2018 Volume 66 Issue 7 Pages 732-740
2018 Volume 66 Issue 7 Pages 732-740
Three 2-fluoroacetonylbenzoxazole ligands 1a–c and their new Zn(II) complexes 2a–c have been synthesized. In addition, syntheses of new metal [Mg(II), Ni(II), Cu(II), Pd(II), and Ag(I)] complexes from 1a have been also described. The molecular and crystal structures of six metal complexes 2b and 2d–h were determined by single-crystal X-ray diffraction analyses. Their antibacterial activities against six Gram-positive and six Gram-negative bacteria were evaluated by minimum inhibitory concentrations (MIC), which were compared with those of appropriate antibiotics and silver nitrate. The results indicate that some metal compounds have more antibacterial effects in comparison with free ligands and have preferred antibacterial activities that may have potential pharmaceutical applications. Noticeably, the Ag(I) complex 2h exhibited low MIC value of 0.7 µM against Pseudomonas aeruginosa, which was even superior to the reference drug, Norfloxacin with that of 1.5 µM. Against P. aeruginosa, 2h is bacteriostatic, exerts the cell surface damage observed by scanning electron microscopy (SEM) and is less likely to develop resistance. The new 2h has been found to display effective antimicrobial activity against a series of bacteria.
Bacterial infection remains a serious threat to human lives due to rapid development of resistance to the existing antibiotics. In order to prevent this major medical problem, the discovery of new types of antimicrobial agents is a very important but challenging task.1,2) Transition metal complexes have received great attention because many drugs such as antibiotics and quinolone antibiotics possess better pharmaceutical properties when they are in the form of metal complexes.3–7)
Benzoxazole heterocycle system is an important pharmacophore and privileged structure in the medicinal chemistry.8–10) Its derivatives and particularly, 2-substituted benzoxazoles exert various biological activities such as antimicrobial, antiviral, antifungal, and anticancer.11–15) In previous studies, we have identified 2-trifluoroacetonylbenzoxazole (1a) with in vitro antimicrobial activity against Helicobacter pylori and Escherichia coli (Ec) from our compound library of trifluoromethyl ketones obtained by our developed reactions.16,17)
The heteroaryl-substituted alkenol systems such as 1a are known to chelate some metal ions such as aluminum18) and to form a stable six-membered metal complexes in a bidentate mode, where the complexes were reinforced by a positive inductive effect of the heterocyclic moiety and a negative inductive effect of the CF3 groups.19–23)
In the light of these knowledge, three 2-fluoroacetonylbenzoxazole ligands 1a–c and their new Zn(II) complexes 2a–c have been synthesized in order to check how the metal ion binding may influence their antibacterial activity (Chart 1). In addition, syntheses of new metal [Mg(II), Ni(II), Cu(II), Pd(II), and Ag(I)] complexes 2d–h from 1a have been also described (Table 1). The structures of six metal complexes 2b and 2d–h were determined by single-crystal X-ray diffraction analyses. In vitro antimicrobial activities of the ligands 1a–c and their metal complexes 2a–h were investigated against six Gram-positive (G(+)) and six Gram-negative (G(−)) bacteria and the results were compared with those of silver nitrate and appropriate antibiotics such as Kanamycin, Gentamicin, Norfloxacin, and/or Linezolid. The most potent Ag(I) complex 2h is bacteriostatic, disrupts membrane integrity observed by scanning electron microscopy (SEM) and is less likely to develop resistance development against Pseudomonas aeruginosa (Pa).
 | |||
|---|---|---|---|
| 2 | Metal salts (3.0 eq) | Time (h) | Yield (%) |
| d | MgBr2 | 3 | 88 |
| e | NiCl2·6H2O | 6 | 82 |
| f | Cu(OAc)2·H2O | 24 | 25 |
| g | PdCl2 | 3 | 23 |
| h | Ag2CO3 | 3 | 30 |
First, three benzoxazole ligands 1a–c and their new Zn(II) complexes 2a–c have been prepared in order to investigate the relationship of the ligands and their Zn(II) complexes with their antibacterial activities.
Our synthetic protocol to obtain the ligand 1a consists of a trifluoroacetylation of 2-methylbenzoxazole with trifluoroacetic anhydride (TFAA) in the presence of pyridine24) (Chart 1). The Zn(II) complex 2a was prepared by the reaction of 1a with zinc dust in MeCN in 78% yield, consisting of 1a with zinc(II) ion in 2 : 1 ratio.
Similarly, 2-chlorodifluoroacetonylbenzoxazole (1b) was obtained in a good yield from a reaction of 2-methylbenzoxazole with (CF2ClCO)2O instead of TFAA. The 2b was obtained from a reaction of 1b with zinc dust in nitromethane in 98% yield.
On the other hand, the reaction of 1b with zinc dust in the solvent of tetrahydrofuran (THF) instead nitromethane underwent the reduction of chlorodifluoromethyl (CF2Cl-) group to difluoromethyl (CF2H-) group and the formation of a new 2c which consisted of a difluoroacetonylbenzoxazole (1c) with zinc(II) ion in 2 : 1 ratio. The ligand 1c was obtained from 2c in a moderate yield by removing the Zn(II) using excess ethylenediaminetetraacetic acid (EDTA)·2Na·2H2O25) (Chart 1).
Second, we have synthesized the magnesium(II), nickel(II), copper(II), palladium(II) and silver(I) complexes 2d–h by the reaction of 1a with the corresponding metal salts [MgBr2, NiCl2·6H2O, Cu(OAc)2·H2O, PdCl2, and Ag2CO3] (Table 1).
