2019 Volume 67 Issue 11 Pages 1201-1207
2019 Volume 67 Issue 11 Pages 1201-1207
Oleanolic acid (OA) was discovered as a mild influenza hemagglutinin (HA) inhibitor in our earlier studies. In the present work, 20 compounds were prepared by structural modifications of OA, and their antiviral activities against influenza A/WSN/33 (H1N1) virus in Madin–Darby canine kidney (MDCK) cells were evaluated. Based on the biological result, structure–activity relationship (SAR) was discussed. Compound 10 with six-carbon chain and a terminal hydroxyl group showed the strongest anti-influenza activity with an IC50 of 2.98 µM, which is an order of magnitude more potent than OA. Hemagglutination inhibition and Surface plasmon resonance (SPR) assay indicated that compound 10 might interfere with influenza invasion by interacting with HA protein.
Influenza A viruses (IAVs) are the main cause of annual epidemics and occasional pandemics of respiratory diseases worldwide.1–3) According to estimates by the WHO, IAVs infect 5–15% of the world’s population,4) killing between 250000 and 500000 people each year.5) Based on the viral surface glycoproteins hemagglutinin (HA) and neuraminidase (NA), IAVs are classified into various subtypes. There are 18 variants of HA (H1–H18) and 11 variants of NA (N1–N11) on the IAVs,6) which may form 198 subtypes of IAVs, theoretically.7,8)
Two classes of anti-influenza drugs, M2 ion-channel inhibitors (amantadine and rimantadine) and NA inhibitors (oseltamivir, zanamivir and peramivir), have been used in clinic for the interruption of specific processes in influenza infections.9,10) In 2018, baloxavir was licensed in Japan for the treatment of both adult and pediatric patients infected with influenza virus. It is approved for use in children older than twelve age with acute uncomplicated influenza.11) However, frequent application of the marketed anti-influenza drugs, in combination with the high mutation rate of the RNA genome of the influenza virus, has led to the rapid emergence of drug-resistant viral strains. Therefore, cost-effective antiviral strategies must be developed to prevent emerging influenza pandemics.
IAVs entry represents a favorable target of drug discovery, because it is the first step of viral infection. Inhibition of this first step should result in the virus propagation effectively blocked.12,13) In our previous studies, we found that oleanolic acid (OA) and its derivatives, which belongs to the family of pentacyclic triterpenes, display inhibitory activity against IAVs entry by interrupting the interaction between HA and sialic acid receptor.14)
As a continuation of our project aimed at discovering novel entry-targeting IAVs inhibitors, a total of 20 OA C-28 derivatives were designed, synthesized, and evaluated for their anti-IAVs activities in vitro.
Certain structural modifications of these triterpenoids, many of which are simple derivatizations of the molecule’s functional groups, can have a significant impact on their biological properties. For example, studies have shown increased biological activities of the C-28 amino conjugates of various pentacyclic triterpenic acids as compared to their natural precursors.
As shown in Chart 1, the C-28 of OA was first activated by 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI) and (CH3CH2)3N in the presence of 1-hydroxybenzotriazole (HOBt), then reacted with amine reagents, giving the corresponding OA derivatives (compounds 1–20) with high yields. The amine reagents were selected with different chain lengths and terminal functional groups to evaluate their influence on the anti-IAVs activity.
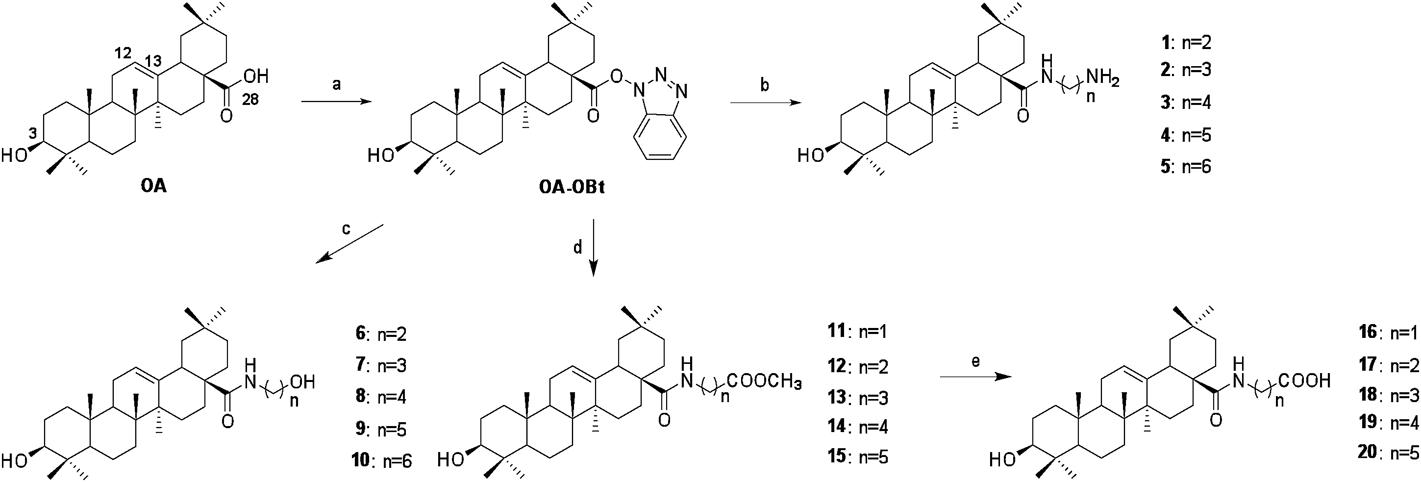
Reagents and conditions: (a) EDCI, HOBt, (CH3CH2)3N, CH2Cl2, 5–10°C, 12 h. (b) Amine compound, Na2CO3, DMF, r.t., 12h. (c) Alcohol compound, Na2CO3, DMF, r.t., 12h. (d) Methyl ester compound, Na2CO3, DMF, r.t., 12h. (e) NaOH, MeOH, r.t., 6h.
