2018 Volume 66 Issue 5 Pages 519-526
2018 Volume 66 Issue 5 Pages 519-526
The Okinawa Islands are a crescent-shaped archipelago and their natural forests hold a huge variety of unique subtropical plants with relatively high endemism. We have performed phytochemical study on Okinawan subtropical plants for many years. In this review, we describe our recent research progress on the isolation of new compounds and their various bioactivities.
The Okinawa Islands are a crescent-shaped archipelago located between Kyushu Island of Japan and Taiwan. The flora and fauna are separated into three regions by currently recognized biogeographic boundaries: the Hachisuka, Watase, and Miyake lines at south of Kyushu, Yakushima, and Okinawa Islands, respectively.1) The plants and animals have evolved in each island independently for an extremely long period of time. Now, the natural forests of Okinawa hold a huge variety of unique subtropical plants with relatively high endemism.2)
The taxonomy of Okinawa Islands includes 286 families, 1961 genera, and 5793 plant species, which represent 81.7% of total species (7087) in Japan, although the area of Okinawa is only 0.6% of that of Japan.3) Okinawa prefectural government published a plant red data book in which 1399 vascular plant species were threatened with extinction through the destruction of forests and environment pollution, although many plant species have not been fully phytochemically investigated so far.4) Comprehensive analysis revealed that approximately 65% of therapeutic small-molecule drugs are related to natural products. Thus natural resources including plants remain a highly important resource for discovering novel candidate drugs.5)
In view of this situation, we have focused on the plant resources of our own country, especially for Okinawan subtropical plants. In this review, we will describe our recent research progress from our various studies.
Microtropis japonica HALLIER f. (Celastraceae) is an evergreen tree of about 5 m in height, and distributed in restricted areas such as southern part of Kanto, Kyushu, and Okinawa Islands in Japan and Taiwan.6)
Constituents of the EtOAc-soluble fraction of the stems and roots of M. japonica have been reported7–9) including sesquiterpenoids (1–15), triterpenoids (16–23), lignans (24–26), and other phenolic compounds (27–37) (Fig. 1). Various dihydroagarofuranoid sesquiterpenes are isolated from the genus Microtropis. Among the isolated compounds, celahin C (1) and salasol A (5) showed potent in vitro antituberculosis activity against Mycobacterium tuberculosis H37Rv (both minimum inhibitory concentration (MIC)=15.0 µg/mL).8) The cytotoxic effects of triterpenes obtained from this plant were evaluated and 12,23-dihydroxy-11α-methoxyurs-12-en-3-one (17) exhibited inhibitory activity against HL60 and HepG2 cells with IC50 values of 5.1 and 7.7 µg/mL, respectively.9)

Because phytochemical analysis on the polar fraction of branch and the leaves has not been performed so far, we have investigated extensively on 1-BuOH-soluble fraction prepared from M. japonica collected in Okinawa.10–13)
First, we isolated nine ent-labdane-type diterpenoid glucosides, named microtropiosides A–I (38–46), an ursane-type triterpene diglucoside (47), and a flavonol glycoside (48) from the 1-BuOH-soluble fraction of the leaves of M. japonica.10,13) The structures of these compounds were determined mainly by spectroscopic methods (1H, 13C-NMR, distortionless enhancement by polarization transfer (DEPT), correlation spectroscopy (COSY), heteronuclear single quantum coherence (HSQC), and heteronuclear multiple bond connectivity (HMBC)) and chemical modification such as acetylation to determine the number of free hydroxy groups for evidence of the presence of unusual two oxirane rings for compound 38. The stereochemistry of these compounds was analyzed by comparison of the chemical shift values with related known compounds (47 and 48), and nuclear Overhauser effect spectroscopy (NOESY)/rotating frame nuclear Overhauser enhancement spectroscopy (ROESY) analysis. Absolute stereochemistry was determined by application of the 13C-β-D-glucosylation-induced shift-trend rule, namely anisotropic effect of glucose on adjacent carbon signals between glucoside and aglycone, and by the modified Mosher’s method after protection of primary alcohol by pivaloylation for these common aglycones. X-Ray crystallographic analysis with a clue of D-glucoside rigidly confirmed the structure (Fig. 2). Further detailed constituent analysis on the 1-BuOH-soluble fraction of branches of M. japonica afforded 21 aliphatic glucosides, named microtropins A–W carrying (2S,3R)-2-ethyl-2,3-dihydroxybutyrate at the 6-position of glucose (52–74)11–13) (Fig. 3). Nitrile functional group was observed for five compounds (54, 55, 58, 61, 63), which suggested by a characteristic IR absorption around 2200 cm−1 and the nitrile carbon resonance at around δC 120 ppm, and their structures including absolute stereochemistry were elucidated by the combination of X-ray crystallographic analysis of 54 with D-glucose and spectroscopic analysis. The acyl moiety was determined to be 2-ethyl-2,3-dihydroxybutyrate by alkaline hydrolysis. Absolute stereochemistry of aglycone was determined by coupling constant and application of β-D-glucopyranosylation-induced shift-trend rule. 2-Ethyl-2,3-dihydroxybutyric acid is probably an intermediate in biosynthesis of isoleucine.14) Isolation of (2S,3R)-2-ethyl-2,3-dihydroxybutyrate is interesting for a point of chemotaxonomy.