All the complexes 2a–h are crystalline powders; as expected, the Zn(II) (2a–c) and Mg(II) (2d) are colorless complexes, but Ni(II) (2e) (dark green), Cu(II) (2f) (brown), Pd(II) (2g) (orange), and Ag(I) (2h) (yellow) complexes are colored. Complexes 2a–g except the Ag(I) complex 2h are stable and can be stored in a sealed flask for months without any change. The presence of fluorine atoms in the ligand periphery, effective steric shielding and bidentate chelating behavior toward the metal centers may impart these complexes 2a–g as remarkable stability against air and moisture. However, the Ag(I) complex 2h is slightly sensitive to light and darkens slowly under the light, especially when dissolved in the solution. Therefore, light stability of 2h was studied in direct light under an air atmosphere at room temperature to mimic their exposure to light under every life conditions.26) Cotton pads were impregnated with 0.025 mol/L aqueous solutions of the compounds and exposed to air and light. The stability was monitored visually within 5 d. The photos of the samples (2h, 2a and AgNO3) are shown in Fig. S1. The 2h started to be a little beige after 18 h and was completely blackened after 24 h. However, under the dark the 2h is stable at room temperature for several months in the form of crystalline powder and their solution in dimethyl sulfoxide (DMSO)-d6 was remained unchanged at room temperature for 24 h. Indeed, the solutions of 2h (3 mg) in DMSO-d6 (0.5 mL) or D2O/DMSO-d6 (5 : 1) (0.5 mL) were standing at room temperature under the shading for 24 h and their NMR spectra remained unaltered. In the antibacterial assay and time–kill assay, the complexes were dissolved in DMSO and then diluted by Muller–Hinton broth. Therefore, the stability of 2h (35 µM) in the buffer solution and DMSO were tested by monitoring their UV spectra at different intervals of time. On the other hand, 24 h of treatment is needed in biological activity studies. As shown in Fig. S2, only slight changes were observed in the UV spectra of the 2h in DMSO and the buffer under the dark at 0 and 24 h.
The structures of new ligands 1b, c and metal complexes 2a–h were established through IR, UV, 1H- and 13C-NMR, and mass spectral studies.
The IR spectra of the ligands 1a–c show a weak and broad band at 3100 cm−1. This might be due to intramolecular hydrogen bonding between the enol hydroxyl hydrogen and nitrogen of the benzoxazole ring forming a six-membered ring.27) The absence of νOH in the complexes of Zn(II) (2a–c) and Cu(II) (2f) complexes suggested the deprotonation of the enolic–OH and its co-ordination through the O atom. Moreover, the νC=N mode which occurred at 1644 cm−1 in the ligand 1a was shifted in the complexes. This shifting of νC=N towards lower frequency (1580–1610 cm−1) suggested that the co-ordination of the ligands occurred through the deprotonated oxygen of the enol-OH group and nitrogen of the benzoxazole.27) However, the existence of water of hydration in the Mg(II) (2d), Pd(II) (2g), Ag(I) (2h) complexes or coordination in the Ni(II) (2e) complex causes difficulty in drawing conclusions from νOH band for the hydroxy groups of the free ligand 1a which would overlap with those of water molecules. In the Ni(II) complex (2e), the absorption band at ca. 3100 cm−1 (ν(O–H)) disappeared, indicating the de-protonation of the enol after coordination Ni(II), while the emerging band at 3400 cm−1 was attributed to the O–H stretch of the coordinated water. Further conclusive evidence of the co-ordination of the ligands with the metals was confirmed by the appearance of weak low frequency bands at 480–510 and 345–370 cm−1 due to metal–oxygen and metal–nitrogen stretching vibrations in the metal complexes and not observable in the spectra of the ligands 1a–c.28)
The Ni(II) (2e) and Pd(II) (2g) complexes would be paramagnetic and their 1H-NMR spectra were appeared at wide range. The 1H- and 13C-NMR spectra of the ligands and diamagnetic Zn(II) (2a–c), Mg(II) (2d), Cu(II) (2f), Ag(I) (2h) complexes were obtained. The 1H-NMR spectra of the Zn(II) complexes 2a–c, assumed to have pseudotetrahedral coordination, affirming the simple spectrum expected for the ligand when all ligands are geometrically equivalent. The signal assigned to vinyl protons in 1H-NMR spectra of three ligands 1a–c and their Zn(II) complexes 2a–c were appeared at a singlet between δ=5.86–6.07 and δ=5.98–6.11, respectively. The most notable changes were observed in the benzoxazole ring signals, particularly those assigned to the atoms of C2 (δ=163.9–164.8 in the three ligands 1a–c, δ=167.3–167.7 in three Zn(II) complexes 2a–c, 170.7 in the Mg(II) complex 2d, 167.7 in the Ag(I) complex 2h and C3a (δ=133.8–134.5 in the three ligands 1a–c, δ=136.8–137.0 in three Zn(II) complexes 2a–c, 140.4 in the Mg(II) complex 2d, 141.7 in the Ag(I) complex 2h.
The UV-Vis spectra data for compounds 1a–c and 2a–h in MeOH are summarized in Table 2. The major absorption bands of three ligands 1a–c and all metal complexes 2a–h are all around at 240 nm and 311–336 nm, which were mainly unaffected (Fig. S2). Peaks at 311–336 nm can be attributed to the π–π* transitions due to the conjugated enol double bond. The molar absorption coefficient (ε) of metal complexes 2a–h were much higher than those of the free ligands 1a–c.
| Compd. | λmax (nm) | ε (M−1 cm−1) |
|---|---|---|
| 1a | 326.5 | 5620 |
| 1b | 333 | 10561 |
| 1c | 320.5 | 1782 |
| 2a | 312 | 47418 |
| 2b | 316 | 46323 |
| 2c | 317 | 33061 |
| 2d | 311 | 40609 |
| 2e | 317 | 38663 |
| 2f | 315.5 | 38921 |
| 2g | 335.5 | —a) |
| 2h | 311.5 | 36049 |
a) The ε value of 2g was not determined due to the low solubility.