All the synthesized OA derivatives were evaluated for their in vitro anti-A/WSN/33 (H1N1) virus in an Madin–Darby canine kidney (MDCK) cell line at a concentration of 100 µM. The inhibition rates of the compounds were calculated and shown in Fig. 1. To exclude the possibility that the observed anti-IAVs virus activity was due to cytotoxicity, a preliminary screening was performed to determine their cytotoxic activity in MDCK cells via the CellTiter-Glo assay and the cytopathic effect (CPE) reduction assay.14) Oseltamivir (OSV, 100 µM) and 1% dimethyl sulfoxide (DMSO) were used as positive and negative controls, respectively.
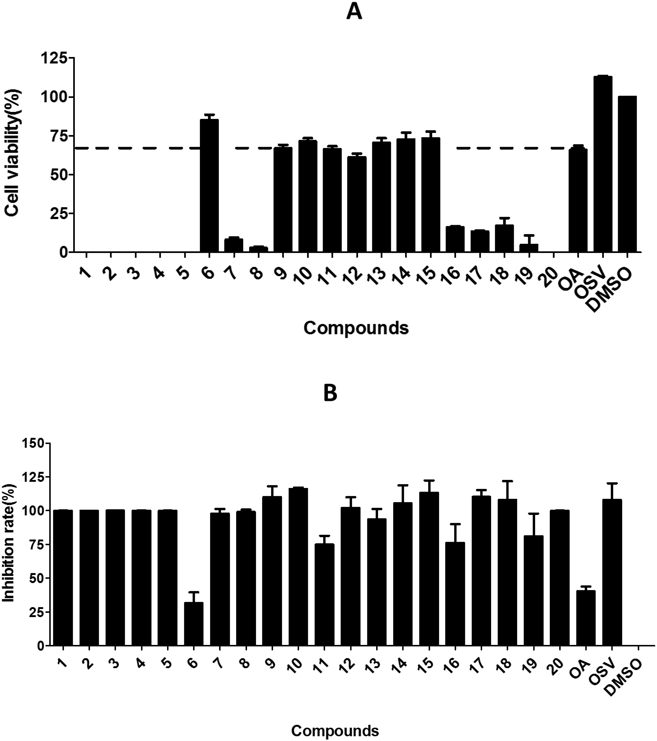
A. Cytotoxicity screening of OA derivatives (final concentration 100 µM) using CellTiter-Glo® assay. B. The CPE-based screening of OA derivatives by using a CellTiter-Glo® assay. MDCK was utilized as the host cell to test A/WSN/33 (H1N1) virus infection; 1% DMSO (final concentration) was used as negative control, and OSV (oseltamivir) was utilized as positive control, respectively. The error bars indicate the standard deviations of triplicate experiments.
In Fig. 1A, compounds 1–5, the diamine conjugates of OA, exhibited strong cytotoxicity. This is in agreement with Parra’s observation that that triterpene derivatives with a diamine, showed extreme cytotoxicity against cancer-cell lines (B16-F10, HT29 and Hep G2).15) On the other hand, no obvious toxicity was observed when the terminal of the side chain is a methylated carboxyl group at the final concentration of 100 µM (compounds 11–15). However, the removal of the methyl group (compounds 16–20) resulted in significant cytotoxicity at the same concentration, indicating that the terminal group of the side chain has a significant effect on cytotoxicity. Interestingly, compounds 7 and 8 display much higher cytotoxicity than 6, 9 and 10, which means the length of the side chain might play a critical role when the terminal is a hydroxyl group (compounds 6–10). Figure 1B shows that most of the compounds displayed a much higher inhibition rate than OA. However, in light of Fig. 1A, this observation might, to a large extent, reflect the cellular toxicity rather than anti-IAVs potency enhancement. According to the results above, six compounds, 9, 10, and 12–15, with high inhibition rates (>80%) and low cytotoxicity (cell viability >70%) in the initial screening, were selected for the dose response assays. As shown in Table 1, compound 10 displayed the strongest inhibitory activity in this study with an IC50 value of 2.98 µM. Compared with DMSO-treated MDCK cells, influenza A/WSN/33 virus causes a severe CPE in MDCK-infected cells. We found that compound 10 (Fig. 2A) could significantly reduce the CPE, which indicating these compounds were able to protect MDCK cells from influenza virus-induced CPE.
| Compound | IC50 (µM)a) | CC50 (µM)b) |
|---|---|---|
| 9 | >50 | >100 |
| 10 | 2.98 | >100 |
| 12 | 5.36 | >100 |
| 13 | 39.49 | >100 |
| 14 | >50 | >100 |
| 15 | >50 | >100 |
| OSV | 1.82 | >100 |
| OA | 72.27 | >100 |
All data presented are averages of at least three separate experiments. a) IC50: compound concentration required to achieve 50% inhibition of replication of virus, as determined by the CellTiter-Glo assay. b) CC50: compound concentration required to cause 50% death of uninfected MDCK cells, as determined by the CellTiter-Glo assay.
