths are reported by the WHO3); however, the current treatment is limited to fluid replacement and supplemental medical care,6,7) and therefore, there is an urgent need for effective anti-DENV drugs. In 2015, the first vaccine, Dengvaxia® (CYD-TDV),8) was approved in Mexico, which heralded the beginning of studies of the DENV vaccine. However, the development of small molecule anti-DENV drugs has been a slow process. To date, only four small molecule anti-DENV drugs (Table 1), chloroquine (ClinicalTrials.gov Identifier: NCT00849602), celgosivir (NCT01619969, NCT02569827), balapiravir (NCT01096576), and UV-4B9) (NCT02061358, NCT02696291) have entered Phase I or Phase II clinical trials.10) Currently, the trial status and outcome of NCT00849602 remains unclear; NCT01619969 and NCT02569827 achieved the required safety profile, but did not reduce viral load as expected,11,12) and the clinical trial of the α-glucosidase inhibitor NCT02696291 was terminated at Phase I.10)
 |
Promising anti-DENV drugs are anticipated to inhibit all serotypes of DENV (DENV1–4 and presumably DENV-5)13,14); although Katzelnick et al. suggested that DENVs should be classified according to antigenic differences,15) in this review, we have followed the widely adopted traditional classification by serotype. The inhibition of all serotypes, and the antibody-dependent enhancement phenomenon observed during DENV infection, complicates the investigation of anti-Dengue drugs: if a patient was re-infected by a heterotypic virus, the antibodies created previously would become pathogenic.16) Moreover, in laboratory testing, the limited availability of preclinical animal models has slowed the pace of drug development. As DENV-infected mice activate the interferon (INF) responses and the severe forms that occur in humans do not develop naturally in laboratory mice, only a few mouse models, such as suckling mice, immunocompetent mice (e.g. C57BL/6 mice), humanized mice (e.g. SCID-huK562 mice), and immunocompromised mice (e.g. AG129, A129, AB6 mice), were developed,17) but it is still hard to generalize these models to the human body.
This review mainly discusses the promising DENV targets and antiviral inhibitors during the previous five years (2012 to 2017). To begin, the history of DENV is described; subsequently, its genome organization and life-cycle are introduced. In the final section, the important anti-DENV targets are summarized and a discussion of emerging inhibitors is conducted.
The word “dengue” is widely reported to originate from the Swahili word “Ki-dinga pepo,” which means “cramp-like pains, produced through the agency of an evil spirit”.18) Between 265 and 420 A.D. (the Jin Dynasty), a record of dengue-like illness was first found in an ancient Chinese medical book as “Shuidu”, which means “water poison”. This record was formally edited in 610 A.D. (Tang Dynasty) and 992 A.D. (the Northern Sung Dynasty).19,20) There was a possibility that the epidemics in 1779 in Batavia (Jakarta), Indonesia, and Cairo, Egypt were dengue; and it was also quite likely that the Philadelphia epidemic in 1780 was dengue.19) Thus, before the 18th century, dengue had already achieved a wide geographic distribution.
In 1906, it was demonstrated that the transmission occurred via the Aedes mosquito. In the following year, a series of studies confirmed that DENVs were transmitted in a fashion similar to the “jungle cycle” of yellow fever virus.21) Before the 1950 s, rare epidemics of DF occurred tropical regions. After World War II, this pattern of the disease was broken. During the 1950 s, the epidemic of DHF was first recognized in Manila, the Philippines. In the subsequent 20 years, the disease had spread throughout Southeast Asia.19) Although the outbreaks of dengue have been reported for hundreds of years, DENV-1 and DENV-2 were isolated in 1943 in Japan and in 1945 in Hawaii, respectively. The Japanese virologists, Dr. R. Kimura and Dr. S. Hotta, have achieved their names in the history through these isolations22); however, more details are beyond of the purpose of this review. In 1953, DENV-3 and DENV-4 were first reported in the Philippines and Thailand. Since then, DENV infections have been found or reported in Asia every year.23)
DENVs belong to the genus Flavivirus of the family Flaviviridae.24) The genome is approximately 11 kilobases long, containing a single open reading frame (ORF) with untranslated regions (UTRs) at both ends. The 5′ terminal is capped, but the 3′ terminal is non-polyadenylated. A large precursor polyprotein of more than 3000 amino acids is encoded by the ORF and is cleaved by host proteases and virus NS3 protease into at least ten proteins, including three structural proteins, capsid (C), membrane precursor (prM), and envelope (E), and seven non-structural (NS) proteins, NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS521,25) (Fig. 1).

The invasion starts from the adhesion to host receptors, and the subsequent hijack of host machinery helps DENV to multiply. The abundant release of mature viruses created by the Golgi Network endanger the host. Viral proteins are synthesized through the cleavage of a large polyprotein into small functional structures. The major active enzymes during the cleavage are viral proteases and host proteases. The red triangles indicate the sites cleaved by the former and the green triangles indicates the sites cleaved by the latter.
The DENV life cycle contains the following major events: viral entry, fusion and disassembly, viral genome replication, viral protein translation and processing, assembly, maturation, budding, and release. After attachment to the susceptible host cell receptors, receptor-dependent endocytosis begins. Subsequently, a low pH-dependent membrane fusion occurs, triggered by conformational change of the E protein (Fig. 2), and a viral core is inserted into the cytoplasm of the host cell. In the cytoplasm, the viral core is uncoated, releasing RNA genome and viral proteins. The positive viral RNA is translated into a polyprotein, which is cleaved to create viral proteins by virus and host proteases. Meanwhile, the positive viral RNA is translated into an intermediate negative RNA template, which is a template for subsequent genomic replication. The created viral proteins and replicated genome are packaged into virions in the endoplasmic reticulum (ER) during viral maturation. After passing the virions into the Golgi vesicles, prM is cleaved into membrane (M) protein by host furin and induces viral maturation. Finally, maturated virions are released by exocytosis21,25–28) (Fig. 1).