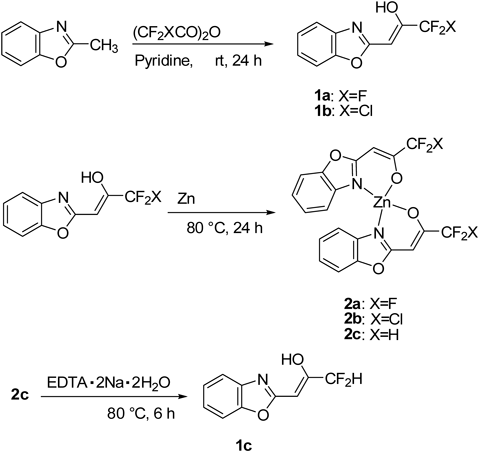
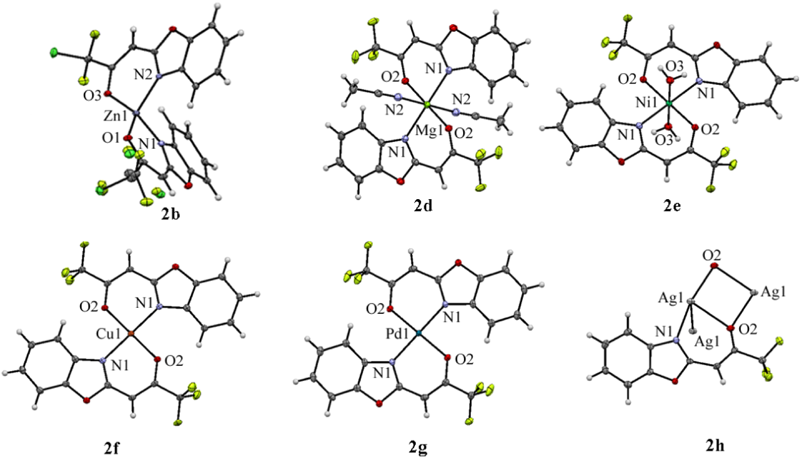
The molecular and crystal structures of six metal complexes 2b and 2d–h were determined by single-crystal X-ray diffraction analysis and are shown in Fig. 1. Their crystallographic data, including selected bond lengths [Å] and bond angles [°], were presented in Tables S1–8. Metal–ligand bond lengths and angles for 2b and 2d–h are in the range of values reported for other metal N–O systems.29–39)
(Color figure can be accessed in the online version.)
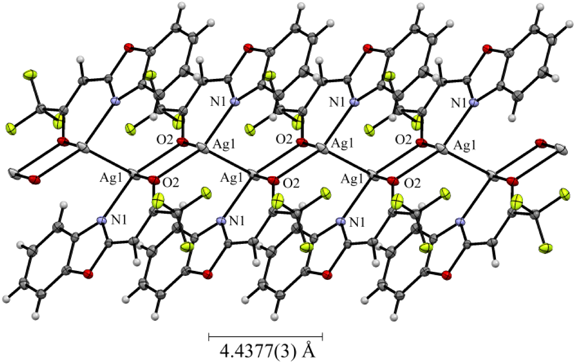
In the Zn(II) complex 2b, the geometry at the Zn(II) center can be best described as a distorted tetrahedron in which two benzoxazole-2-chlorodifluoroacetonate (1b) ligands are placed in a similar disposition about the zinc center.29–31) One coordination plane (N–O–Zn) in 2b is approximately perpendicular to the other with a dihedral angle of 89.2°. The molecular structure of 2b was incorporated with hexane molecule. In the Mg(II) complex 2d, the central Mg(II) atom was coordinated by two N atoms and two O atoms from two 1a bidentate ligands and the axial sites were occupied by two coordinated acetonitrile molecules, forming a distorted N4O2 octahedral geometry.32) The nitrogen atoms of the benzoxazole ring situated mutually in the trans position. The Ni(II) complex 2e exhibits a distorted octahedral geometry, in which two 1a ligands are located in the equatorial plane, forming two stable six-membered rings with metal ion, and the axial sites are occupied by two coordinated water molecules.33,34) In the Cu(II) complex 2f, the Cu(II) atom was coordinated by two N atoms and two O atoms from two 1a, forming a strictly square planar geometry (dihedral angle between two N–Cu–O planes: 0°).35–37) In the Pd(II) complex 2g, the geometry at Pd(II) center was coordinated as nearly perfect square planar which are similar to that of the Cu(II) atom in 2f.38) The crystal structure of Ag(I) complex 2h revealed to consist a 1 : 1 metal–ligand molar ratio and the Ag(I) ion exhibits a distorted trigonal pyramidal geometry by two 1a and the another Ag(I) atom, forming a 4-membered parallelogram Ag2O2 ring.39) Thus, the complex 2h crystallizes in a dimeric form with two [Ag2O2] units held together by an Ag–Ag contact of 3.1340(4) Å which is longer than the Ag–Ag one in metallic silver (2.88 Å), but much shorter than the sum of the van der Waals radii of two Ag(0) atom (3.44 Å), implying the existence of d10–d10 interactions between two closed-shell Ag(I) ions. Such argentrophilic interactions have been observed in some multinuclear Ag(I) complexes.40,41) The crystal of 2h formed a zigzag structure along the crystallographic a axis, with a pitch of 4.4377(3) Å (Fig. 2).
(Color figure can be accessed in the online version.)
The synthesized compounds 1a–c and 2a–h were evaluated for their antimicrobial activities in vitro six G(+) bacteria of Bacillus subtilis (Bs), Enterococcus faecalis (Ef), Staphylococcus aureus (Sas), Methicillin-resistant Staphylococcus aureus (Sar), Streptococcus pneumoniae (Spn), and Streptococcus pyogenes (Spy) and six G(−) bacteria of Acinetobacter baumannii (Ab), Escherichia coli (Ec), Klebsiella pneumoniae (Kp), Pseudomonas aeruginosa (Pa), Serratia marcescens (Sm), and Vibrio parahaemolyticus (Vp). The minimum inhibitory concentrations (MIC, µM) were determined by means of standard serial dilution method according to the National Committee for Clinical Laboratory Standards (NCCLS)42) and are presented in Table 3. Currently available antimicrobial drugs Kanamycin, Gentamicin, Linezolid, or Norfloxacin were used as the positive control. In addition, AgNO3 was also evaluated for its antimicrobial activities in order to compare with those of the Ag(I) complex 2h. The MIC value of 1a against Ec was already reported to be 8 µg/mL (34 µM).17) In order to assess the effect of three ligands and their metal complexes on the antibacterial activity, the three ligands 1a–c and their Zn(II) complexes 2a–c were first evaluated for in vitro antibacterial activity against six G(+) and six G(−) bacteria. The data revealed that the complexes 2a and b showed more potent antibacterial activities against not only G(−) bacteria but also G(+) bacteria than the ligands 1a and b, respectively. However, 2c and 1c showed higher MIC values against both G(−) and G(+) bacteria than 2a, b or 1a, b, respectively.