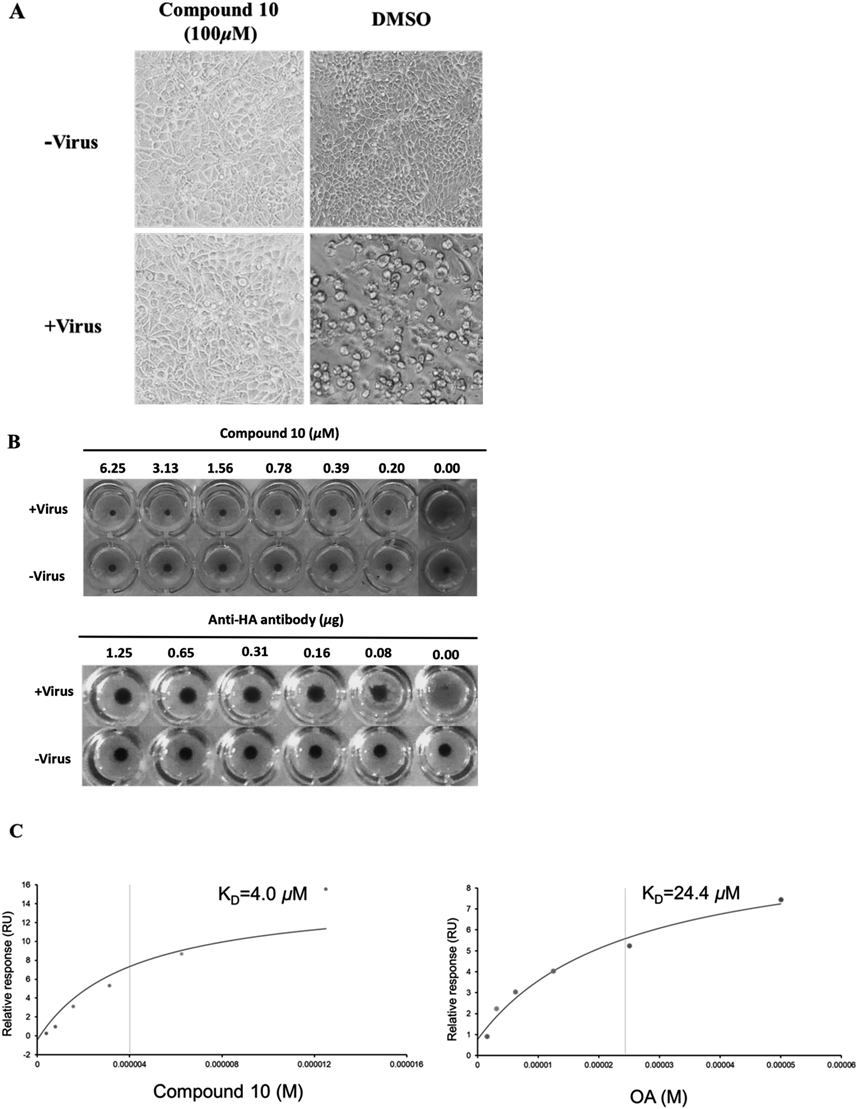
B. Identify HA as the potential target of compound. Comparisons of the behaviors of compound 10 vs. anti-HA antibody in inhibition of influenza virus-induced aggregation of chicken erythrocytes. Compound 10 exerted identical capability as anti-HA antibody in inhibition of hemagglutination in a dose dependent manner. C. Characterization of the affinity between compounds (10 and OA) and HA protein, which were immobilized on a CM5 sensor chip, based on the SPR assay. Their KD values are labeled on the corresponding curves.
Hemagglutinin (HA) plays an important role in the early stages of IAVs infection and mediates attachment of IAVs to host cells via the sialic acid receptor.16,17) HA can also bind to sialic acid on the surface of red blood cells (RBCs), causing agglutination.18) To investigate whether compound 10 can block the ability of viral particles to bind to cell membrane receptors, a hemagglutination inhibition (HI) assay was performed. We found that compound 10 inhibited the binding of influenza virus A/WSN/33 to RBCs, suggesting that compound 10 may directly act on the HA protein (Fig. 2B).
In addition, surface plasmon resonance (SPR) assay was used to characterize the affinity between HA protein and the compounds 10, OA.19) We found that compound 10 could bind tightly to the HA immobilized on the sensor chip (CM5) and at concentrations of 0.39–15 µM exerted dose-dependent responses. As shown in Fig. 2C, the binding curves are fitted well with the Langmuir equation for monovalent binding, which allows the determination of the apparent dissociation constant, KD. The calculated KD values for compound 10 and OA were 4 µM and 24.4 µM, respectively, indicating that compound 10 bound more tightly to HA than OA.
In summary, we have designed and synthesized 20 compounds by structural modification of OA, a mild influenza HA inhibitor discovered in our previous work. Among them, compound 10, which contains a six-carbon chain with a hydroxyl terminal group, was less cytotoxic and much more potent than other compounds with an IC50 of 2.98 µM. Hemagglutination inhibition and SPR assays indicated that compound 10 might interfere with influenza invasion by interaction with the HA protein.
To OA-OBt (0.2 mmol) and Na2CO3 (0.6 mmol) stirring in DMF (20 mL) was added the corresponding amine (0.2 mmol) or alcohol (0.4 mmol) or methyl ester (0.6 mmol). The mixture was stirred at room temperature for 12 h. After completion (TLC) the solvent was removed under reduced pressure. The mixture was dissolved in EtOAc and washed with water and brine twice. The organic layer was dried over Na2SO4, then filtered and concentrated. The crude product was purified by column chromatography.
General Procedure B for the Synthesis of Oleanolic Acid Derivatives (16–20)To compounds 11–15 (0.1 mmol) stirring in methanol (10 mL) was added 1N NaOH (3 mL). The mixture was stirred at rt. After completion (TLC) the reaction mixture was neutralized with 1 mol/L HCl (3 mL). Water was added and the resulting suspension was filtered. Crude product was purified by column chromatography.
OA-OBtThis compound was prepared from OA (10 mmol), EDCI (13 mmol), (CH3CH2)3N (13 mmol) and 1-hydroxybenzotriazole (HOBt) according to the Lei’s studies.20)
Compound 1This compound was prepared from OA-OBt (0.2 mmol) and ethylenediamine (2 mmol) according to the general procedure A. The residue was purified by column chromatography (dichloromethane/methanol, 15 : 1 v/v). Yield: 62 mg, 62%; white solid. Mp 202.5–203.3°C. 1H-NMR (400 MHz, CDCl3) δ: 0.76, 0.78, 0.92, 0.94, 0.98, 1.18 (7 × CH3), 0.76–2.00 (m, other aliphatic ring protons), 2.66 (d, J = 11.4 Hz, 1H), 2.81 (t, J = 6 Hz, 2H), 3.11–3.21 (m, 2H), 3.36–3.43 (m, 2H), 5.40 (s, 1H); 13C-NMR (100 MHz, CDCl3) δ: 15.00, 15.31, 16.67, 18.07, 23.15, 23.18, 23.27, 25.57, 26.48, 27.07, 27.73, 30.42, 32.26, 32.66 (2C), 33.85, 36.74, 38.35, 38.51, 39.18, 40.44, 40.80, 41.47, 41.69, 46.23, 46.31, 47.35, 55.01, 78.43, 122.79, 144.00, 179.73. Electrospray ionization (ESI)-HRMS (m/z) [M + H]+ Calcd for C32H55N2O2, 499.4258. Found, 499.4257.
