Abstract
Terreinlactone A (1a/1b), a pair of 3-substituted δ-lactone enantiomers, and terreinlactone B (2), a new biosynthetic intermediate of 1a/1b, were isolated from Aspergillus terreus, along with their biosynthetic precursor (+)-terrein (3) and (+)-isoterrein (4). Compounds 1a and 1b were separated by using a Daicel chiral-pak ASH column eluting with n-hexane–EtOH (80 : 20). The structures of 1a/1b with absolute configurations were determined by comprehensive spectroscopic analyses and electronic circular dichroic (ECD) calculations. Terreinlactone A (1) represents the first example of 1,5-seco-terrein and a biogenetic pathway is proposed from the precursor terrein via the intermediated terreinlactone B (2).
Aspergillus terreus is a filamentous fungus distribute widely in natural world, and from the culture broth of it, a variety of natural products have been isolated, such as alkaloids,1,2) meroterpenoids,3,4) sesterterpenoids,5) lignans,2,6,7) and so on. The most famous metabolite from A. terreus is lovastatin (named as mevinolin at first), which is an important hydroxymethylglutaryl (HMG)-CoA reductase inhibitor clinically used.8,9) Terrein represents another type of notable metabolites of A. terreus, which was firstly isolated in 1935 and structural determined in 1955.10–12) Then, terrein and its analogues attracted considerable attention for their biosynthetic investigations,13–15) and until 2014, Hertweck and colleagues illustrated the gene cluster responsible for the biosynthesis of terrein.16) Besides, terrein and analogues also attracted much attention for their total syntheses17–20) for their versatile bioactivities including inhibitor of plant growth, anti-microbial, anti-proliferative, and anti-oxidative activities.6,21–24)
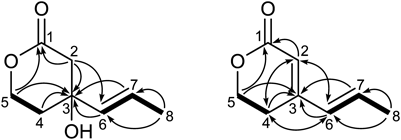
In our previous research, asperterpenes A and B, two meroterpenoids with unusual skeletons and β-Site Amyloid Precursor Protein Cleaving Enzyme 1 (BACE1) inhibiting activity, were isolated from the fungus A. terreus isolating from the soil collected in the bottom of Yangzi River.4) In the further search for bioactive metabolites from A. terreus, a chemical investigation of another A. terreus was carried out, which was obtained from China Forestry Culture Collection Center (CFCC 81836). Unexpectedly, a pair of 3-substituted δ-lactone enantiomers, named (±)-terreinlactone A (±1), derived from terrein, were isolated, along with their biosynthetic intermediate terreinlactone B (2) and precursors (+)-terrein (3) and (+)-isoterrein (4)25,26) (Fig. 1).
Results and Discussion
Terreinlactone A (1) was isolated as a colorless oil. Its molecular formula was determined to be C8H12O3 by high resolution-electrospray ionization (HR-ESI)-MS spectrum with an ion peak at m/z 179.0664 [M+Na]+ (Calcd for C8H12O3Na, 179.0684), suggesting three degrees of unsaturation. The IR spectrum of 1 showed absorption bands at 3410 and 1714 cm−1, suggesting the presence of a hydroxyl and an ester carbonyl. The 1H- and 13C-NMR data (Table 1) along with the heteronuclear single quantum coherence (HSQC) spectrum showed signals of an ester carbonyl (δC 173.4), a disubstituted double bond with trans-configuration (δC 137.0 and 125.4; δH 5.75, dq, J=15.5, 6.4 Hz and 5.61, dq, J=15.5, 1.5 Hz), an oxygenated quaternary carbon (δC 70.3), three methylenes including an oxygenated one (δC 67.5, 43.8, and 35.2; δH 4.56, ddd, J=11.2, 10.5, 4.2 Hz; 4.37, ddd, J=11.2, 5.6, 3.8 Hz; 2.69, d, J=17.3 Hz; 2.52, dd, J=17.3, 2.2 Hz; 2.04, ddd, J=14.2, 10.5, 5.6 Hz; 1.84, dddd, J=14.2, 4.2, 3.8, 2.2 Hz), and a methyl (δC 17.8; δH 1.73, dd, J=6.4, 1.5 Hz). These data combined with the degrees of unsaturation revealed one ring system in the structure of 1.
Table 1.