| Compd. | MIC (µM) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G(+) bacteria | G(−) bacteria | |||||||||||
| Bs | Ef | Sas | Sar | Spn | Spy | Ab | Ec | Kp | Pa | Sm | Vp | |
| 1a | 9 | 34 | 17 | 17 | 17 | ≧280 | 69 | 34b) | 69 | ≧280 | 140 | 8 |
| 1b | 4 | 32 | 16 | 8 | 8 | 26 | 32 | 32 | 65 | ≧522 | 130 | 8 |
| 1c | 19 | 75 | 75 | 75 | 37 | 75 | 75 | 37 | 75 | 303 | 75 | 18 |
| 2a | 2 | 15 | 7 | 7 | 4 | 30 | 30 | 15 | 15 | ≧122 | 30 | 3 |
| 2b | 2 | 28 | 2 | 14 | 4 | 15 | 28 | 28 | 28 | ≧115 | 57 | 3 |
| 2c | 8 | 131 | 65 | 32 | 16 | ≧131 | 65 | 65 | ≧131 | ≧131 | ≧131 | 16 |
| 2d | 8 | 67 | 17 | 33 | 33 | ≧133 | ≧133 | 67 | ≧133 | ≧133 | ≧133 | 8 |
| 2e | 4 | 31 | 15 | 15 | 7 | 63 | 62 | 31 | 62 | ≧124 | ≧124 | 3 |
| 2f | 4 | 30 | 15 | 15 | 4 | 31 | 61 | 30 | 61 | ≧123 | ≧123 | 3 |
| 2g | 57 | ≧114 | ≧114 | ≧114 | ≧114 | ≧114 | ≧113 | ≧113 | ≧113 | ≧113 | ≧113 | 56 |
| 2h | 4 | 7 | 7 | 7 | 28 | 56 | 3 | 7 | 3 | 0.7 | 7 | 3 |
| Kanamycin | ND | ND | ND | ND | ND | ND | 8 | 4 | 8 | ≧132 | 66 | 4 |
| Gentamicin | 0.5 | 67 | 2 | 1 | 8 | 16 | 4 | 1 | 2 | 16 | 2 | 4 |
| Linezolid | ND | ND | ND | 3 | ND | ND | ND | ND | ND | ND | ND | ND |
| Norfloxacin | ND | ND | ND | ND | ND | ND | ND | ND | ND | 1.5 | ND | ND |
| AgNO3 | 11 | 47 | 94 | 94 | 189 | 94 | 11 | 11 | 11 | 23 | 23 | 11 |
a) Bs: B. subtillis ATCC6633, Ef: E. faecalis IID622, Sas: S. aureus 209P (MSSA), Sar: S. aureus N315 (MRSA), Spn: S. pneumoniae IID555, Spy: S. pyogenes 244, Ab: A. baumannii ATCC17978, Ec: E. coli TG1, Kp: K. pneumoniae IID5209, Pa: P. aeruginosa O1, Sm: S. marcescens, Vp: V. parahaemolyticus RIMD221051. b) The data was previously reported in Ref. 17.
Among all the tested metal complexes 2a–h, the bioactivity against the tested G(+) bacteria for various complexes decreased in the sequence Ag>Zn>Cu, Ni>1a>Mg>>Pd. On the other hand, the bioactivity against the tested G(−) bacteria for various complexes decreased in the sequence Ag>>Zn>1a>Cu, Ni>>Mg, Pd. The Ag(I) complex 2h showed broad antibacterial spectrum and potent antibacterial activities in comparison with the other metal complexes. In addition, all the MIC values estimated for the 2h are considerably lower than AgNO3. Particularly, the 2h was found to exhibit the strongest inhibitory activity against G(−) bacteria, such as Pa, Ab, Ec, Kp, Sm, and Vp, with MIC values of 0.7, 3, 7, 3, 7, and 3 µM, respectively. It is noted that the 2h was the only one to display outstanding effect against Pa (MIC=0.7 µM) and was found to be considerably stronger than the inhibitory concentration of the benchmark drug, Norfloxacin with MIC=1.5 µM. On the other hand, Zn(II) complexes 2a and b exhibited the strongest inhibitory activities against Bs, Sas, and Spn with MIC values of 2–4 µM.
Generally, G(+) bacteria are more susceptible to the antibacterial effects of the ligands 1a–c and the metal complexes 2a–g than G(−) bacteria. On the other hand, G(−) bacteria are more susceptible to the Ag(I) complex 2h and AgNO3 than G(+) bacteria, presumably due to their thinner wall, which may allow more rapid absorption or adhesion of the silver into the cell.43–45) The cell wall of G(−) bacteria contains lipopolysaccharide (LPS) as the outer leaflet of the outer membrane bilayer. However, the G(+) bacteria are surrounded by a thick peptidoglycan layer, which explains the elevated activity of the metal complex over G(−) bacteria than G(+) bacteria species.