Compound 2This compound was prepared from OA-OBt (0.2 mmol) and 1,3-propanediamine (2 mmol) according to the general procedure A. The residue was purified by column chromatography (dichloromethane/methanol, 15 : 1 v/v). Yield: 79 mg, 77%; white solid. Mp 187.6–188.4°C. 1H-NMR (400 MHz, CD3OH) δ: 0.76, 0.78, 0.91, 0.98, 1.17 (7 × CH3), 0.76–2.00 (m, other aliphatic ring protons), 2.56 (d, J = 12.4 Hz, 1H), 2.69 (t, J = 6.56 Hz, 2H), 3.04–3.10 (m, 1H), 3.17–3.21 (m, 1H), 3.37–3.44 (m, 1H), 5.33 (s, 1H), 5.38 (s, 1H), 7.38 (s, 1H); 13C-NMR (100 MHz, CD3OH) δ: 15.90, 16.32, 17.93, 19.48, 23.98, 24.03, 24.56, 26.49, 27.86, 28.54, 28.76, 31.62, 33.10, 33.56, 33.88, 34.50, 35.12, 37.91, 38.15, 39.81, 39.84, 40.68, 42.55, 42.93, 47.55, 47.67, 56.71, 79.66, 124.03, 145.27, 180.48. ESI-HRMS (m/z) [M + H]+ Calcd for C33H57N2O2, 513.4415. Found, 513.4413.
Compound 3This compound was prepared from OA-OBt (0.2 mmol) and 1,4-diaminobutane (2 mmol) according to the general procedure A. The residue was purified by column chromatography (dichloromethane/methanol, 15 : 1 v/v). Yield: 80 mg, 76%; white solid. Mp 179.3–180.2°C. 1H-NMR (400 MHz, (CD3)2SO) δ: 0.66, 0.84, 0.86, 0.87, 0.88, 1.07 (7 × CH3), 0.66–2.00 (m, other aliphatic ring protons), 2.77 (d, J = 13.8 Hz, 1H), 2.89–3.05 (m, 6H), 5.20 (s, 1H), 7.24 (t, J = 5.4 Hz, 1H), 8.31 (s, 1H); 13C-NMR (100 MHz, (CD3)2SO) δ: 15.08, 16.00, 16.81, 18.01, 22.27, 22.92, 23.56, 25.66, 26.59, 26.95(2C), 28.22, 30.41, 30.54, 32.45, 32.74, 32.95, 33.66, 36.57, 38.08, 38.36, 38.74, 40.45, 41.23, 41.27, 45.15, 46.04, 47.12, 54.83, 76.80, 79.18, 121.34, 144.12, 176.01. ESI-HRMS (m/z) [M + H]+ Calcd for C34H59N2O2, 527.4571. Found, 527.4572.
Compound 4This compound was prepared from OA-OBt (0.2 mmol) and 1,5-pentanediamine (2 mmol) according to the general procedure A. The residue was purified by column chromatography (dichloromethane/methanol, 15 : 1 v/v). Yield: 94 mg, 86%; white solid. Mp 142.3–143.2°C. 1H-NMR (400 MHz, (CD3)2SO) δ: 0.65, 0.66, 0.83, 0.85, 0.87, 0.88, 1.07 (7 × CH3), 0.65–2.00 (m, other aliphatic ring protons), 2.54–2.56 (m, 2H), 2.77 (d, J = 9.54 Hz, 1H), 2.87–3.05 (m, 4H), 5.20 (t, J = 4.92 Hz, 1H), 7.24 (t, J = 5.64 Hz, 1H); 13C-NMR (100 MHz, (CD3)2SO) δ: 15.15, 16.10, 16.90, 18.06, 22.30, 22.99, 23.64, 23.92, 25.72, 27.00, 28.30, 28.96, 30.49, 31.21, 32.53, 32.82, 33.02, 33.72, 36.63, 38.13, 38.44, 38.84, 38.96, 40.06, 40.50, 40.80, 41.30, 45.25, 46.11, 47.17, 54.88, 76.89, 121.40, 144.20, 176.18. ESI-HRMS (m/z) [M + H]+ Calcd for C35H61N2O2, 541.4728. Found, 541.4728.
Compound 5This compound was prepared from OA-OBt (0.2 mmol) and 1,6-hexylenediamime (2 mmol) according to the general procedure A. The residue was purified by column chromatography (dichloromethane/methanol, 15 : 1 v/v). Yield: 86 mg, 77%; white solid. Mp 138.9–139.5°C. 1H-NMR (400 MHz, (CD3)2SO) δ: 0.67, 0.85, 0.87, 0.88, 0.89, 1.09 (7 × CH3) 0.67–2.00 (m, other aliphatic ring protons), 2.6 (t, J = 6.8 Hz, 2H), 2.79 (d, J = 10.64 Hz, 1H), 2.91–3.07 (m, 4H), 5.21 (br s, 1H), 5.76 (s, 1H), 7.22 (t, J = 5.52 Hz, 1H); 13C-NMR (100 MHz, (CD3)2SO) δ: 15.07, 16.03, 16.85, 18.00, 22.27, 22.93, 23.58, 25.66, 26.02, 26.43, 26.95, 28.23, 29.09, 30.84, 32.47, 32.77, 32.95, 33.66, 38.07, 38.76, 39.78, 40.43(2C), 40.46, 46.06, 47.11, 54.81, 76.81, 121.32. ESI-HRMS (m/z) [M + H]+ Calcd for C36H63N2O2, 555.4884. Found, 555.4883.