1H- and
13C-NMR Data of
1 and
2 in CD
3OD
a)| No. | 1 | 2 |
|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC |
|---|
| 1 | | 173.4 | | 165.7 |
| 2a | 2.69 d (17.3) | 43.8 | 5.76 s | 115.7 |
| 2b | 2.52 dd (17.3, 2.2) | | | |
| 3 | | 70.3 | | 153.7 |
| 4a | 2.04 ddd (14.2, 10.5, 5.6) | 35.2 | 2.53 td (6.2, 0.9) | 24.0 |
| 4b | 1.84 dddd (14.2, 4.2, 3.8, 2.2) | | | |
| 5a | 4.56 ddd (11.2, 10.5, 4.2) | 67.5 | 4.38 t (6.2) | 66.0 |
| 5b | 4.37 ddd (11.2, 5.6, 3.8) | | | |
| 6 | 5.61 dq (15.5, 1.5) | 137.0 | 6.20 m | 130.3 |
| 7 | 5.75 dq (15.5, 6.4) | 125.4 | 6.20 m | 135.4 |
| 8 | 1.73 dd (6.4, 1.5) | 17.8 | 1.89 d (4.9) | 18.9 |
a) 400 MHz for 1H and 100 MHz for 13C, δ in ppm.
The whole structure of 1 was further elucidated by analyses of the 1H–1H correlation spectroscopy (COSY) and heteronuclear multiple bond connectivity (HMBC) spectra (Fig. 2). Elucidation of the 1H–1H COSY spectrum disclosed the spin systems of C(4)H2–C(5)H2 and C(6)H–C(7)H–C(8)H3. In addition, the δ-lactone ring was further established by the HMBC correlations from H-4 and H-5 to C-3, from H-2 to C-1 and C-3, and from H-5 to C-1. And then, the allyl group of C-6–C-7–C-8, which was elucidated by 1H–1H COSY above and further confirmed by HMBC interactions from Me-8 to C-6 and C-7, was located at C-3 as determined by HMBC correlations from H-6 and H-7 to C-3 and from H-2 to C-6. Thus, the structure of 1 was finally determined.
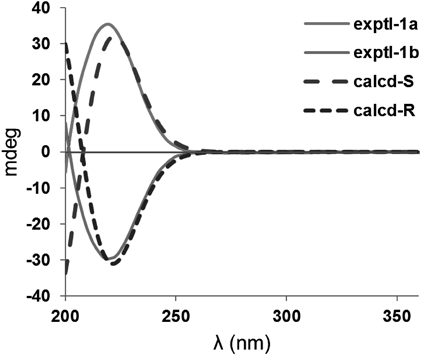
We tried to determine the absolute configuration of 1 by electronic circular dichroism (ECD) as there is an ester carbonyl close to the stereogenic center of C-3. However, the ECD spectrum showed nearly no Cotton effect, suggesting the racemic feature of 1. The optical rotation of 1 is almost zero, which further indicates that 1 is a racemic mixture. Finally, chiral separation of 1a/1b was performed by using a Daicel chiral-pak ASH column eluting with n-hexane–EtOH (80 : 20) (Fig. 3) after many attempts with various chiral columns and solvent systems. As expected, 1a and 1b exhibited mirror-like ECD curves (Fig. 4) and totally opposite optical rotations (1a: [α]D20 +2.4; 1b: [α]D20 −3.1). Time-dependent density functional theory (TDDFT) calculations of the theoretical ECD spectra of 1-S and 1-R were then carried out, which assigned the absolute configurations of 1a and 1b as S and R, respectively. Thus, compounds 1a and 1b were elucidated, and named terreinlactones A1 and terreinlactones A2, respectively.
Terreinlactone B (2) was also isolated as a colorless oil. The HR-ESI-MS spectra showed an ion peak at m/z 161.0566, 18 mass units less than 1, indicating a molecular formula of C8H10O2. Further comparing the 1H- and 13C-NMR data (Table 1) of 2 with those of 1 revealed the presence of an additional double bond (δC 115.7 and 153.7; δH 5.76, s), which was located between C-2 and C-3 by HMBC correlations from H-2 to C-1, C-4, and C-6 (Fig. 2). The whole structure of 2 was assigned by analyses of two dimensional (2D)-NMR spectra.
The origin of terrein from five acetate units was preliminarily illuminated in the 1960 to 1980s by using classic isotope-labeling studies.13–15) Until 2014, Hertweck and colleagues discovered the polyketide synthase biosynthetic pathway of terrein and revealed the gene terA as essential one be responsible for the biosynthesis of terrein.16) (±)-Terreinlactone A (1a/1b) was proposed to originate from terrein by reduction to obtain the intermediate iii and then followed by a Baeyer–Villiger oxidation to yield the lactone intermediate 2 (Chart 1). Finally, compound 2 underwent a hydrolysis reaction to form the enantiomer 1a and 1b.