A potent tumor-promotor, phorbol 12-myristate 13-acetate, has been isolated from Croton oil and used for biological study as an activator of protein kinase C. Thus Croton species have potential as a source of phytochemical investigation. Croton cascarilloides (Euphorbiaceae) is an evergreen shrubby tree of about 0.5–2 m in height and distributed around southern China, Taiwan, Vietnam, Malaysia, and Okinawa Islands, Japan. C. cascarilloides is the only Croton species growing wild in Japan. The leaves are oblong and shiny white ramenta covers their undersurface.6)
Several studies have reported the isolation of diterpenoids, triterpenoids, and flavonoid from C. cascarilloides, such as 3-acetyl aleuritolic acid, rubiadin-1-methyl ether, and julocrotine, ent-8,9-seco-7α-hydroxy-11β-acetoxykaura-8(14),16-dien-9,15-dione, ent-8,9-seco-8,14-epoxy-7α-hydroxy-11β-acetoxy-16-kauren-9,15-dione, ent-8,9-seco-7α-hydroxykaura-8(14),16-dien-9,15-dione, luteolin-7-O-α-L-rhamnoside.15)
Our further extensive study on the leaves and stems of C. cascarilloides revealed the presence of many structurally interesting compounds including megastigmane glycosides, crotonionosides A–G (75–81), crotofolane-type diterpenoids, crotocascarins A–Q (82–98), isocrotofolane glucoside (99), neocrotocascarin (100), and crotocascarin α–γ (101–103)16–20) (Fig. 5).
Crotonionosides A–G (75–81) were isolated from 1-BuOH-soluble fraction of the leaves of C. cascarilloides by means of various chromatographic procedures including a highly porous synthetic resin (Diaion HP-20), normal and reversed-phase octadecyl silica gel (ODS) column chromatography, droplet counter-current chromatography (DCCC), and HPLC. The structures of the new megastigmane glycosides (75–81) were determined by detailed inspection of spectral data, and the absolute structures were evidenced by application of modified Mosher’s method. The absolute stereochemistry of dendranthemoside A (DA) and turpinionoside A (TA) have already been determined unambiguously as a pair of C-9 epimers by our research.21,22) The NMR spectra of these compounds are essentially identical and the specific optical rotation values are also similar [DA: 44.2 (c 0.54, MeOH) and TA: 34.8 (c 0.86, MeOH)], but the HPLC retention times are significantly different (DA: 16.0 min and TA: 18.5 min). Thus crotonionoside A (75) having partial structure of DA was hydrolyzed to remove acyl moiety, and HPLC analysis clearly revealed the presence of DA, not TA. Thus crotonionoside A (75) was elucidated to be (3S,5R,6S,7E,9R)-7-megastigmene-3,6,9-triol 3-O-β-D-(6′-O-feruloyl)glucopyranoside, as shown in Fig. 4.17)

From the stems of C. cascarilloides, structurally rare crotofolane-type diterpenoids (82–98) and three new skeletons, i.e., isocrotoforane (99), neocrotocascarin (100), and rearranged nor-crotofolanes α–γ (101–103), were isolated18–20) (Fig. 5). The structures were determined by precise inspection of spectroscopic data and X-ray crystallographic analyses (Fig. 6). Crotofolane-type diterpenoids have 5/6/7 tricyclic skeleton. The carbon skeleton is biosynthesized from cembrane via lathyrane skeleton by cross annular cyclization. Crotoforane-type diterpenoids have been isolated only from Croton corylifoius, Croton dichogamus, and Croton humanianus.23–26)
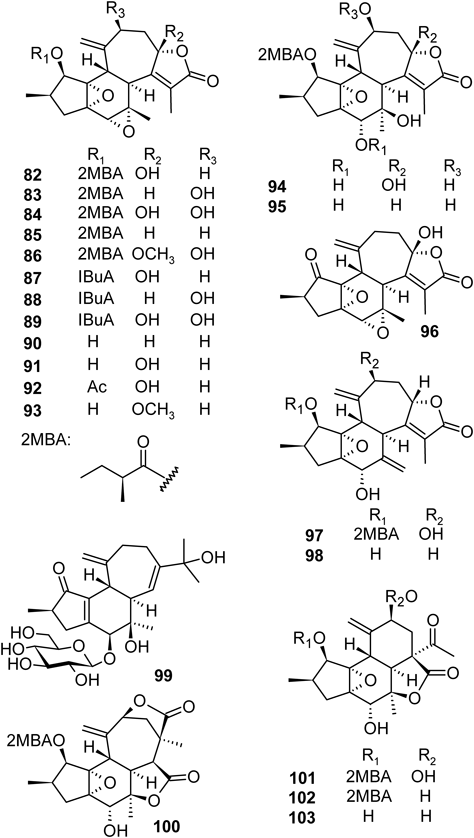