Compound 6This compound was prepared from OA-OBt (0.2 mmol) and 2-aminoethanol (0.4 mmol) according to the general procedure A. The residue was purified by column chromatography (petroleum ether/EtOAc, 1 : 1 v/v). Yield: 76 mg, 76%; white solid. Mp 232.4–233.8°C. 1H-NMR (400 MHz, (CD3)2SO) δ: 0.67, 0.84, 0.86, 0.87, 0.89, 1.08 (7 × CH3), 0.67–2.00 (m, other aliphatic ring protons), 2.75 (d, J = 9.28 Hz, 1H), 2.943.02 (m, 2H), 3.12–3.40 (m, 1H), 3.33–3.36 (m, 2H), 4.27 (d, J = 5.16 Hz, 1H), 4.59 (t, J = 5.32 Hz, 1H), 5.21 (br s, 1H), 7.15 (t, J = 5.56 Hz, 1H); 13C-NMR (100 MHz, (CD3)2SO) δ: 15.08, 16.01, 16.72, 17.97, 22.34, 22.91, 23.51, 25.64, 26.93(3C), 28.21, 30.41, 32.36, 32.68, 32.89, 33.61, 36.55, 38.36, 40.54, 41.23, 41.54(2C), 45.24, 46.02, 47.09, 54.78, 59.84, 76.80, 121.48, 144.00, 176.50. ESI-HRMS (m/z) [M + Na]+ Calcd for C32H53NO3Na, 522.3918. Found, 522.3919.
Compound 7This compound was prepared from OA-OBt (0.2 mmol) and 3-aminopropanol (0.4 mmol) according to the general procedure A. The residue was purified by column chromatography (petroleum ether/EtOAc, 1 : 1 v/v). Yield: 80 mg, 77%; white solid. Mp 210.9–211.3°C. 1H-NMR (400 MHz, (CD3)2SO) δ: 0.67, 0.84, 0.86, 0.88, 0.89, 1.08 (7 × CH3), 0.67–2.00 (m, other aliphatic ring protons), 2.76 (d, J = 9.84 Hz, 1H), 2.96–3.04 (m, 2H), 3.07–3.16 (m, 1H), 3.38 (q, J = 5.44 Hz, 2H), 4.27 (d, J = 5.12 Hz, 1H), 4.41 (t, J = 5.24 Hz, 1H), 5.21 (br s, 1H), 7.22 (t, J = 5.56 Hz, 1H); 13C-NMR (100 MHz, (CD3)2SO) δ: 15.06, 15.99, 16.79, 17.97, 22.27, 22.90, 23.51, 25.63, 26.93, 28.20, 30.40, 32.25, 32.40, 32.77, 32.89, 33.63, 36.32, 36.55, 38.05, 38.35, 40.06, 40.46(2C), 41.21, 45.17, 46.04, 47.08, 54.78, 58.84, 76.80, 121.43, 144.01, 176.21. ESI-HRMS (m/z) [M + Na]+ Calcd for C33H55NO3Na, 536.4074. Found, 536.4074.
Compound 8This compound was prepared from OA-OBt (0.2 mmol) and 4-amino-1-butanol (0.4 mmol) according to the general procedure A. The residue was purified by column chromatography (petroleum ether/EtOAc, 1 : 1 v/v). Yield: 86 mg, 81%; white solid. Mp 205.7–206.9°C. 1H-NMR (400 MHz, (CD3)2SO) δ: 0.66, 0.67, 0.84, 0.86, 0.88, 0.89, 1.08 (7 × CH3), 0.66–2.00 (m, other aliphatic ring protons), 2.78 (d, J = 9.68 Hz, 1H), 2.90–3.08 (m, 3H), 3.38 (q, J = 6 Hz, 2H), 4.28 (d, J = 5.16 Hz, 1H), 4.37 (t, J = 4.96 Hz, 1H), 5.20 (br s, 1H), 7.21 (t, J = 4.96 Hz, 1H); 13C-NMR (100 MHz, (CD3)2SO) δ: 15.08, 16.00, 16.80, 18.00, 22.25, 22.91, 23.56, 25.66, 25.78, 26.95, 28.22, 30.07, 30.42, 32.45, 32.75, 32.94, 33.66, 36.57, 38.07, 38.36, 38.62, 38.74, 40.44, 41.23, 45.14, 45.16, 46.03, 47.11, 54.81, 60.54, 76.82, 121.34, 144.11, 176.00. ESI-HRMS (m/z) [M + Na]+ Calcd for C34H57NO3Na, 550.4231. Found, 550.4231.
Compound 9This compound was prepared from OA-OBt (0.2 mmol) and 5-amino-1-pentanol (0.4 mmol) according to the general procedure A. The residue was purified by column chromatography (petroleum ether/EtOAc, 1 : 1 v/v). Yield: 74 mg, 68%; white solid. Mp 195.9–200.9°C. 1H-NMR (400 MHz, (CD3)2SO) δ: 0.69, 0.84, 0.86, 0.88, 0.89, 1.08 (7 × CH3), 0.67–2.00 (m, other aliphatic ring protons), 2.78 (d, J = 10.2 Hz, 1H), 2.93–3.03 (m, 3H), 3.17 (d, J = 5.36 Hz, 1H), 3.31–3.38 (m, 3H), 4.28 (d, J = 3.96 Hz, 1H), 4.33 (t, J = 5.04 Hz, 1H), 5.20 (s, 1H), 7.19 (t, J = 5.24 Hz, 1H); 13C-NMR (100 MHz, (CD3)2SO) δ: 15.07, 16.01, 16.82, 18.01, 22.26, 22.92, 23.10, 23.56, 25.66, 26.95, 28.23, 29.01, 30.42, 32.25, 32.30, 32.47, 32.75, 32.95, 33.67, 36.57, 38.08, 38.37, 40.46, 41.24, 45.16, 46.05, 47.12, 54.83, 60.58, 60.70, 76.70, 76.82, 121.34, 144.12, 176.00. ESI-HRMS (m/z) [M + Na]+ Calcd for C35H59NO3Na, 564.4387. Found, 564.4385.