a) The structure of capsid constructed by copies of envelope proteins (1k4r.pdb). b) Top: an illustration of the membrane fusion step. Left bottom: the structure of dimeric E protein before conformational change (1k4r.pdb). Right bottom: the structure of trimeric E protein after conformational change (4gsx.pdb). Domain I, Domain II, and Domain III are colored in red, yellow, and blue, respectively. c) Octyl-β-D-glucoside (β-OG) binds to E proteins (1oke.pdb). The two β-OG molecules are presented in space filling format and colored magenta. The van der Waals surface map of dimeric E proteins was calculated by MOE.
Among the essential proteins, the NS3/NS2B protease, NS3 helicase, E protein, methyltransferase (MTase), and RNA-dependent RNA polymerase (RdRp) of NS5, and the host factors in the life-cycle of DENV are potential targets for the design of antiviral inhibitors in the past 5 years.
4.1. NS3/NS2B ProteaseThe success of the NS3 protease (NS3pro) as a drug target against human immunodeficiency virus (HIV)-1 and hepatitis C virus (HCV) inspired a similar study against DENV. NS3pro is a trypsin-like serine protease with catalytic a triad, His51, Asp75, and Ser135 (Fig. 3a), which cleaved the precursor protein and the host proteases (Fig. 1); thus, the inhibition of NS3pro could stop the processing of viral proteins and decrease the viral load. However, it is difficult for the flat and hydrophilic catalytic center of NS3pro (Fig. 3b) to bind inhibitors. Recent research discovered that the cleavage site had a preference for dibasic residues29) and that the NS2B protein contributed to the enhancement of protease activities30,31) (Fig. 3d). In addition, an allosteric site (Fig. 3c) behind the catalytic center was also reported to be promising.32)

a) A cartoon representation of superposed DENV and HCV NS3pro structures (Yellow: 2fom.pdb; Water Blue: 4ktc.pdb). The residues of the catalytic triad, His51, Asp75, and Ser135, are shown in stick representation. b) A superposition of the surfaces of DENV and HCV NS2B/NS3pro (Yellow: 2fom.pdb; Water Blue: 4ktc.pdb). c) A cartoon representation of superposed DENV and WNV NS3pro structures (Yellow: NS3pro form 2fom.pdb; Orange: NS2B from 2fom.pdb; Blue: NS3pro form 2ijo.pdb Water Blue: NS2B from 2ijo.pdb). The catalytic triad, His51, Asp75, and Ser135, is shown in stick representation. The surface of DENVpro was shown in pink and α balls expressing binding sites found by MOE are shown in red and blue. A blue circle indicates the catalytic site. d) A different angle from C and an allosteric site behind the catalytic site are shown in blue circles and blue spheres. e) A prediction of the binding mode of SK-12 and NS3pro is shown. SK-12 is presented as a space-filling representation and the carbon atoms are colored green. SK-12 is predicted to insert into a space occupied by NS2B.
Dengue NS3 helicase (NS3heli), located at the C-terminal domain, is a superfamily 2 DEAH-box helicase with three sub-domains, D1, D2, and D3. The functions of NS3heli in DENV infection have not been fully determined, but the major functions were assumed to be the ATP driven separation of annealed viral RNA and double-strand unwinding. One mutagenesis study of viral recombinants recently reported that the special positions 225, 268, and 538 required basic amino acids to enhance viral RNA replication, and that Arg560 and Arg599 were important to retain the structure of NS3heli.33)
4.3. Envelope ProteinThe envelope (E) protein, the basic unit of the DENV surface, consists of a transmembrane anchor, a membrane-proximal stem region, and hydrophobic domains I, II, and III (Fig. 2b). A total of 180 E protein copies yields the icosahedral symmetry of surface structure (Fig. 2a). The E protein, a class II fusion protein, acts on virion assembly and viral-target cell membrane fusion. In the mediator action of the E protein, the pH-dependent change from an antiparallel dimer to trimer triggers the fusion process: domain II is spanned to bare fusion peptides (or fusion loops) by the rearrangement of stems, domain I, and domain III; subsequently, fusion peptides interact with the host membrane, the so-called “zip up” step bundles three hydrophobic trans-membrane anchors and domain II, and gathers hydrophobic domain I and III; finally, the amphipathic structure is a trigger in the late stages of fusion34) (Fig. 2b).
The successful example of enfuvirtide (the first anti-HIV drug targeting the virus-host fusion process) indicated the opportunities in targeting membrane fusion. The X-ray structures of the E protein revealed the existence of a hydrophobic pocket, to which octyl-β-D-glucoside (β-OG) could bind (Fig. 2c); thus, both the β-OG pocket and complex per se (involving the dimer or triad) became promising targets for anti-DENV inhibitors.
4.4. Methyltransferase and RNA-Dependent RNA Polymerase Domains of NS5As the largest NS protein, NS5 consisted of 900 residues and acted on genome replication, transcription, and capping. NS5 has two domains: methyltransferase (MTase) and RNA-dependent RNA polymerase (RdRp).
The MTase domain, located at the N-terminal (amino acids 1–262), could methylate the N7 of the guanine cap in the capping/methylation of the 5′ of viral positive RNA and the internal methylation of 2′-OH of the first nucleotide ribose. Both methylation and internal methylation are S-adenosine-L-methionine (SAM)-dependent and can generate the by-product S-adenosine-L-homocysteine (SAH).
The RdRp domain, located at the C-terminals (amino acids 272–900), could synthesize intermediate RNA during DENV genome replication and semi-conservative transcription. The crystal structures of the RdRp domain and full-length NS5 revealed a right-hand structure with fingers, palm, and a thumb (Fig. 4). The catalytic center is located in the RNA template tunnel, with a priming loop that assists in moving the thumb domain towards the finger domain to form an initiation complex for the de novo synthesis of primers. This structure is considered to be a “closed” form. A large structural rearrangement, in which the priming loop moves outside the tunnel to create a more “open” form, is thought to occur in RNA elongation. Therefore, when targeting the RdRp domain, the structural rearrangement should be taken into account.