Time–kill studies were performed with 107 colony-forming units (CFU/mL) of Pa at baseline (Fig. 3A). Pa was incubated in the presence of the Ag(I) complex 2h at 0, 1, 2, 4 and 8 times respective MIC (MIC=0.7 µM) and the number of surviving bacteria were determined by colony counting. The data are judged relative to the conventional definition of bactericidal activity, that is, a 3 log10 CFU/mL or greater reduction in the initial inoculum by 24 h.42,46)

In growth curve studies (Fig. 3B), no influence was observed on the growth of Pa in liquid medium supplemented with up to 2 times the MIC of 2h. However, Pa was submitted to 8 times the MIC of 2h, the growth was suppressed until 12 h and then the regrowth occurred. This regrowth was attributed to adaptation,47,48) because the doubling rate of the cell collected after 24 h in the experiment of 8 times MIC of 2h was the same as that for the growth curve when the same experiment was repeated at 8 times MIC of 2h. This bacterial regrowth was not observed for AgNO3. We conclude that 2h is bacteriostatic against Pa (<3 log10 CFU/mL of drop). On the other hand, AgNO3 exhibited bactericidal activity against Pa with MIC of 23 µM, demonstrating killing of approximately 3 to 4 log units. It is also reported that Ag(I)-coated carbon nanotubes are bactericidal against Pa.49)
Considering that some Ag complexes can interfere with the cell wall synthesis,50,51) scanning electron microscopy (SEM) was used to visualize the outer surface change of Pa in the presence of the Ag(I) complex 2h. Pa cell morphology changes were examined after 1 h treatment of exponential phase bacteria (O.D.600 of about 1.4) with 2, 5, 10×MIC of 2h (Fig. 4). When 2×MIC of 2h was allowed to act, small morphological changes (marked by the arrow) were observed on the cell surface as compared with the control (Figs. 4a, b). Furthermore, obvious morphologic alternations on the cell surface, irregular, pitted, and shriveled, were observed in the cells treated with the concentration of more than 5×MIC of 2h (Figs. 4c, d). The morphological alternations on the surface might result in the leakage of contents of the cells. These images suggest a probable mechanism of action whereby the Ag(I) complex 2h interacts with the cell membrane, causing membrane permeabilization and degradation, with accompanying changes in cell morphology.

Indicated photographs are an electron micrograph of untreated cells (a) and cells treated with 2×(b), 5×(c), 10×(d) MIC of the Ag(I) complex 2h for 1 h at 37°C. The arrows represent the observed small morphological changes. The scale bar indicates 2 µm. Independent experiments were carried out three times and a similar result was provided.
Next, in order to assess the ability of Pa to develop resistance to the Ag(I) complex 2h, we exposed a standard strain of Pa to liquid medium supplemented with 4×MIC of 2h. We passed the strain 4 times in the same concentration medium and passaged to stepwise increasing concentration of 2h. The MIC of 2h for each passage of Pa was measured, but it showed the same MIC as the standard strain. Furthermore, we cultured a standard strain of Pa (4×109 CFU cells) on an agar medium supplemented with 4 and 8×MIC of 2h and isolated several candidate strains growing on the agar medium. The MIC of 2h for these strains was measured, but it showed the same MIC as the standard strain. These assay indicates that Pa do not develop significant resistance against 2h, as easily as the bacteria do against Norfloxacin.52)
Indeed, bacterial resistance to silver has been rarely reported.53,54) The reason why 2h is less likely to develop resistance may be that the multiple actions of the Ag complex make it difficult for bacteria to simultaneously employ existing strategies for resistance. The probable mechanisms of action of Ag(I) ions are reported to include binding to DNA, interaction with the cell membrane, interference with the electron transport chain and reaction with the thiol group in vital enzymes.50,51) However, we cannot exclude the conclusion that antibacterial activity is connected to silver ion release.55)
The 2-trifluoroacetonylbenzoxazole ligand (1a) coordinates with monovalent Ag(I) metal in 1 : 1 and divalent metals [Zn(II), Mg(II), Ni(II), Cu(II), and Pd(II)] in 1 : 2 metal–ligand ratios, as confirmed by IR, MS and X-ray. In the antimicrobial screening, it has been observed that the ligands were found to be biologically active and the antibacterial activity increased on complexation with Ag(I) or Zn(II). Such increased activity of the metal complexes can be explained on the basis of Overtone concept and Tweedy chelation theory.56,57) According to the overtone concept of cell permeability, the lipid membrane that surrounds the cell favors the passage of only lipid-soluble materials; therefore, lipophilicity is an important factor which controls the antibacterial activity. On chelation, polarity of the metal ion is reduced to a greater extent due to the overlapping of ligand orbital and partial sharing of the positive charge of the metal ion with donor groups. Moreover, delocalization of the π-electrons over the whole chelate ring is increased and lipophilicity enhances the penetration of the complexes into the lipid membranes and blocks the metal binding sites in the enzymes of microorganisms. Therefore, a significant increase in antimicrobial activity with Ag(I) complex can be explained on the basis of the above described Overtone concept and Tweedy chelation theory.
The Ag(I) complex 2h displayed significant antibacterial activities. In particular, the inhibition against Pa was observed at low micromolar concentration, which was found to be considerably stronger than the inhibitory concentration of the benchmark drug, Norfloxacin with MIC=1.5 µM. The 2h is bacteriostatic and exerts the cell surface damage against Pa. Serial transfer of Pa on drug gradient plates produced no evidence of rapid resistance development. Compared to small-molecular antibiotics, the Ag(I) complex may be less likely to develop resistance partly because multiple actions of Ag(I) complex may not be easily overcome by strategies employed by bacteria to develop resistance simultaneously. These results can be utilized for developing a new class of novel antibiotics.7) Furthermore, it guides us to synthesize some analogs of substituted benzoxazole ligands and their metal complexes and to study their biological activities.
All melting points were determined using a Yanagimoto hot-stage melting point apparatus and are uncorrected. 1H-NMR and 13C-NMR spectra were measured on Bruker AVANCE500 (500 MHz for 1H and 126 MHz for 13C) spectrometer. Both 1H- and 13C-NMR spectral data are reported in parts per million (δ) relative to tetramethylsilane (Me4Si). IR spectra were recorded on a JASCO FT/IR-4100 spectrometer. Low- and high-resolution (LR/HR)-MS were obtained with a JEOL JMS-GC mate II or a JEOL JMS-700 spectrometer with a direct inlet system at 70 eV. X-ray crystallographic data were recorded on a Rigaku VariMax SaturnCCD724/α diffractometer using graphite monochromated Mo-Kα radiation at the Advanced Research Support Center, Ehime University.