Compound 10This compound was prepared from OA-OBt (0.2 mmol) and 6-amino-1-hexanol (0.4 mmol) according to the general procedure A. The residue was purified by column chromatography (petroleum ether/EtOAc, 1 : 1 v/v). Yield: 90 mg, 80%; white solid. Mp 183.8–184.5°C. 1H-NMR (400 MHz, (CD3)2SO) δ: 0.66, 0.67, 0.84, 0.86, 0.88, 0.89, 1.08 (7 × CH3), 0.66–2.00 (m, other aliphatic ring protons), 2.78 (dd, J = 3.96, 13.38 Hz, 1H), 2.92–3.04 (m, 3H), 3.36 (dd, J = 6.6, 11.76 Hz, 2H), 4.29 (d, J = 5.22 Hz, 1H), 4.33 (t, J = 5.16 Hz, 1H), 5.20 (br s, 1H), 7.20 (t, J = 5.52 Hz, 1H); 13C-NMR (100 MHz, (CD3)2SO) δ: 15.07, 16.04, 16.85, 18.00, 22.26, 22.94, 23.59, 25.38, 25.67, 26.62, 26.96, 28.24, 29.20, 30.45, 32.47, 32.63, 32.77, 32.97, 33.67, 36.58, 38.08, 38.39, 38.83, 38.91, 40.06, 40.46, 41.25, 45.19, 46.06, 47.12, 54.83, 60.69, 76.83, 121.33, 144.18, 176.02. ESI-HRMS (m/z) [M + Na]+ Calcd for C36H61NO3Na, 578.4544. Found, 578.4545.
Compound 11This compound was prepared from OA-OBt (0.2 mmol) and glycine methyl ester hydrochloride (0.8 mmol) according to the general procedure A. The residue was purified by column chromatography (petroleum ether/EtOAc, 3 : 1 v/v). Yield: 94 mg, 89%; white solid. Mp 137.8–138.6°C. 1H-NMR (600 MHz, (CD3)2SO) δ: 0.64, 0.67, 0.84, 0.87, 0.88, 0.89, 1.08 (7 × CH3), 0.64–2.00 (m, other aliphatic ring protons), 2.76(d, J = 9.6 Hz, 1H), 2.97–3.00 (m, 1H), 3.59 (s, 3H), 3.64 (dd, J = 5.52 Hz, 17.04 Hz, 1H), 3.80 (dd, J = 6 Hz, 16.98 Hz, 1H), 4.29 (d, J = 5.16 Hz, 1H), 5.17 (t, J = 3.36 Hz, 1H), 7.76 (t, J = 5.7 Hz, 1H); 13C-NMR (150 MHz, (CD3)2SO) δ: 15.13, 16.05, 16.60, 18.02, 22.40, 22.94, 23.50, 25.64, 26.84, 26.97, 28.24, 30.42, 32.42, 32.50, 32.90, 33.61, 36.59, 38.09, 38.40, 38.86, 40.35, 40.93, 41.24, 45.19, 46.05, 47.14, 51.51, 54.83, 76.83, 121.42, 143.95, 170.54, 176.94. ESI-HRMS (m/z) [M + Na]+ Calcd for C33H53NO4Na, 550.3867. Found, 550.3862.
Compound 12This compound was prepared from OA-OBt (0.2 mmol) and methyl 3-aminopropionate hydrochloride (0.8 mmol) according to the general procedure A. The residue was purified by column chromatography (petroleum ether/EtOAc, 3 : 1 v/v). Yield: 90 mg, 83%; white solid. Mp 218.8–219.6°C. 1H-NMR (600 MHz, (CD3)2SO) δ: 0.65, 0.67, 0.84, 0.86, 0.87, 0.89, 1.07 (7 × CH3), 0.65–2.00 (m, other aliphatic ring protons), 2.40–2.43 (m, 2H), 2.74 (dd, J = 3.96, 13.38 Hz, 1H), 2.97–3.00 (m, 1H), 3.15–3.21 (m, 1H), 3.25–3.21 (m, 1H), 3.57 (s, 3H), 4.28 (d, J = 4.74 Hz, 1H), 5.19 (t, J = 3.42 Hz, 1H), 7.35 (t, J = 5.58 Hz, 1H); 13C-NMR (150 MHz, (CD3)2SO) δ: 15.10, 16.03, 16.77, 17.99, 22.27, 22.92, 23.52, 25.65, 26.86, 26.96, 28.23, 30.42, 32.40, 32.61, 32.92, 33.57, 33.61, 35.02, 36.57, 38.08, 38.38, 38.89, 40.43, 41.22, 45.22, 46.01, 47.10, 51.26, 54.81, 76.82, 121.48, 143.93, 171.93, 176.47. ESI-HRMS (m/z) [M + Na]+ Calcd for C34H55NO4Na, 564.4023. Found, 564.4025.
Compound 13This compound was prepared from OA-OBt (0.2 mmol) and methyl 4-aminobutyrate hydrochloride (0.8 mmol) according to the general procedure A. The residue was purified by column chromatography (petroleum ether/EtOAc, 3 : 1 v/v). Yield: 87 mg, 78%; white solid. Mp 190.4–191.7°C. 1H-NMR (400 MHz (CD3)2SO) δ: 0.54, 0.64, 0.67, 0.84, 0.86, 0.89, 1.08 (7 × CH3), 0.54–1.97 (m, other aliphatic ring protons), 2.27 (t, J = 7.48 Hz, 2H), 2.78 (d, J = 10.00 Hz, 1H), 2.97–3.04 (m, 3H), 3.58 (s, 3H), 4.28 (d, J = 5.08 Hz, 1H), 5.21 (s, 1H), 7.31 (t, J = 5.72 Hz, 1H); 13C-NMR (100 MHz, (CD3)2SO) δ: 15.12, 16.08, 16.86, 18.08, 22.27, 22.98, 23.66 (2C), 24.47, 25.77, 27.03 (2C), 28.30, 30.51, 30.93, 32.53, 32.87, 33.03, 33.71 (2C), 36.64, 38.16, 38.20, 38.45, 40.52, 41.30, 45.32, 46.07, 47.19, 51.28, 54.90, 76.90, 121.47, 144.16, 173.22, 176.33. ESI-HRMS (m/z) [M + Na]+ Calcd for C35H57NO4Na, 578.4180. Found, 578.4183.