The RdRp domain adopts a typical right-hand structure and the parts are colored separately.
Other viral proteins that play an important role in DENV infection can be targeted. With a deeper understanding of DENV, many different targets on different viral proteins, such as NS1, NS2B, and NS4B, have been proposed.
DENV recruits host proteins and machinery to breed offspring in its life-cycle, so host proteins could also be targets. However, it is a “double-edged sword”: the host proteins that survive long after the evolution of the organism are highly likely to have essential or beneficial effects, which makes inhibition or interference risky owing to the undesirable drug-induced side effects; nevertheless, compared with the frequently mutated viral proteins, host proteins are much stable and provide high barriers to drug resistance, which makes non-permanent inhibition a rational approach.
Several herbal medicine targets and newly discovered potent by-products were also discovered against DENV, but the targets or mechanisms have not been fully validated. The ingredients of herbal medicines are complex and may interact with targets in a synergistic or additive manner; additionally, owing to the unintentional nature of the discovery of the anti-DENV effects from by-products, more validation experiments are needed.
As research into antiviral inhibitors has been a hotspot for the discovery of anti-DENV drug, many compounds have been identified as anti-DENV inhibitors in the past five years. Based on the targets mentioned before, we have summarized the NS3/2B inhibitors in Table 2 and presented other potent inhibitors that bind to different targets in Table 3.
| Name of the Inhibitors | Structure | Inhibitory Activities for Dengue Virus 2 | Notes | References |
|---|---|---|---|---|
| 1 | Ac–Phe–Ala–Ala–Gly–Arg–Arg-αketo-Ser–Leu–CONH2 | Ki=47 µM | [D. Leung, 2001] | |
| 4 | Ac–Phe–Ala–Ala–Gly–Arg–Arg–CHO | Ki=16 µM | [D. Leung, 2001] | |
| 35 |  |
Ki=0.4 µM | [M. Behnam, 2014] | |
| IC50=0.6 µM | ||||
| 42a |  |
IC50=0.21 µM | [L.F. Weigel, 2015] | |
| 45a |  |
IC50=0.26 µM | [L.F. Weigel, 2015] | |
| 104 |  |
EC50=3.42 µM | [M. Behnam, 2015] | |
| 9 (or 1) |  |
Ki=2.2 µM | [S. Xu, 2012] | |
| IC50=114.2 µM | [Y. Takagi, 2017] | |||
| 29 |  |
IC50=1.7 µM | CC50=134.1 µM | [Y. Takagi, 2017] |
| EC50=11.8 µM | ||||
| Anthraquinone (ARDP0006, 5) |  |
EC50=4.2 µM | permeability at pH 7.4: 4.45 *10−6 cm s−1 | [S.M. Tomlinson, 2009] |
| reduced viral titer more than 1 log PFU/mL at 1 µM | [J.J. Chu, 2015] | |||
| 23i |  |
IC50=9.45 µMa | a: fluorometric enzyme assays | [J. Deng, 2012] |
| IC50=24.7 µMb | b: biochemical inhibition of protease in cell culture | |||
| BP13944 |  |
EC50=1.03 µM | assay using DENV replicon reporter | [C.C. Yang, 2014] |
| ZINC04321905 |  |
Ki=7 µM | [U. Viswanathan, 2014] | |
| MB21 |  |
IC50=5.95 µM | [R. Raut, 2015] | |
| Compound 14 |  |
EC50=5.0 µMa | a: fluorometric enzyme assays (BHK21) | [L. Li, 2015] |
| EC50=5.0 µMb | b: biochemical inhibition of protease in cell culture (HuH7) | |||
| 16 |  |
IC50=48.2 µM | [A.K. Timiri, 2015] | |
| 19 |  |
IC50=121.9 µM | [A.K. Timiri, 2015] | |
| 7 |  |
10% inihibition at 50 mMa | a: fluorometric enzyme assays | [H. Wu, 2015] |
| IC50=9.3 µMb | b: biochemical inhibition of protease in cell culture | |||
| EC50=2.5 µMc | c: antiviral activity, inhibition of viral replication | |||
| 8 |  |
IC50=3.6 mMa | a: fluorometric enzyme assays | [H. Wu, 2015] |
| IC50>3 µMb | b: biochemical inhibition of protease in cell culture | |||
| EC50>3 µMc | c: antiviral activity, inhibition of viral replication | |||
| 4e |  |
IC50=15.22 µM | fluorometric enzyme assays | [H. Osuman, 2017] |
| 4j |  |
IC50=16.23 µM | fluorometric enzyme assays | [H. Osuman, 2017] |
| Policresulen |  |
IC50=0.48 µMa | a: fluorometric enzyme assays | [D.W. Wu, 2015] |
| IC50=4.99 µMb | b: biochemical inhibition of protease in cell culture | |||
| SK-12 |  |
EC50=0.98 µM | EC50=0.97 µM (DENV-1) | [S. Pambudi, 2013] |
| EC50=2.43 µM (DENV-3) | ||||
| EC50=0.74 µM (DENV-4) | ||||
| NSC135618 |  |
IC50=1.8 µM | CC50=48.8 µM | [M. Brecher, 2017] |
| EC50=0.81 µM | ||||
| Biliverdin |  |
Ki=8.55 µM | [C. K. Tseng, 2016] |