Materials and MethodsDimethyl sulfoxide (DMSO) (Wako Pure Chemical Industries, Ltd., Osaka, Japan) was used as solvent control. Kanamycin (Wako Pure Chemical Industries, Ltd.), Gentamicin (Wako Pure Chemical Industries, Ltd.), Linezolid (Wako Pure Chemical Industries, Ltd.), Norfloxacin (Wako Pure Chemical Industries, Ltd.) and AgNO3 (Nacalai Tesque, Kyoto, Japan) were used as standard drugs. (Z)-1-(Benzo[d]oxazol-2-yl)-3,3,3-trifluoroprop-1-en-2-ol (1a) was prepared by the literature method.24) 1a: mp 160–162°C (mp24) 165°C).
Procedures for the Preparation of Benzoxazoles and Their Zinc(II) Complexes(Z)-1-(Benzo[d]oxazol-2-yl)-3-chloro-3,3-difluoroprop-1-en-2-ol (1b)Chlorodifluoroacetic anhydride (23.4 mL, 138.6 mmol) was added dropwise to a solution of 2-methylbenzoxazole (5.4 mL, 46.2 mmol) and pyridine (18 mL, 230.7 mmol) in anhydrous toluene (60 mL) at 0°C. The mixture was stirred at room temperature for 24 h and diluted with 3% Na2CO3 (80 mL) and extracted with EtOAc (80 mL)×3, washed brine, dried over Na2SO4, filtered, and concentrated under reduced pressure. Recrystallization gave the pure product (1b). Brown solid, 81% yield. mp 182°C. IR (KBr) 3191, 1642, 1619, 1576, 1298, 1198, 1141, 1094, 975, 955, 831, 748, 718, 500 cm−1; 1H-NMR (500 MHz, CDCl3) δ: 5.98 (s, 1H, CH), 7.26–7.39 (m, 2H, ArH), 7.50 (t, J=9.2 Hz, 2H, ArH); 13C-NMR (125 MHz, CDCl3) δ: 78.5, (t, 3JC-F=3.4 Hz, CH), 120.8, 114.9, 121.7 (t, 1JC-F=295.4 Hz, CF), 125.0, 125.7, 133.1, 147.4, 164.8, 169.4 (t, 2JC-F=29.3 Hz, CO); LR-MS (electron ionization (EI)+): m/z=245 (M+, 13.7), 160 (100); HR-MS (EI) Calcd for C10H6ClF2NO2 (M+): 245.0055. Found: 245.0047.
Bis((Z)-1-(benzo[d]oxazol-2-yl)-3,3,3-trifluoroprop-1-en-2-ate)zinc(II) (2a)A mixture of 1a (245.5 mg, 1 mmol) and zinc dust (196.6 mg, 3 mmol) in CH3CN (5 mL) was stirred for 24 h at 80°C. After being cooled to room temperature, the reaction mixture was filtered through a pad of Celite and the filtrate was concentrated in vacuo. Column chromatography of the crude product on silica gel eluting with hexane–ethyl acetate provided a pure product (2a). Colorless solid, 78% yield. mp 201°C. IR (KBr) 3096, 1594, 1562, 1461, 1431, 1328, 1232, 1204, 1137, 1121, 1021, 881, 754, 947, 718 cm−1; 1H-NMR (500 MHz, CDCl3) δ: 6.11 (s, 1H, CH), 7.18–7.34 (m, 3H, ArH), 7.52 (dd, 1H, J=7.6 and 7.3 Hz, ArH); 13C-NMR (125 MHz, DMSO-d6) δ: 81.1, 110.9, 115.7, 119.4 (q, 1JC-F=279.6 Hz, CF), 125.5, 125.8, 136.7, 147.6, 166.9 (q, 2JC-F=33.6 Hz, CO), 167.2; LR-MS (EI+): m/z=520 (M+, 3.7), 160 (100); HR-MS (EI) Calcd for C20H10F6N2O4Zn (M+): 519.9836. Found: 519.9825.
Bis((Z)-1-(benzo[d]oxazol-2-yl)-3-chloro-3,3-difluoro-prop-1-en-2-ate)zinc(II) (2b)A mixture of 1b (245.5 mg, 1 mmol) and zinc dust (196.6 mg, 3 mmol) in nitromethane was stirred for 24 h at 80°C. After being cooled, the reaction mixture was filtered through a pad of Celite and the filtrate was concentrated in vacuo. Column chromatography of the crude product on silica gel eluting with hexane–ethyl acetate provided pure product (2b). Colorless solid, 98% yield. mp 165°C. IR (KBr) 3136, 3106, 3077, 2922, 2852, 1578, 1549, 1459, 1424, 1310, 1205, 1112, 1024, 962, 931, 831, 771, 744, 727, 678, 635, 522 cm−1; 1H-NMR (500 MHz, CDCl3) δ: 6.09 (s, 1H, CH), 7.22–7.34 (m, 3H, ArH), 7.52 (dd, J=7.2 and 6.9 Hz, 1H, ArH); 13C-NMR (125 MHz, CDCl3) δ: 79.4 (t, 3JC-F=3.6 Hz, CH), 110.9, 115.9, 122.8 (t, 1JC-F=298.5 Hz, CF), 125.5, 125.8, 136.8, 147.8, 167.4, 169.9 (t, 2JC-F=27.6 Hz, CO); LR-MS (EI+): m/z=552 (M+, 16.0), 159 (100); HR-MS (EI) Calcd for C20H10Cl2F4N2O4Zn (M+): 551.9245. Found, 551.9230.