Compounds 1a, 1b, and 2 were tested for cytotoxic activities against five human cancer cell lines (HL-60, SMMC-7721, A-549, MCF-7, SW480) by the 3-(4,5-dimethylthiazol-2-yl)-5(3-carboxymethoxyphenyl)-2-(4-sulfopheny)-2H-tetrazolium (MTs) method, in vitro. However, both compounds showed no obvious cell proliferation up to a concentration of 40 µM.
In summary, terreinlactone A (1) and B (2) represent the first examples of terrein derivatives with an unusual δ-lactone formed by reduction and Baeyer–Villiger oxidation. The enantiomeric property of (±)-terreinlactone A (1a/1b) was tackled by an enantioseparation procedure and their absolute configurations were determined by ECD calculations.
Experimental
GeneralHR-ESI-MS was conducted in the positive-ion mode on a Thermo Fisher LC-LTQ-Orbitrap XL spectrometer. UV spectra were recorded with a PerkinElmer, Inc. Lambda 35 spectrophotometer. IR spectra were measured by a Bruker Vertex 70 FT-IR spectrophotometer. Optical rotations were obtained in a 0.7 mL cell on a Rudolph Autopol IV automatic polarimeter. ECD spectra were obtained with a JASCO J-810 spectrometer. NMR spectra were obtained on a Bruker AM-400 spectrometer, and the 1H- and 13C-NMR chemical shifts were referenced to the solvent peaks for CD3OD at δH 3.31 and δC 49.0 and dimethyl sulfoxide (DMSO)-d6 at δH 2.50 and δC 39.5. Semipreparative HPLC was performed using a Dionex HPLC system equipped with an Ultimate 3000 pump, an Ultimate 3000 autosampler injector, and an Ultimate 3000 diode array detector (DAD) detector controlled by the Chromeleon software (version 6.80) using a reversed-phase (RP) C18 column (5 µm, 10×250 mm, Welch Materials, Inc.), and a Ultimate AQ-RP18 column. Column chromatography (CC) was performed on silica gel (100–200 mesh and 200–300 mesh; Qingdao Marine Chemical, Inc., Qingdao, China), Sephadex LH-20 (40–70 µm, Amersham Pharmacia Biotech AB, Uppsala, Sweden), and octadecylsilyl (ODS, 50 µm, YMC Co., Ltd., Japan). TLC was performed with RP-C18 F254 plates (Merck, Germany) and silica gel 60 F254 (Yantai Chemical Industry Research Institute).
Fungal MaterialThe fungus of Aspergillus terreus was provided by China Forestry Culture Collection Center (CFCC 81836).
Extraction and IsolationThe strain was cultured on potato dextrose agar (PDA) at 28°C for 5 d to prepare the seed culture, then inoculated into 300 Erlenmeyer flasks (1 L) which were previously sterilized by autoclaving and each containing 200 g rice and 180 mL distilled water. The flasks were incubated at 28°C for 28 d. After that, the solid culture was extracted with EtOH, and the EtOH was removed under reduced pressure to yield a crude extract. The crude extract was partitioned with EtOAc against water to obtain the EtOAc fraction (650 g).
The EtOAc fraction was separated by chromatography on a silica gel column chromatography (CC, petroleum ether to EtOAc, 50 : 1–0 : 1) to furnish seven fractions (Fr. 1–7). Fraction 3 (12.0 g) was further separated by the ODS column (MeOH–H2O, 30–100%) to yield eight subfractions (Fr. 3.1–3.8). Fraction 3.1 (720.0 mg, eluted with MeOH–H2O, 30 : 70) was chromatographed on a Sephadex LH-20 column (CH2Cl2–MeOH, 1 : 1) to afford three subfractions (Fr. 3.1.1–3.1.3). Fraction 3.1.1 was subjected to a silica gel CC (CH2Cl2–MeOH, 1 : 0–0 : 1) to give four fractions (Fr. 3.1.1.1–3.1.1.4). Fraction 3.1.1.2 was purified by semipreparative HPLC (MeCN–H2O, 51 : 49) to afford compound 2 (15.4 mg, tR 14.1 min).
Fraction 4 (18.0 g) was subjected to a silica gel CC (CH2Cl2–MeOH, 100 : 1–0 : 1) to give four fractions (Fr. 4.1–4.4). Fraction 4.1 was separated by the ODS column (MeOH–H2O, 30–100%) to yield six subfractions (Fr. 4.1.1–4.1.6). Fraction 4.1.1 (157.0 mg, eluted with MeOH–H2O, 30 : 70) was chromatographed on a Sephadex LH-20 column (CH2Cl2–MeOH, 1 : 1) to afford three subfractions (Fr. 4.1.1.1–4.1.1.3). Fraction 4.1.1.2 was purified by semipreparative HPLC (MeOH–H2O, 28 : 72) to afford 1 (a mixture of 1a and 1b) (16.6 mg, tR 20.4 min). Chiral resolution of 1 was performed on Daicel chiral-pak ASH column (eluted with n-hexane–EtOH, 80 : 20, flow rate 1.0 mL/min, column temperature 29°C) to give 1a (8.0 mg, tR 8.3 min) and 1b (7.8 mg, tR 11.4 min).