| Target | Name of the Inhibitors | Structure | Inhibitory Activities for Dengue Virus 2 | Notes | References |
|---|---|---|---|---|---|
| NS3heli | ST-610 |  |
EC50=0.272 µM | EC50=0.236 µM (DENV-1) | [C.M. Byrd, 2013] |
| EC50=0.256 µM (DENV-3) | |||||
| EC50=0.203 µM (DENV-4) | |||||
| NS3heli | Suramin | 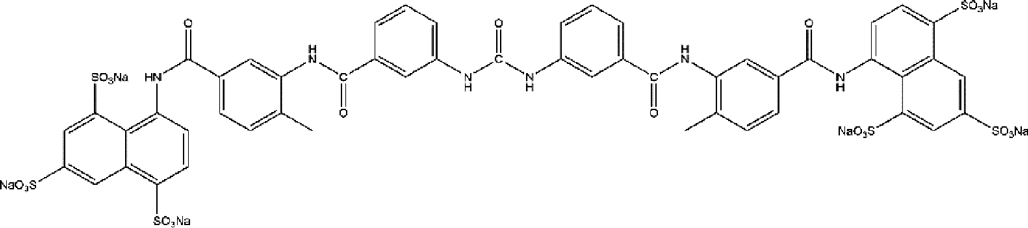 |
— | IC50=0.4 µM (DENV4NS3FL) | [C. Basavannacharya, 2014] |
| IC50=0.6 µM (DENV3NS3FL) | |||||
| IC50=0.8 µM (DENV3NS3H) | |||||
| IC50=0.8 µM (DENV4NS3H) | |||||
| Entry Inhibitor | DN59 | 692MAILGDTAWDFGSLGGVFTSIGKALHQVFGAIY724 | IC50 in 10 µM range | [Y. M. Hrobowski, 2005] | |
| Entry Inhibitor | DV2419-447 | 419AWDFGSLGGVFTSIGKALHQVFGAIYGAA447 | IC90=0.3 µM | with a solubility tag RGKGR at C-terminal | [A. G. Schmidt, 2010] |
| Entry Inhibitor | DV2419-447(www 442-444) | 419AWDFGSLGGVFTSIGKALHQVFGWWWGAA447 | IC90<25 nM | with a solubility tag RGKGR at C-terminal | [A. G. Schmidt, 2010] |
| Entry | EF | EF | IC50=96.8 µM | [A. Panya, 2014] | |
| Entry | MLH40 | 1SVALVPHVGMGLETRTETWMSSEGAWKHVQRIETWILRHPG40 | IC50=31.41 µM | based on the ectodoman of M protein | [A. Panya, 2015] |
| Entry (β-OG) | Compound 6 |  |
EC50=0.068 µM | [Q. Y. Wang, 2009] | |
| Entry (β-OG) | 1662G07 |  |
IC90=16.9 µM | [A. G. Schmidt, 2012] | |
| Entry (β-OG) | 3-110-22 |  |
IC90=0.74 µM | [A. G. Schmidt, 2012] | |
| Entry (β-OG) | 1 |  |
— | IC50=116 µM (DENV-1) | [T. Abe, 2014] |
| Capsid | ST-148 |  |
EC50=0.052 µM | [P.Scaturro, 2014] | |
| RdRp | NITD-1 |  |
IC50=1.5 µM | [P. Niyomrattanakit, 2010] | |
| RdRp | NITD-2 |  |
IC50=0.7 µM | [P. Niyomrattanakit, 2010] | |
| RdRp | SOF |  |
EC50=9.9 µM | [H.T. Xu, 2017] | |
| RdRp | HeE1-2Tyr |  |
IC50=15 µM | IC50=6.8 µM (DENV-1) | [D. Tarantino, 2016] |
| IC50=10 µM (DENV-3) | |||||
| IC50=7.7 µM (DENV-4) | |||||
| RdRp | DMB220 |  |
EC50=2.8 µM | EC50=2.7 µM (DENV-1) | [H.T. Xu, 2016] |
| EC50=2.7 µM (DENV-3) | |||||
| EC50=2.2 µM (DENV-4) | |||||
| RdRp | 66E2 |  |
— | 90% inhibition of replication were obatined at 5 µM (48hpi) | [A. Madhvi, 2017] |
| NS4B | 1a |  |
EC50=0.012 µM | EC50>1 µM (DENV-1) | [Q.Y. Wang, 2015] |
| EC50=0.032 µM (DENV-3) | |||||
| [B. Zou, 2015] | |||||
| EC50>1 µM (DENV-4) | |||||
| NS4B | 14a |  |
EC50=0.042 µM | EC50>20 µM (DENV-1) | [Q.Y. Wang, 2015] |
| EC50=0.076 µM (DENV-3) | |||||
| [B. Zou, 2015] | |||||
| EC50>20 µM (DENV-4) | |||||
| IMPDH | 10-Allyl-7-cloro-9(10H)-acridone |  |
EC50=13.5 µM | [M.B. Mazzucco, 2015] | |
| NS5-IMPα/β1 | 4-HPR |  |
EC50=2.1 µM | EC50=2.6 µM (DENV-1) | [J.E. Fraser, 2014] |
| EC50=1.4 µM (DENV-3) | |||||
| EC50=2.1 µM (DENV-4) | |||||
| INF-α | Celastrol |  |
EC50=0.12 µM | EC50=0.19 µM (DENV-1) | [J.S. Yu, 2017] |
| EC50=0.16 µM (DENV-3) | |||||
| EC50=0.17 µM (DENV-4) | |||||
| Host ribosome (E-stie) | LTM |  |
EC90=0.4 µM | [M. Carocci, 2016] | |
| Host S1P | PF-429242 |  |
EC50=1.2 µM | [A. Hyrina, 2017] | |
| [L. Uchida, 2016] | |||||
| Unknown (BTK? Other host factor?) | QL47 |  |
IC90=0.343 µM | [M. Wispelaere, 2017] | |
| Unknown (host factor?) | YKL-04-085 |  |
IC90=0.555 µM | [Y. Liang, 2017] | |
| c-Src/Fyn | SaracatinibAZD0530 |  |
EC90=12.2 µM | [P.L. Yang, 2013] | |
| c-Src/Fyn | Dasatinib |  |
EC90=4.7 µM | [P.L. Yang, 2013] | |
| NS5-NS3 interaction & c-Src/Fyn | 16i |  |
EC50=5.3 µM | [P. Vincetti, 2015] | |
| Furin | 45 |  |
Κι=77.3 µM | [J. Kouretova, 2017] | |
| Furin | 46 |  |
Κι=83.1 µM | [J. Kouretova, 2017] | |
| HO-1 | CoPP |  |
— | showed improved survival rates in ICR suckling mice at 50 mg/kg | [C.K. Tseng, 2016] |
| HO-1 | Andrographolide |  |
— | showed improved survival rates in ICR suckling mice at 50 mg/kg | [C.K. Tseng, 2016] |
| 2K peptide? | 1-Acetyllycorine |  |
EC50=0.4 µM | [P. Wang, 2014] | |
| Unknown (against vial replication) | BRC |  |
EC50=1.2 µM | EC50=1.6 µM (DENV-1) | [F. Kato, 2016] |
| EC50=0.8 µM (DENV-3) | |||||
| EC50=0.8 µM (DENV-4) | |||||
| Unknown | N-Desmethylclozapine |  |
IC50=1.0 µM | [G.R. Medigeshi, 2016] | |
| Unknown | Fluoxetine |  |
IC50=0.38 µM | [G.R. Medigeshi, 2016] | |
| Unknown | Salmeterol |  |
IC50=0.67 µM | [G.R. Medigeshi, 2016] |