Compound 14This compound was prepared from OA-OBt (0.2 mmol) and methyl 5-aminopentanoate hydrochloride (0.8 mmol) according to the general procedure A. The residue was purified by column chromatography (petroleum ether/EtOAc, 3 : 1 v/v). Yield: 77 mg, 67%; white solid. Mp 178.8–179.6°C. 1H-NMR (400 MHz, (CD3)2SO) δ: 0.65, 0.67, 0.84, 0.86, 0.88, 0.89, 1.08 (7 × CH3), 0.65–2.00 (m, other aliphatic ring protons), 2.27 (t, J = 7.24 Hz, 2H), 2.76 (d, J = 9.84 Hz, 1H), 2.93–3.05 (m, 3H), 3.57 (s, 3H), 4.26 (d, J = 5.16 Hz, 1H), 5.20 (s, 1H), 7.24 (t, J = 5.52 Hz, 1H); 13C-NMR (100 MHz, (CD3)2SO) δ: 15.01, 15.99, 16.79, 17.97, 22.01, 22.22, 22.87, 23.55, 25.64, 26.94, 28.21, 28.50, 30.42, 32.43, 32.77, 32.93, 33.02(2C), 33.63, 36.55, 38.06, 38.31, 38.35, 38.88, 40.44, 41.22, 45.19, 46.02, 47.10, 51.11, 54.79, 76.80, 121.36, 144.08, 173.21, 176.08. ESI-HRMS (m/z) [M + Na]+ Calcd for C36H59NO4Na, 592.4336. Found, 592.4333.
Compound 15This compound was prepared from OA-OBt (0.2 mmol) and methyl 6-aminocappoate hydrochloride (0.8 mmol) according to the general procedure A. The residue was purified by column chromatography (petroleum ether/EtOAc, 3 : 1 v/v). Yield: 95 mg, 81%; white solid. Mp 116.2–117.4°C. 1H-NMR (600 MHz (CD3)2SO) δ: 0.66, 0.67, 0.84, 0.86, 0.87, 0.89, 1.08 (7 × CH3), 0.66–2.00 (m, other aliphatic ring protons), 2.27 (t, J = 7.44 Hz, 2H), 2.77 (dd, J = 4.02, 13.44 Hz, 1H), 2.91–3.04 (m, 3H), 3.57 (s, 3H), 4.27 (d, J = 5.16 Hz, 1H), 5.20 (t, J = 3.42 Hz, 2H), 7.21 (t, J = 5.58 Hz, 2H). 13C-NMR (150 MHz, (CD3)2SO) δ: 15.06, 16.03, 16.85, 18.00, 22.26, 22.93, 23.59, 24.23, 25.67, 26.06, 26.96, 28.24, 28.77, 30.45, 32.46, 32.77, 32.97, 33.30, 33.66, 36.58, 38.08, 38.39, 38.60, 38.91, 40.06, 40.46, 41.25, 45.20, 46.05, 47.12, 51.17, 54.82, 76.83, 121.34, 144.17, 173.26, 176.07. ESI-HRMS (m/z) [M + Na]+ Calcd for C37H61NO4Na, 606.4493. Found, 606.4493.
Compound 16This compound was prepared from 11 (0.2 mmol) and sodium hydroxide (0.6 mmol) according to the general procedure B. Yield: 84 mg, 82%; white solid. Mp 176.0–177.8°C. 1H-NMR (600 MHz, (CD3)2SO) δ: 0.64, 0.67, 0.83, 0.87, 0.87, 0.89, 1.08 (7 × CH3), 0.64–2.00 (m, other aliphatic ring protons), 2.76 (dd, J = 3.84, 13.38 Hz, 1H), 2.97–3.00 (m, 1H), 3.56 (dd, J = 3.54, 17.28 Hz, 1H), 3.73 (dd, J = 6.0, 17.28 Hz, 1H), 4.28 (d, J = 4.44 Hz, 1H), 5.19 (t, J = 3.36 Hz, 1H), 7.57 (t, J = 5.58 Hz, 1H), 12.37 (s, 1H); 13C-NMR (150 MHz, (CD3)2SO) δ: 15.12, 16.05, 16.60, 18.00, 22.45, 22.95, 23.50, 25.65, 26.85, 26.97, 28.24, 30.42, 32.39, 32.48, 32.91, 33.62, 36.58, 38.10, 38.39, 38.88, 40.47, 40.90, 41.24, 45.14, 46.05, 47.15, 54.82, 76.83, 121.48, 143.97, 171.48, 176.68. ESI-HRMS (m/z) [M + Na]+ Calcd for C32H51NO4Na, 536.3710. Found, 536.3713.
Compound 17This compound was prepared from 12 (0.2 mmol) and sodium hydroxide (0.6 mmol) according to the general procedure B. Yield: 90 mg, 85%; white solid. Mp >250°C. 1H-NMR (600 MHz (CD3)2SO) δ: 0.65, 0.67, 0.84, 0.86, 0.86, 0.88, 1.07 (7 × CH3), 0.65–2.00 (m, other aliphatic ring protons), 2.33 (t, J = 7.08 Hz, 2H), 2.72 (dd, J = 3.78, 13.80 Hz, 1H), 2.97–3.00 (m, 1H), 3.123.15 (m, 1H), 3.23–3.29 (m, 1H), 3.37 (s, 1H), 5.19 (br s, 1H), 7.30 (t, J = 5.58 Hz, 1H), 12.20 (s,1H); 13C-NMR (150 MHz, (CD3)2SO) δ: 15.16, 16.09, 16.83, 18.05, 22.33, 22.97, 23.55, 25.69, 26.93, 27.00, 28.28, 30.47, 32.43, 32.66, 32.96, 33.64, 33.82, 35.02, 36.61, 38.13, 38.43, 38.95, 40.56, 41.28, 45.23, 46.05, 47.15, 54.84, 76.87, 121.62, 143.93, 173.21, 176.44. ESI-HRMS (m/z) [M + Na]+ Calcd for C33H53NO4Na, 550.3867. Found, 550.3865.
Compound 18This compound was prepared from 13 (0.2 mmol) and sodium hydroxide (0.6 mmol) according to the general procedure B. Yield: 94 mg, 87%; white solid. Mp 228.7–229.5°C. 1H-NMR (400 MHz, (CD3)2SO) δ: 0.65, 0.67, 0.84, 0.86, 0.89, 1.08 (7 × CH3), 0.65–2.00 (m, other aliphatic ring protons), 2.18 (t, J = 7.4 Hz, 2H), 2.78 (d, J = 9.68 Hz, 1H), 2.96–3.03 (m, 3H), 3.32 (d, J = 8.92 Hz, 2H), 4.28 (d, J = 5.08 Hz, 1H), 5.21 (brs, 1H), 7.29 (t, J = 5.44 Hz, 1H), 12.0 (s, 1H); 13C-NMR (100 MHz, (CD3)2SO) δ: 15.16, 16.10, 16.92, 18.08, 22.27, 22.99, 23.67, 24.53, 25.76, 27.04, 28.31, 30.52, 31.29, 32.53, 32.87, 33.03, 33.71, 36.65, 38.23, 38.43, 38.46, 40.50, 41.31, 45.29, 45.30, 46.09, 47.19, 54.89, 76.79, 76.90, 121.48, 144.17, 174.36, 176.24. ESI-HRMS (m/z) [M + Na]+ Calcd for C34H55NO4Na, 564.4023. Found, 564.4024.