Fraction 6 (28.6 g) was subjected to a silica gel CC (CH2Cl2–MeOH, 100 : 1–0 : 1) to give three fractions (Fr. 6.1–6.3). Fraction 6.2 was separated by the ODS column (MeOH–H2O, 20–100%) to yield seven subfractions (Fr. 6.2.1–6.2.7). Fraction 6.2.1 (261.6 mg, eluted with MeOH–H2O, 20 : 80) was chromatographed on a Sephadex LH-20 column (CH2Cl2–MeOH, 1 : 1) to afford three subfractions (Fr. 6.2.1.1–6.2.1.3). Fraction 6.2.1.2 was purified by semipreparative HPLC (MeOH–H2O, 17 : 83) to afford compounds 3 (20.5 mg, tR 32.6 min) and 4 (12.7 mg, tR 36.2 min).
Terreinlactone A (1)Colorless oil, IR νmax=3410, 1714 cm−1; UV (MeOH) λmax (log ε)=203 (2.75) nm; for 1H-NMR (400 MHz) and 13C-NMR (100 MHz) data see Table 1; HR-ESI-MS [M+Na]+ m/z 179.0664 (Calcd for C8H12O3Na, 179.0684); 1a: [α]D20 +2.4 (c=3.6, MeOH); ECD (MeOH) λ (Δε) 219 (+0.84) nm; 1b: [α]D20 −3.1 (c=0.7, MeOH); ECD (MeOH) λ (Δε) 219 (–0.78) nm.
Terreinlactone B (2)Colorless oil, IR νmax=1717 cm−1; UV (MeOH) λmax (log ε)=265 (4.28) nm; for 1H-NMR (400 MHz) and 13C-NMR (100 MHz) data see Table 1; HR-ESI-MS [M+Na]+ m/z 161.0566 (Calcd for C8H10O2Na, 161.0578).
Computational DetailsThe conformations of 1-S and 1-R generated by BALLOON were subjected to semiempirical PM3 quantum mechanical geometry optimizations using the Gaussian 09 program. Duplicate conformations were identified and removed when the root-mean-square (RMS) distance was less than 0.5 Å for any two geometry-optimized conformations. The remaining conformations were further optimized at the B3LYP/6-31G(d) level in MeOH with the IEFPCM solvation model using Gaussian 09, and the duplicate conformations emerging after these calculations were removed according to the same RMS criteria above. The harmonic vibrational frequencies were calculated to confirm the stability of the final conformers. The electronic circular dichroism (ECD) spectrum were calculated for each conformer using the TDDFT methodology at the B3LYP/6-311++G(d,p)//B3LYP/6-31G(d) level with MeOH as solvent by the IEFPCM solvation model implemented in Gaussian 09 program. The ECD spectra for each conformer were simulated using a Gaussian function with a bandwidth σ of 0.4 eV. The spectra were combined after Boltzmann weighting according to their population contributions.
Cytotoxic Activities EvaluationFive human cancer cell lines, including HL-60, SMMC-7721, A-549, MCF-7, and SW-480, together with one noncancerous cell line (Beas-2B), were used in the cytotoxic activity assay as described in our previous report.27)
Acknowledgments
This work was financially supported by the Program for Changjiang Scholars of Ministry of Education of the People’s Republic of China (No. T2016088); National Natural Science Foundation for Distinguished Young Scholars (No. 81725021); Innovative Research Groups of the National Natural Science Foundation of China (81721005); the National Natural Science Foundation of China (Nos. 81502943 and 31600266); the Academic Frontier Youth Team of Huazhong University of Science and Technology (HUST) the Integrated Innovative Team for Major Human Diseases Program of Tongji Medical College (HUST); We thank the Analytical and Testing Center at Huazhong University of Science and Technology for assistance in testing of ECD, UV and IR analyses.
Conflict of Interest
The authors declare no conflict of interest.
Supplementary Materials
The online version of this article contains supplementary materials.
References
- 1) Cai S., Du L., Gerea A. L., King J. B., You J., Cichewicz R. H., Org. Lett., 15, 4186–4189 (2013).