NS3/2Bpro inhibitors are required to compete with substrate peptides with P1 and P2 positions, which are basic Arg residues. α-Keto amides and tri-/tetra-peptides (three or four residue peptides) containing electrophilic groups were discovered to interact with the catalytic pocket. In the NS3/2B expression model CF40.NS3pro, four α-keto amides were reported by Leung et al.35); the best were 1 (Ki=47 µM) and 4 (Ki=16 µM).
After the CF40.NS3pro model, several researchers from Novartis and academia reported active tri-/tetrapeptide inhibitors.36–41) The sequences of these inhibitors were modified from natural substrates by using different caps, electrophilic warheads, and unnatural amino acids to improve inhibitory activity or reduce cellular toxicity. The major basic sequences were Bz-Nle-Lys-Arg-x, X-Lys-Arg-(Lys/Arg)-Arg-H, and Bz-X-Lys-Phg-NH2; where x stands for different electrophilic warheads (e.g., aldehydes, trifluoromethyl ketones, and boronic acids) and X stands for N-terminal cap and substituted residues, respectively.
Yin et al. from Novartis studied a series of Bz-Nle-Lys-Arg-x compounds and found that Bz-Nle-Lys-Arg-B(OH)2 showed the strongest inhibitory activity (Ki=43 nM), but had poor bioavailability.36) Among the P1–P4 residues, P4 was the weakest contributor and P2 was the strongest37); consequently, 35, 42a, 45a, and 104 were designed. Among the designed inhibitors, only 42a and 45a were tested for membrane permeability by using Parallel Artificial Membrane Permeation Assay (PAMPA), with 45a additionally tested for metabolic rate in vivo. However, neither of the two compounds showed membrane permeability, and metabolism of 45a was fast (t1/2=22 min).40) Considering the pharmacodynamics and pharmacokinetics drug candidates, the membrane permeability should be tested on other reported inhibitors.
5.2. Non-linear Peptide NS3/2B Protease InhibitorsCyclic peptides, which restrict the freedom of residues, were another candidate for NS3/2Bpro inhibitors. Xu et al.42) tested the peptide library of conotoxins from Conus and found MeIA was active against DENV NS3/2Bpro. An eight-residue peptidyl ring named 9 showed cell-permeability and stability, with the lowest Ki of 2.2 µM, whereas the linear peptide showed dramatically decreasing activity.
Shionogi Co., Ltd. performed the structure–activity relationship (SAR) studies of Xu’s compound 9 (renamed as 143)). After conducting alanine scanning on every residue in 1, they found that P1–3 strongly affected NS3/2Bpro activity. Through the introduction of the aromatic rings of L-homoPhe and D-Phe into the P2′ and P4′ positions and D-Pro or D-Ala into P4, they obtained one optimum peptide, with the lowest IC50 (114.2 mM) in the fluorometric enzyme assays; however, this peptide was not active in the BHK-21 cell based assay. Through the replacement of the sequence with the combination of Arg and hydrophobic residues and the introduction of high affinity for the membrane and endocytosis, 29 and 32 were developed, with low IC50 and EC50 values, and a Selectivity Index (SI) greater than 10.43)
Neither of the above cyclic peptidyl inhibitors were tested in vivo. Compared with the linear peptide inhibitors, there are fewer reports on cyclic peptidyl inhibitors, which suggests there is scope for the discovery of novel cyclic peptidyl inhibitors.
5.3. Non-peptide NS3/2B Protease InhibitorsNon-peptide inhibitors can be classified into three major types: catalytic center inhibitors, the most commonly reported type; allosteric site inhibitors; and NS2B binding inhibitors.
Owing to the progress in Computer Aided Drug Design (CADD) during the past five years, most inhibitors were first selected via in silico or high-throughput screening, which was followed by the evaluation of their antiviral activities via in vitro or in-cell based assays. Catalytic center inhibitors, such as 23i, ZINC04321905, MB21, compound 14, and policresulen, and compounds with various skeletons, such as anthracene derivate (ARDP0006), quinolone contained scaffold, quaternary ammonium bromide (BP13944), piperidone-phthalimides (16 and 19), diaryl thioethers (7 and 8), sulfonamides (4e and 4j), and heme catabolism (biliverdin), exhibited anti-DENV activities.44–54) Among the above, MB21 was discovered in a carefully designed two-step docking study.