Compound 19This compound was prepared from 14 (0.2 mmol) and sodium hydroxide (0.6 mmol) according to the general procedure B. Yield: 79 mg, 71%; white solid. Mp 139.1–140.9°C. 1H-NMR (600 MHz (CD3)2SO) δ: 0.65, 0.66, 0.83, 0.86, 0.87, 0.88, 1.07 (7 × CH3), 0.65–2.00 (m, other aliphatic ring protons), 2.17 (t, J = 7.26 Hz, 2H), 2.78 (dd, J = 4.08, 13.56 Hz, 1H), 2.94–3.02 (m, 3H), 3.41 (s, 1H), 5.20 (br s, 1H), 7.26 (t, J = 5.52 Hz, 1H), 11.98 (s, 1H); 13C-NMR (150 MHz, (CD3)2SO) δ: 15.16, 16.09, 16.83, 18.05, 22.33, 22.97, 23.55, 25.69, 26.93, 27.00, 28.28, 30.47, 32.43, 32.66, 32.96 (2C), 33.64, 33.82, 35.02, 36.61, 38.13, 38.43, 38.95, 40.06, 40.56, 41.28, 45.23, 46.05, 47.15, 54.84, 76.87, 121.62, 143.93, 173.21, 176.44. ESI-HRMS (m/z) [M + Na]+ Calcd for C35H57NO4Na, 578.4180. Found, 578.4180.
Compound 20This compound was prepared from 15 (0.2 mmol) and sodium hydroxide (0.6 mmol) according to the general procedure B. Yield: 82 mg, 72%; white solid. Mp 119.8–120.7°C. 1H-NMR (600 MHz, (CD3)2SO) δ: 0.66, 0.67, 0.84, 0.86, 0.87, 0.88, 1.08 (7 × CH3), 0.66–2.00 (m, other aliphatic ring protons), 2.17 (t, J = 7.38 Hz, 2H), 2.78 (dd, J = 3.9, 13.32 Hz, 1H), 2.91–3.04 (m, 3H), 5.20 (brs, 1H), 7.22 (t, J = 5.58 Hz, 2H), 11.98 (s, 1H); 13C-NMR (150 MHz, (CD3)2SO) δ: 15.08, 16.05, 16.86, 18.01, 22.27, 22.94, 23.60, 24.30, 25.68, 26.17, 26.97, 28.25, 28.86, 30.46, 32.47, 32.77, 32.98, 33.67(2C), 36.59, 38.08, 38.39, 38.66, 38.91, 40.05, 40.47, 41.26, 45.20, 46.06, 47.13, 54.83, 76.84, 121.35, 144.16, 174.40, 176.06. ESI-HRMS (m/z) [M + Na]+ Calcd for C36H59NO4Na, 592.4346. Found, 592.4344.
BioassaysCell Activity AssayMDCK cells were seeded into 96-well plates in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) cultured overnight at 37°C in 5% CO2. Then, cells were suspended in DMEM supplemented with 1% FBS, containing test compound and 2 µg/mL TPCK-treated trypsin, and the cells were further incubated at 37°C in 5% CO2 for 40 h. Cell viability was assessed using the CellTiter-Glo assay kit (Promega Corp., Madison, WI, U.S.A.) as recommended by the supplier, and the plates were read using a plate reader (Molecular Devices SpectraMax M2). Viability was calculated using the background corrected absorbance as follows: Viability (%) = (CA of experiment well/DA of control well) × 100, where CA, DA represent the reading values of test compound and DMSO, respectively.
Cytopathic Effect (CPE) Reduction AssayMDCK cells were seeded into 96-well plates, incubated overnight and infected with influenza virus (MOI = 0.1) suspended in DMEM supplemented with 1% FBS, containing test compound and 2 µg/mL TPCK-treated trypsin, with a final DMSO concentration of 1% in each well. After 40 h of incubation, CellTiter-Glo reagent was added and the plates were read using a plate reader (Molecular Devices SpectraMax M2).
Hemagglutination Inhibition (HI) AssayCompound from a 2-fold serial dilution in saline was mixed with an equal volume of influenza virus (The HA titers of A/WSN/33 (H1N1) virus is 1 : 2) in the V-bottomed 96-well microplate. Subsequently, 50 µL of freshly prepared chicken RBCs (1% v/v in saline) were added to each well. The mixture was incubated for 30 min at RT before observing RBCs aggregation on the plate.
SPRInteractions between the influenza HA and the compounds were analyzed using the Biacore T200 system (GE Healthcare, Uppsala, Sweden) at 25°C. Recombinant influenza HA (Sino Biological Inc., Beijing, China) was immobilized on a sensor chip (CM5) using an amine coupling kit (GE Healthcare, Buckinghamshire, U.K.). Final HA-immobilized levels were typically approx. 16000 RU. Subsequently, compounds were injected as analytes at various concentrations, and PBS-P (10 mM phosphate buffer with 2.7 mM KCl and 137 mM NaCl, 0.05% surfactant P20, pH 4.5) was used as running buffer. For binding studies, analytes were applied at corresponding concentrations in running buffer at a flow rate of 30 mL/min with a contact time of 60s and a dissociation time of 60s. Chip platforms were washed with running buffer and 50% DMSO. Data were analyzed with the Biacore evaluation software (T200 version 1.0) by curve fitting using a binding model of 1 : 1.
This work was supported by the National Natural Science foundation of China (Grant No. 81560560) and the Scientific Research foundation of Kunming University of Science and Technology (No. KKSY20169517).
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.