- 2) He F., Bao J., Zhang X. Y., Tu Z. C., Shi Y. M., Qi S. H., J. Nat. Prod., 76, 1182–1186 (2013).
- 3) Liaw C. C., Yang Y. L., Lin C. K., Lee J. C., Liao W. Y., Shen C. N., Sheu J. H., Wu S. H., Org. Lett., 17, 2330–2333 (2015).
- 4) Qi C., Bao J., Wang J., Zhu H., Xue Y., Wang X., Li H., Sun W., Gao W., Lai Y., Chen J. G., Zhang Y., Chem. Sci., 7, 6563–6572 (2016).
- 5) Liu Z., Chen Y., Chen S., Liu Y., Lu Y., Chen D., Lin Y., Huang X., She Z., Org. Lett., 18, 1406–1409 (2016).
- 6) Liao W. Y., Shen C. N., Lin L. H., Yang Y. L., Han H. Y., Chen J. W., Kuo S. C., Wu S. H., Liaw C. C., J. Nat. Prod., 75, 630–635 (2012).
- 7) Sun K., Zhu G., Hao J., Wang Y., Zhu W., Tetrahedron, 74, 83–87 (2018).
- 8) Alberts A. W., Chen J., Kuron G., Hunt V., Huff J., Hoffman C., Rothrock J., Lopez M., Joshua H., Harris E., Patchett A., Monaghan R., Currie S., Stapley E., Albers-Schonberg G., Hensens O., Hirshfield J., Hoogsteen K., Liesch J., Springer J., Proc. Natl. Acad. Sci. U.S.A., 77, 3957–3961 (1980).
- 9) Tobert J. A., Nat. Rev. Drug Discov., 2, 517–526 (2003).
- 10) Raistrick H., Smith G., Biochem. J., 29, 606–611 (1935).
- 11) Barton D. H. R., Miller E., J. Chem. Soc., 1955, 1028–1029 (1955).
- 12) Grove J., J. Chem. Soc., 1954, 4693–4694 (1954).
- 13) Birch A. J., Cassera A., Jones A. R., Chem. Commun., 1965, 167–168 (1965).
- 14) Hill R. A., Carter R. H., Staunton J., J. Chem. Soc., Chem. Commun., 1975, 380–381 (1975).
- 15) Hill R. A., Carter R. H., Staunton J., J. Chem. Soc., Perkin Trans. 1, 1981, 2570–2576 (1981).
- 16) Zaehle C., Gressler M., Shelest E., Geib E., Hertweck C., Brock M., Chem. Biol., 21, 719–731 (2014).
- 17) Auerbach J., Weinreb S. M., J. Chem. Soc., Chem. Commun., 1974, 298–299 (1974).
- 18) Klunder A. J. H., Bos W., Zwanenburg B., Tetrahedron Lett., 22, 4557–4560 (1981).
- 19) Kolb H. C., Martin H., Hoffmann R., Tetrahedron Asymmetry, 1, 237–250 (1990).
- 20) Altenbach H. J., Holzapfel W., Angew. Chem. Int. Ed. Engl., 29, 67–68 (1990).
- 21) Park S. H., Kim D. S., Kim W. G., Ryoo I. J., Lee D. H., Huh C. H., Youn S. W., Yoo I. D., Park K. C., Cell. Mol. Life Sci., 61, 2878–2885 (2004).
- 22) Phattanawasin P., Pojchanakom K., Sotanaphun U., Piyapolrungroj N., Zungsontiporn S., Nat. Prod. Res., 21, 1286–1291 (2007).
- 23) Arakawa M., Someno T., Kawada M., Ikeda D., J. Antibiot., 61, 442–448 (2008).
- 24) Demasi M. A., Felicio L., Pacheco A. O., Leite H. G., Lima C., Andrade L. H., J. Braz. Chem. Soc., 21, 299–305 (2010).
- 25) Wakana D., Hosoe T., Itabashi T., Nozawa K., Kawai K., Okada K., Campos–Takaki G. M., Yaguchi T., Fukushima K., Mycotoxins, 56, 3–6 (2006).
- 26) Trabolsy Z. B. K. A., Anouar E. H., Zakaria N. S. S., Zulkeflee M., Hasan M. H., Zin M. M., Ahmad R., Sultan S., Weber J. F. F., J. Mol. Struct., 1060, 102–110 (2014).
- 27) Zhu H., Chen C., Yang J., Li X. N., Liu J., Sun B., Huang S. H., Li D., Yao G., Luo Z., Li Y., Zhang J., Xue Y., Zhang Y., Org. Lett., 16, 6322–6325 (2014).