The allosteric site inhibitor NSC13561832) was screened from the “split luciferase complementation (SLC) assay” and was the only compound to demonstrate allosteric activity in the NIC library. The NS2B binding inhibitor, SK-12,31) was the first found to target the interface between NS2B and NS3 and displayed anti-DENV1–4 activity (EC50=0.98 µM; SI=68.63).
Many other in silico studies also revealed potent inhibitors. Mirza et al.55) conducted molecular docking and dynamic simulations on 18 million compounds and identified five top-ranked compounds (ZINC95518765, ZINC44921800, ZINC71917414, ZINC39500661, and ZINC36681949) with potent anti-DENV1-4 activity; Dwivedi et al.56) screened 20000 pure compounds from a traditional Chinese medicine (TCM) database and found five compounds (eriodictyol 7-O-glucuronide, luteolin 8-C-beta-glucopyranoside, [−]-epicatechin-3-O-gallate, 6-O-trans-p-coumaroylgeniposide, and luteolin-7-O-glucoside) that exhibited promising activity against DENV. However, the above in silico results are unconvincing until sufficient experimental validation has been obtained.
The shortcomings of these NS3/2Bpro studies are unavoidable: many results were merely based on biochemical assays without confirmation in cell-based assays; sometimes, even the biochemical assays were not conducted to a standard protocol, which made the direct comparisons of IC50 or Ki impossible.
The study of Chu et al.57) revealed that validation studies cannot be neglected. They conducted a validation study on 15 compounds with reported activity in human cell-based viral quantification assays to test the anti-DENV activities and membrane-penetrating capabilities. Most of the compounds failed owing to low cell permeability and high cytotoxicity. The most promising compound found was anthraquinone (ARDP0006 or 5), which was highly membrane permeable and reduced the viral titer by more than 1 log PFU/mL at 1 µM without the induction of cytotoxicity effects.57)
One further limitation was that most structure-based docking studies were performed on the “open form” of the protease, owing to the lack of NS2B, or a partial length NS2B in the structure. However, the “closed form” is considered to be the active form. Several groups constructed two noncovalent component proteases by using independent vectors of NS2B and NS3 but co-expressed them in Escherichia coli cells. Although this is not the natural state, this unlinked NS2B-NS3pro is thought to result in a “closed form”. The activities of the compounds to this “closed form” should be reevaluated.58)
5.4. NS3 Helicase InhibitorsByrd et al.59) reported a benzoxazole inhibitor, ST-610, which was a potent inhibitor of DENV2 (EC50=0.272 µM; EC90=3.59 µM) in the viral titer reduction assay. The compound was non-toxic against DENV1–4 in cell culture and was mutagenic in Ames tests. A mutation of A263T mapping at a basic patch within a channel in NS3heli was correlated with the susceptibility of ST-610. Another report from Basavannacharya et al. constructed a molecular beacon helicase assay for NS3heli. Through screening a library of 1600 compounds, they identified suramin as a potent non-competitive inhibitor.60)
5.5. Entry InhibitorsThe first mimic peptide entry inhibitor was created by Hrobowski et al.61) A 33-residue peptide, DN59, was derived from a helix parallel to the membrane of the E protein (99% inhibition of plaque formation at a concentration of <25 µM). This peptide was effective for DENV1–4, but not specific to DENV.
Schmidt et al.62) truncated residues 419 to 447 of the E peptides from DENV1–4, WNV, and other flaviviruses. They found that DV2419–447 had the strongest anti-DENV activity (IC90≈0.3 µM). Despite the high similarities between DV419–447 and WNV419–447, a significant variation in inhibitory activities was observed. After testing many mutations, they identified a peptide, DV2419–447(www 442–444), with IC90<25 nM.
Panya et al.63) tested small peptides (i.e., tri-/di-peptides) on the β-D-glycoside binding pocket by using in silico methods. After the evaluation of seven peptides, they found that Glu-Phe (EF) had anti-DENV activity, with an IC50 value of 96.8 µM against DENV2. Panya et al.64) also published the mimic peptidyl inhibitor, MLH40, which was active against DENV1–4. This was a 40-residue peptide derived from the M protein of the dengue virus, with an IC50 value of 31.41 µM against DENV2. As the target of MLH40 was M–E interaction, its antiviral activities and inhibition of viral entry were secure.
Small molecular weight inhibitors have superior absorption; the development of these was focused on to avoid the costly and inconvenient route of drug injection. Wang et al.65) reported a promising compound, compound 6, which contained a thiophene ring attached to a quinazoline core ring (0.0680 µM<EC50<0.496 µM). The effects of the compound remained for 75 min after virus infection, which supported its interaction in the entry stages of the virus without acidification effects. The colocalization of a biotinylated compound 6 (called compound 7 in the original paper) and viral particles showed that compound 7 and compound 6 interacted with E proteins.
Most structure-based in silico drug design was conducted by docking studies and these compound–protein interactions were score function based. Abe et al. conducted computational design by using the fragment molecular orbital (FMO) method,66) an approximate molecular orbital method for the calculation of large molecules that was developed by Kitaura et al.67) The starting structure was octyl-β-D-glucose, an inhibitor that binds to the β-OG pocket. Through the creation of rational interaction models and the assessment of the interactions between octyl-β-D-glucose and the E protein with FMO, the authors found the 1, octyl-2-O-sulfo-β-D-glucose was active against DENV1 (IC50=116 µM).
A group of computational chemists from India used de novo design to identify four compounds that targeted β-OG; subsequently, they conducted pharmacophore analysis, docking studies, and molecular dynamics (MD) simulations. They mentioned that compound #3 could counteract the movement of the kl β-hairpin during the MD simulation, which indicated that it may be a candidate compound for further studies.68)
Another group conducted in silico screening on 828 compounds from the ZINC, ChemSpider, and PubChem databases. They presented the structures and the predicted ΔG0 and pKi of the top nine compounds and five reference compounds. Although the newly found compounds showed weaker binding ability compared with the reference compounds, better pharmacological activity was predicted for 26124033 (5-(3,4-dichlorophenyl)-N-[2-(p-tolyl)benzotriazol-5-yl]furan-2-carboxamide).