Bis((Z)-1-(benzo[d]oxazol-2-yl)-3,3-difluoroprop-1-en-2-ate)zinc(II) (2c)A mixture of 1b (245.5 mg, 1 mmol) and zinc dust (196.6 mg, 3 mmol) in THF (5 mL) was stirred for 24 h at 80°C. After being cooled to room temperature, the reaction mixture was filtered through a pad of Celite and the filtrate was concentrated in vacuo. Column chromatography of the crude product on silica gel eluting with hexane–ethyl acetate provided a pure product (2c). Colorless solid, 97% yield. mp 183°C. IR (KBr) 1590, 1557, 1432, 1297, 1121, 765 cm−1. 1H-NMR (500 MHz, CDCl3) δ: 5.98 (s, 1H, CH), 6.05 (t, J=55 Hz, CF2H), 7.15 (d, J=7.5 Hz,1 H, ArH), 7.23–7.30 (m, 2H, ArH), 7.50 (d, J=7 Hz, 1H, ArH); 13C-NMR (125 MHz, CDCl3) δ: 80.3, 110.7, 111.5 (t, 1JC-F=245.3 Hz, CF), 115.7, 125.0, 125.6, 137.1, 147.6, 167.7, 173.1 (t, 2JC-F=22.5 Hz, CO); LR-MS (EI+) m/z=484 (M+, 100); HR-MS (EI) Calcd for C20H12F4N2O4Zn (M+): 484.0024. Found: 484.0001.
(Z)-1-(Benzo[d]oxazol-2-yl)-3,3-difluoroprop-1-en-2-ol (1c)A mixture of 2b (242.8 mg, 0.5 mmol) and EDTA·2Na·2H2O (1.7 g, 5 mmol) in EtOH (5 mL) was stirred for 6 h at 80°C. After being cooled to room temperature, the reaction mixture was diluted with 3% NaOH (30 mL) and extracted with EtOAc (30 mL×3), washed brine, dried over Na2SO4, filtered and concentrated under reduced pressure. Column chromatography of the crude product on silica gel eluting with hexane–ethyl acetate provided a pure product (1c). White solid, 66% yield. mp 133–135°C. IR (KBr) 3109, 1638, 1553, 1446, 1347, 1296, 1251, 1190, 1163, 1094, 1069, 1005, 977, 813, 740, 484 cm−1; 1H-NMR (500 MHz, CDCl3) δ: 5.26 (br s, 1H, OH), 5.86 (s, 1H, CH), 6.36 (t, J=54 Hz, 1H, CF2H), 7.32–7.41 (m, 2H, ArH), 7.60 (d, J=6.9 Hz, ArH); 13C-NMR (125 MHz, CDCl3) δ: 79.1 (t, 3JC-F=3.4 Hz, CH), 111.0, 111.3 (t, 1JC-F=243.0 Hz, CF2H), 115.2, 124.9, 125.8, 133.8, 146.9, 163.9, 172.9 (t, 2JC-F=23.0 Hz, CO); LR-MS (EI+) m/z=211 (M+, 49.4), 160 (100); HR-MS (EI) Calcd for C10H7F2NO2 (M+): 211.0445. Found: 211.0450.
General Procedure for Synthesis of Metal Complexes (2d–h)A mixture of 1a (229 mg, 1 mmol), metal salts (3 mmol) in THF (5 mL) was stirred at room temperature. Reaction time was described in Table 1. The reaction mixture was filtered through a Celite and filtrate was concentrated under vacuum. The resulting product was recrystallized several times from CHCl3 and hexane. Or chromatography (hexane/AcOEt) on silica gel gave the pure product.
Bis((Z)-1-(benzo[d]oxazol-2-yl)-3,3,3-trifluoroprop-1-en-2-ate)magnesium(II)· 2(MeCN) (2d)Purified by chromatography on silica gel. Colorless solid, 88% yield. mp 274–275°C. IR (KBr) 3173, 1603, 1552, 1429, 1317, 1232, 1188, 1127, 874, 778, 751 cm−1; 1H-NMR (500 MHz, DMSO-d6) δ: 5.73 (s, 1H, CH), 7.20–7.24 (m, 2H, ArH), 7.53–7.55 (m, 1H, ArH), 7.87 (br s, 1H, ArH); 13C-NMR (125 MHz, DMSO-d6) δ: 79.1, 110.2, 118.3, 120.6 (q, 1JC-F=284.4 Hz, CF), 124.0, 124.6, 140.4, 148.2, 163.9 (q, 2JC-F=31.5 Hz, CO), 166.9; LR-MS (EI+): m/z=480 (M+, 100); HR-MS (EI) Calcd for C20H10F6MgN2O4 (M+): 480.0395. Found: 480.0389.
Bis((Z)-1-(benzo[d]oxazol-2-yl)-3,3,3-trifluoroprop-1-en-2-ate)nickel(II)·2H2O (2e)Purified by chromatography on silica gel. Dark green solid, 82% yield. mp 172–173°C. IR (KBr) 3399, 1595, 1560, 1458, 1429, 1318, 1231, 1186, 1129, 876, 775, 749 cm−1. The compound exhibit ferromagnetism and unmeasurable by 1H-NMR and 13C-NMR. LR-MS (EI+): m/z=514 (M+, 88.9), 160 (100); HR-MS (EI) Calcd for C20H10F6N2NiO4 (M+): 513.9898. Found: 513.9870.
Bis((Z)-1-(benzo[d]oxazol-2-yl)-3,3,3-trifluoroprop-1-en-2-ate)copper(II) (2f)Purified by chromatography on silica gel. Brown solid, 25% yield. mp 214–215°C. IR (KBr) 3162, 1609, 1554, 1430, 1347, 1330, 1235, 1186, 1143, 1021, 933, 883, 782, 758 cm−1; 1H-NMR (500 MHz, CDCl3) δ: 5.19 (br s, 1H), 6.44 (br s, 1H). 13C-NMR signals could not be unambiguously assigned due to a set of broad signals for aromatic carbons. LR-MS (EI+): m/z=519 (M+, 93.7), 291 (100); HR-MS (EI) Calcd for C20H10CuF6N2O4 (M+): 518.9841. Found: 517.9837.