The absolute configuration at the C-9 position was determined as S by the positive Cotton effect in the circular dichroism (CD) spectrum.18) The absolute configuration of 2-methylbutanoic acid (2MBA) was elucidated as S by HPLC analysis with optical rotation detector as shown in Fig. 5. In addition, X-ray crystallographic analysis revealed the structure rigidly (82, 83, 94, 98, 100, and 101) (Fig. 6).
Compounds 99, 100, 101–103 have new skeletons. A plausible biosynthetic pathway for the new skeletons is summarized in Chart 1. Geranylgeranylpyrophosphate is cyclized to form cembrane skeleton and further cross annular cyclization of cembrane produces casbane, lathyrane, jatropholane, and tigliane. The cyclopropane ring of jatropholane is cleaved in two pathways. In pathway 1, the C-9 and C-15 bonds are cleaved to form crotofolane whereas in pathway 2, cleavage of C-8 and C-15 bonds leads to the formation of a new skeleton, named isocrotofolane such as 99. The crotofolane then rearranges to form two new skeletons as follows. The migration of C-9 and C-10 bond to C-8 (route a) and following decarbonation results to form the rearranged nor-crotofolane, crotocascarins α–γ (101–103). While the C-9 and C-10 bond migration through route b gives new skeletal compound, neocrotocascarin (100).18–20)

Macaranga tanarius (L.) MÜLL.–ARG. (Euphorbiaceae) is one of the pioneer trees that grows faster than other plants species on impoverished soil.27) M. tanarius produces ant-attracting food body for inviting ants as a defense mechanism against herbivores, which is the feature of ant-plant.28) M. tanarius is a small evergreen tree with a height of 4–5 m and is distributed in Korea, southern China, and Okinawa in Japan. In China, the bark and root of this plant are used as a folk medicine for dysentery and hemoptysis, respectively.29)
The light petroleum ether extract of the stems and bark of M. tanarius yielded pimarane-type diterpenoids (104 and 105), a lathyrane (106), and cytotoxic taraxerane-type triterpenoids (107–110)30–32) (Fig. 7).

These structures were identified by spectroscopic methods including NMR and MS analyses. The triterpenoids (107–110) showed cytotoxicities against human lung carcinoma A549 with IC50 values of 3.6–13.8 µM with in vitro inhibitory activity toward DNA topoisomerase II (ca. 2 µM for 107–110).
The bark and leaves of M. tanarius are known to be rich in tannins,33) and the aqueous acetone extract of the leaves was subjected to a combination of column chromatography to isolate a variety of tannins (111–138)33) (Fig. 8).

We have also isolated many new compounds from this plant such as megastigmane glucosides, named macarangiosides A–F (147, 148, 150, 151, 144, 149, respectively) (Fig. 9), and prenylated flavanones, macaflavanones A–G (173, 174, 177, 180, 179, 181, 182, respectively), from the leaves of M. tanarius together with known compounds34–36) (Figs. 9, 10). The absolute stereochemistries of macarangiosides B (148), C (150), and F (149) were determined by chemical conversion to each other and application of the modified Mosher’s method (Chart 2).



a) 0.1 M NaOMe in MeOH, r.t., 2 h, b) PtO2/H2, r.t., 1 h, c) crude hesperidinase, 37°C, 12 h, d) NaBH4 in MeOH, 0 to 25°C, 30 min, e) EDC, DMAP, (R) and (S)-MTPA in CH2Cl2, r.t., 30 min.
2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical-scavenging activity and cytotoxicity of these compounds were also investigated. As a result, (+)-pinoresinol 4-O-[6″-O-galloyl]-β-D-glucopyranoside (158), and macarangiosides A, B, C, and E (147, 148, 150, 144, respectively) possessed potent DPPH radical-scavenging activity without significant cytotoxity.34,35) These results suggest that macarangiosides A, B, C, and E have a potential for non-toxic safe antioxidant agents.
Several prenylated flavonoids were also isolated from M. tanarius.36–39) Tanariflavanones A (175), B (178), and nymphaeol C (170) were determined as allelopathic substances from the fallen leaves of M. tanarius.37) Our phytochemical investigation of the leaves of M. tanarius resulted in the isolation of seven new prenylated flavanones, macaflavanones A–G (173, 174, 177, 180, 179, 181, 182, respectively).36) The absolute structure of tanariflavanone B (174) was also resolved in our study by chemical conversion and application of CD spectra.36) The cytotoxic activities of isolated flavanones were assayed using two cell lines, KB and A549, and macaflavanone G (182) was the most active among the isolated compounds with IC50 values of 12.3 and 13.4 µM, respectively.
In conclusion, our phytochemical investigation clearly revealed diverse chemical constituents from Okinawan plants with a variety of biological activities. Thus subtropical plants in Okinawa are a promising resource for phytochemical research toward the discovery of new drug candidate.
The authors declare no conflict of interest.