Another group from India focused on the dissimilarities of β-OG pockets among DENV1–4 and created homological models for all serotypes. Through the assessment of the pocket, the previous docking reported β-OG inhibitors, 18466 plants, and a few fungal alkaloids from all the models. They predicted drug-likeness, such as pharmacokinetics, aqueous solubility, CYP interaction, and optimization of the virtual leads. Several compounds were predicted to be promising candidates.69) Although these in silico studies led to the identification of potent inhibitors, in vitro and on-virus tests are also needed.
Some natural compounds or extracts from plants, such as sulfated galactomannans,70) were also reported to have anti-DENV activity. ST-148, reported as a potent DENV capsid inhibitor (EC50=0.052 µM), enhanced capsid-protein interaction and caused steric hindrance and/or structural rigidity that inhibited both the entry and assembly or release of infectious virions.71,72)
5.6. RdRp InhibitorsTo inhibit DENV viral RdRp, several approaches were introduced: nucleosides or their analogs that terminate the replication of RNA; non-nucleosides that block allosteric sites and affect the activities of RdRp (e.g., N-sulfonylanthranilic acid derivatives [NITD-1 and NITD-2], sofosbuvir [SOF], and HeE1-2Tyr, 66E2); and metal chelating agents that target essential ions in the catalytic process (DMB220).73–78)
Two studies reported new findings from the re-conformation of active compounds and demonstrated a new approach for the discovery of inhibitors. One group from Spain conducted an in silico study based on the previous study45) of NITD-1 and NITD-2 rather than laboratory testing. They found 39 NITD-like compounds and predicted their pharmacokinetic properties and toxicity potential.75) Xu et al. evaluated the inhibitory activity of (SOF) against DENV replication. SOF is an anti-HCV prodrug that can be metabolized to the active triphosphate form (SOF-TP). They revealed that SOF-TP was incorporated into the nascent RNA and terminated the elongation of RNA to induce drug mutation in RdRp (S600T).76)
Owing to the high degree of conformational freedom, the inhibitor HeE1-2Tyr77) was assumed to participate in intermolecular π–π stacking. This wrapped conformation interacted with Trp795 located in the NITD-binding site, as supported by the crystal structure. The point mutation of Trp795Ala and Asn492Phe in the NITD-binding site residue did not affect the activity of HeE1-2Tyr. Tarantino et al. focused on the discrepancy and pointed out that another pocket, Site 2, was formed by the movement of a priming loop during RdRp reaction with dsRNA.
5.7. MTase InhibitorsTambunan et al. adopted an in silico approach to find proper cyclic peptidyl and SAH-like inhibitors of NS5 MTase,79,80) but did not conduct any experimental tests. To find peptidyl inhibitors, they conducted docking studies on a pool of 300 commercial cyclic peptides. The binding modes of the hit peptides were further examined by MD simulations and absorption, distribution, metabolism, excretion, and toxicity (ADMET) predictions were conducted. For SAH-like inhibitors, 3460 modified SAH-like compounds were validated in the same way and SAH-M1356 was discovered as a promising candidate.80)
5.8. Inhibitors Targeting Other Viral ProteinsA research group from Novartis reported the activity of spiropyrazolopyridones against NS4B. The first hit was a racemate obtained from screening in the DENV2 replicon assay. After separation of the enantiomers, they found the R enantiomer possessed superior activity (1a). 1a inhibited DENV2 and DENV3, but was not as effective against DENV1 and DENV4. In DENV2 and DENV3, valine was conserved at position 63 of NS4B, where resistant mutations were found, which suggested V63 might be responsible for the anti-DENV activities. Although 1a is active, it has poor solubility. SAR studies were performed and the potent inhibitor 14a was synthesized, with better physicochemical properties. Through assays on the host proteins, 14a was demonstrated to be safe and efficacious at both the time of infection and at 24 h after in vivo infection.81,82) However, the spectrum of 14a was not so broad that no effects were detected in DENV1 and DENV4.
5.9. Inhibitors Targeting Host ProteinsThe anti-DENV activity of molecules was also demonstrated through interaction with various host factors, such as inosine monophosphate dehydrogenase (IMPDH), importin α (IMPα)/β1, IFN-α, ribosome, site-1 protease (S1P), Bruton’s tyrosine kinase (BTK), Fyn and c-Src kinases, furin, heme oxygenase-1 (HO-1), and the hydroxymethylglutaryl (HMG)-CoA reductase pathway.
Mazzucco et al. reported that 10-allyl-7-chloro-9(10H)-acridone inhibited all four dengue serotypes83); however, the target of this compound was not clear. The addition of exogenous guanosine partially recovered DENV2 infectivity, which indicated that the host IMPDH enzyme was one of the targets. This compound was proven to be safe in host cell toxicity experiments, but additional animal experimental tests are still required.
Fraser et al. reported N-(4-hydroxyphenyl)retinamide (4-HPR) as an inhibitor of NS5-IMPα/β1 and showed the inhibitory activity against DENV1–4. 4-HPR enhanced three branches (the PERK, ATF6, and inositol-requiring enzyme 1 (IRE1) pathways) of the UPR, which is a cell stress response that exists in both the presence and absence of DENV2 inhibition, and decreased the cytokine level.84) However, 4-HPR did affect ADE-mediated DENV protection in vivo.