Bis((Z)-1-(benzo[d]oxazol-2-yl)-3,3,3-trifluoroprop-1-en-2-ate)palladium(II) (2g)Washed by CHCl3 and hexane. Orange solid, 23% yield. mp 200–204°C. IR (KBr) 3466, 1604, 1557, 1427, 1344, 1235, 1207, 1136, 1123, 895, 775, 753 cm−1. 1H-NMR and 13C-NMR were unmeasurable. LR-MS (EI+): m/z=562 (M+, 100); HR-MS (EI) Calcd for C20H10F6N2O4Pd (M+): 561.9580. Found: 561.9580.
Silver(I) (Z)-1-(benzo[d]oxazol-2-yl)-3,3,3-trifluoroprop-1-en-2-ate (2h)Recrystallized by CHCl3 and hexane. Yellow solid, 30% yield. mp 209°C. IR (KBr) 3434, 1684, 1595, 1556, 1458, 1438, 1250, 1225, 1184, 1123, 1103, 863, 768, 749 cm−1; 1H-NMR (500 MHz, DMSO-d6) δ: 5.36 (s, 1H, CH), 7.19 (t, J=7.5 Hz, ArH), 7.25 (t, J=7.6 Hz, ArH), 7.52 (dd, J=16.9 and 16.9 Hz, ArH); 13C-NMR (125 MHz, DMSO-d6) δ: 74.2, 109.9, 116.9, 119.9 (q, 1JC-F=287.3 Hz, CF), 123.4, 124.5, 141.7, 147.3, 166.3 (q, 2JC-F=29.0 Hz, CO), 167.7.
The molecular ion peak was not observed by MS. The molecular weight of 2h was estimated to be 672 as Ag2L2 and the MIC value was calculated based on the estimated molecular weight.
X-Ray Single-Crystal Structure DeterminationThe single crystals of 2b, e, f and h were prepared at room temperature by the vapor diffusion method using two solvents, AcOEt and hexane for 2b and f, acetone and hexane for 2e, and CHCl3 and hexane for 2h, respectively.58) The single crystals of 2d and g were recrystallized from acetonitrile or THF, respectively. Their crystal structures were determined by single-crystal X-ray diffraction. Data collection was carried out at 100–296 K on a Rigaku VariMax SaturnCCD724/α diffractometer using graphite monochromated Mo-Kα radiation at the Advanced Research Support Center, Ehime University. Basic information pertaining to crystal parameters and structure refinement is summarized in Table S1-7. Further crystallographic details for the structure reported in this paper may be obtained from the Cambridge Crystallographic Data Center, on quoting the depository numbers CCDC 1574811(2b), CCDC 1574812 (2d), CCDC 1574814 (2e), CCDC 1574815 (2f), CCDC 1574816 (2g), and CCDC 1574817 (2h).
Light Stability of the Ag(I) Complex 2hLight stability of the Ag(I) complex 2h was studied in direct light under an air atmosphere at room temperature to mimic their exposure to light under every life conditions.26) The positive controls were AgNO3 and Zn(II) complex (2a). Cotton pads were impregnated with 0.025 M aqueous solutions of the compounds and exposed to air and light. The stability was monitored visually within 5 d. The photos of the samples are shown in Fig. S1. The complex 2h became slightly darkened already after 5 h whereas 2a remained unaltered until 24 h.
Evaluation of Antibacterial Activity in VitroBacterial StrainsGram-negative bacteria of Acinetobacter baumannii ATC C17978, Escherichia coli TG1, Klebsiella pneumoniae IID5209, Pseudomonas aeruginosa O1, Serratia marcescens (clinical isolate), and Vibrio parahaemolyticus RIMD221051 and Gram-positive bacteria of Bacillus subtilis ATC C6633, Enterococcus faecalis IID622, Staphylococcus aureus FDA209P, methicillin-resistant Staphylococcus aureus N315, Streptococcus pneumoniae IID555, and Streptococcus pyogenes 124 were used in this study.
Antimicrobial ActivityThe MICs of all compounds (1a–c and 2a–h) for all 12 strains were determined by the standard two-fold dilution method.42) Briefly, the bacterial cells (105 CFU/mL) were added into 100 µL of the compounds dilutions in Muller-Hinton broth, and then incubated at 37°C for 24 h. The microbial growth was examined by visual inspection. In this assay, all compounds were dissolved in DMSO. The final concentration of DMSO in broth did not affect the microbial growth.
Time–Kill Curve and Growth CurvePa cells, in the stationary growth phase, were diluted to 1.0×107 CFU/mL in Muller Hinton broth (Becton, Dickinson and Company, Sparks, MD, U.S.A.) and incubated at 37°C. The concentrations equivalent to 0, 1, 2, 4 and 8 times MIC of compounds 2h and 4 times MIC of AgNO3 were added to cell suspension after 2 h of incubation. In the time–kill curve, CFU/mL after 0, 2, 4, 6, 8, 12, and 24 h of incubation was determined by counting colonies of diluted cell suspension at each time point on Muller Hinton agar plates. On the other hand, in the growth curve, the optical density at 600 nm (O.D.600) of the cell suspension was measured over time.59)
Scanning Electron Microscopy (SEM) DetectionThe morphology of Pa after incubation with 0×, 2×, 5× and 10×MIC (0, 1, 5 and 10 µg/mL) of Ag(I) complex 2h was observed under SEM. Pa was aerobically cultured in LB medium at 37°C. The Ag(I) complex 2h was added when the absorbance at 600 nm was about 1.5 and then aerobically cultured another 1 h at 37°C. One milliliter of Cells was harvested by centrifugation at 21500×g for 30 s and then suspended the cells in 0.5 mL of PBS. The Cells were subsequently fixed by adding 0.5 mL of 5% (v/v) glutaraldehyde (Grade for electron microscope, Wako Pure Chemical Industries, Ltd.) in PBS and then incubated overnight at 4°C. The fixed cells were spotted onto ISOPORE™ membrane filter (pore size: 0.2 µM, MILLIPORE), washed with PBS and dehydrated with a graded ethanol series on filter. After lyophilized and platinum coating, the samples were visualized by Hitachi S-3400N Scanning Electron Microscope (Hitachi High-Technologies, Tokyo, Japan).60)
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.