Yu et al. reported that the Tripterygium wilfordii extract, celastrol, upregulated IFN-α, suppressed DENV RNA replication, and protected against DENV in mice models.85)
One group from Harvard Medical School reported that lactimidomycin (LTM) from Streptomyces amphibiosporus inhibited DENV2 in cell culture (EC90=0.4 µM). The antiviral effects were mediated by the binding of the E-site of host ribosome. The authors also mentioned that although the target was a host protein, LTM was non-toxicity at the active concentration.86)
Uchida et al. reported a S1P inhibitor, PF-429242. S1P is a host protease that cleaves sterol regulatory element-binding proteins (SREBPs), which positively regulate cellular lipids, such as lipid droplets (LDs). Uchida et al. reported the antiviral activities of PF-429242 against DENV1–4; however, the inhibition was not restored by the addition of exogenous lipids.87) Another paper on the anti-DENV effects of PF-429242, by Hyrina et al., used a different cell line (Huh-7.5.1) and found that PF-429242 exerted anti-DENV activity through the inhibition of S1P and the depletion of LDs.88) The addition of exogenous oleic acid recovered the antiviral activity. The above studies showed that S1P was a promising target for the design of anti-DENV molecules.
A covalent host BTK inhibitor, QL47, was reported by Wispelaere et al. to inhibit DENV2 (IC90=0.343 µM; CC50=67 µM).89) Liang performed SAR studies on QL47 and identified the improved efficacy of YKL-04-085. Unlike QL47, YKL-04-085 lacked hinge-connecting nitrogen and lost all the kinase inhibitory activities, but it retained potent anti-DENV potential (IC90=0.555 µM; CC50=20 µM) and in vivo studies showed that intraperitoneal administration was sufficient to achieve a good drug profile.90)
Two host-cell-targeted inhibitors, saracatinib (AZD0530) and dasatinib, were reported by Yang’s group.91,92) Interactions were found between these two inhibitors and the fyn and c-Src kinases, but the latter interaction was more critical.92) Another group found a multitarget c-Src/Fyn inhibitor, 16i,93) which interacted with both the allosteric site of RdRp and the host proteins.
Kouretova et al. reported that the peptidyl inhibitors 45 and 4694) of furin had both anti-DENV and anti-WNV activities.95)
Tseng et al. reported that HO-1 inhibited DENV replication, which indicated that HO-1 may be a host target for anti-DENV agents. The authors also reported the anti-DENV activity of two HO-1 inducers, cobalt protoporphyrin (CPP) and andrographolide.54)
Soto-Acosta et al. reported that DENV increased HMG-CoA reductase activity via AMP activated protein kinase (AMPK) inactivation and metformin (AMPK activator) and that lovastatin (HMG-CoA reductase inhibitor) altered the co-localization between HMG-CoA reductase and viral proteins; this pathway was therefore a potential new target.96)
5.10. Other InhibitorsAnti-DENV compounds with unclarified targets were also reported, such as the methanol extracts of A. paniculata and M. charanita,97) curcumin,98) and the methanolic extract of Cissampelos pareira, but the anti-DENV effective components and mechanism are still under study.99)
Lee et al. evaluated the anti-DENV2 effect of a cocktail of aqueous extracts from four Phyllanthus spp., and found that that the cocktail altered the expression of 13 regulatory viral and host proteins that act on viral attachment, viral entry, polyprotein production, replication, assembly, and maturation.100) The molecular mechanism and the effect on other serotypes were not reported.
Medigeshi et al. reported a repositioning study on LOPAC1280 database and found that N-desmethylclozapine, fluoxetine, and salmeterol were active against DENV2,101) with IC50 values in the range from 0.3–1.0 µM.
2-Bromo-α-ergocriptine (Bromocriptine: BRC) was reported as an active compound targeting DENV replication. The effect was independent from previously reported antagonist activity to dopamine receptors. The major resistance mutation was detected as N374H in the NS3 protein. BRC was inactive against proteases, NTPases, RdRp, and other viral enzymes in vitro; hence, other viral proteins or host factors were assumed to be the target.102)
Derivatives of lycorine, isolated from Amaryllidaceae,103) were studied. More than 40 derivatives were chemically synthesized and 1-acetyllycorine was selected as the best derivative (EC50=0.4 µM in the Cell-Based Flavivirus Infection (CFI) assay, SI>750). In addition, they conducted CoMFA analyses and identified the steric and electrostatic contributions of the functional groups. The CoMFA model indicated that the surroundings of C1 were negatively charged (r2=0.998, q2=0.575); hence, the oxidization of the hydroxyl group at the C1 of 1-acetyllycorine would further improve the activity. However, no such compound was synthesized and tested.
Viral infection may cause considerable lethality. As a mosquito-borne viral infection, dengue has high infectivity and breakouts occur frequently in poor urban regions. Dengue prevention strategies have been widely publicized by the WHO; however, few treatment strategies and drug therapies have been developed and promoted. Although clinical trials of some agents have been started, the anti-DENV drugs have insufficient efficacy and are unavailable in the market. The clinical trials of anti-DENV drugs have been beset by difficulties, but successful results have accumulated in the discovery of antiviral inhibitors, leading to the provision of abundant resources for drug design in the future.
In this review, we have detailed the recent research achievements in anti-DENV inhibitors and summarized the significant target proteins. Arranged by target, we thoroughly investigated the emerging anti-DENV inhibitors. In addition to the inhibitors, a detailed exploration of the experiments and simulations was also described and the mechanisms were explained, to provide a solid reference for future work. In silico approaches to find inhibitors were also discussed; together with necessary explanation and discussion, this review hopes to have provided some useful tips.
Although the number of countries with a clinically approved anti-DENV vaccine is increasing, the combined usage of a vaccine and a yet-to-be-investigated anti-DENV drugs would present a promising therapy. Pre-clinical and clinical research into anti-DENV drugs is still underway, and many lessons can be learned from the previous studies. In future, we are bound to overcome the challenges, and expect our ongoing work to yield a potent anti-DENV therapy.
Y.-S. Tian is financially supported by the Hirose International Scholarship Foundation. We thank the editor, Prof. A. Otaka of Tokushima University, for inviting us to write this review and Editage (www.editage.jp) for English language editing supports.
The authors declare no conflict of interest.