2019 Volume 67 Issue 12 Pages 1337-1346
2019 Volume 67 Issue 12 Pages 1337-1346
The 1-BuOH-soluble fraction of the methanol (MeOH) extract of Diospyros maritima was separated by chromatographic techniques to give three new oleanane-type and one new ursane-type triterpene glucoside, named ebenamariosides A–D (1–4); two megastigmanes were also isolated. The structures of triterpene glucosides was elucidated with extensive investigation by one and two dimensional NMR spectroscopy and the structures were confirmed by partial enzymatic hydrolyses to give the corresponding mono-glucosides and aglycones. The structures of the megastigmanes, including their absolute stereochemistries, were elucidated by spectroscopic evidence and by the modified Mosher’s method. Two megastigmanes were chemically correlated and their absolute structures were unambiguously determined. The cytotoxicity of the triterpene glucosides and their degradation products were assayed. They did not show any significant activity.
Diospyros maritima Blume (Ebenaceae) is a tall evergreen tree with a height of about 10 m, distributed in Okinawa, Taiwan, Malaysia, Micronesia and Australia.1) In summer, it bears green sap fruits of 2 to 3 cm in diameter, which then turn to a dark orange colour in autumn. It is known that the fruits contain a toxic naphthoquinone derivative, plumbagin, and their constituents have been extensively investigated by Higa et al.2–4) Recently, from the leaves and branches of a related Thai medicinal plant, D. mollis, the isolation of naphthoquinone glycosides was reported.5) In our continuing work on Okinawan resource plants, the constituents of the leaves of D. maritima were investigated to give eight ent-kaurane-type diterpenoid glycosides, called diosmariosides A–H.6) Further extensive work resulted in the isolation of four new triterpene saponins, named ebenamariosides A–D (1–4) and two megastigmanes (5, 6), along with two known flavonol glycosides, kaempferol 3-O-β-D-(2″,6″-di-O-α-L-rhamonopyranosyl)glucopyranoside (7)7) and 3-O-β-D-(2″,6″-di-α-L-rhamonopyranosyl)galactopyranoside (8)8) (Fig. 1). The cytotoxicity of the triterpenoids was assayed using the human lung adenocarcinoma cell line A549 and the parasitic protozoan Leishmania major.

The leaves of D. maritima extracted with MeOH and the MeOH extracts were separated by solvent partition according to the polarity of the constituents. The relatively polar 1-BuOH-soluble fraction was separated by various kinds of chromatography to afford four new triterpene saponins (1–4) and two new megastigmanes (5, 6), together with two known flavonol glycosides (7, 8). The structures of new triterpene derivatives were elucidated using one- and two-dimensional spectroscopies. The structures of megastigmanes were elucidated by NMR and circular dichroism (CD) spectroscopies, and as well as by the modified Mosher method. The biological activity of four new triterpene saponins and their derivatives was assayed against the human lung adenocarcinoma cell line A549 and the parasitic protozoan Leishmania major.
Ebenamarioside A (1), [α]D25 +15.1, was isolated as a colorless amorphous powder and its molecular formula was determined to be C42H68O14 by observation of a quasi-molecular ion peak ([M + Na]+) in the high-resolution (HR) electrospray-ionisation (ESI) mass spectrometry. The IR spectrum showed strong absorption bands assignable to hydroxy (3439 cm−1) and ester carbonyl (1745 cm−1) functional groups. In the 1H-NMR spectrum, signals assignable to six singlet methyls, oxymethylene protons (δH 3.40 and 3.91), oxymethine proton (δH 3.45), olefinic proton (δH 5.43) and two anomeric protons [δH 6.35 (d, J = 8.3 Hz) and 4.85 (d, J = 7.8 Hz)] were observed (Table 1). Since HPLC analysis of the hydrolysate of 1 revealed the presence of D-glucose as a sole sugar component, two D-glucose molecules were expected to be present in 1. In the 13C-NMR spectrum, other than 12 signals assignable to those of glucopyranose units, 30 signals observed comprised of six methyls, eleven methylenes including one oxymethylene, four methines with an oxygenated one, six quaternary carbons, one trisubstituted double bond and a carboxyl functional group. From the above evidence and the degrees of unsaturation (Δ = 7), ebenamarioside A (1) was assumed to be an oleanolic acid derivative with a primary hydroxy group. In the heteronuclear multiple bond connectivity (HMBC) spectrum, geminal oxymethylene protons showed correlation with a methyl carbon (C-30, δC 19.7), methylene carbons, C-19 (δC 41.1) and 21 (δC 29.2) as well as a quaternary carbon at δC 35.5 (Fig. 2). The significant correlation in the phase sensitive-nulear Overhauser effect spectroscopy (PS-NOESY) spectrum between H-18 (δH 3.27) and H3-30 (δH 1.10) on C-30 enabled us to place the oxymethylene carbon at the C-29 position (Fig. 2). Similarly, the oxymethine proton was placed at the 3-position from diagnostic HMBC between H-3 and C-4, C-23 and C-24 (Fig. 2). From the axial (10.2 Hz) and equatorial (4.6 Hz) coupling constants of H-3, the hydroxy group at the 3-position was in a β equatorial orientation. The positions of the sugar linkages were established to be on the carboxyl group at the C-28 and the hydroxy group at C-29 from HMBC correlations H-1′ (δH 6.35) on δC 95.8 and C-28 (δC 176.4), and H-1″ (δH 4.85) on δC 105.5 and C-29 (δC 81.4), respectively. The mode of linkage was assigned to be β from the coupling constants of the anomeric protons. Therefore, the structure of ebenamarioside A (1) was elucidated to be 3β,29-dihydroxyolean-12-en-28-oic acid 28-O-β-D-glucopyranosyl ester 29-O-β-D-glucopyranoside, as shown in Fig. 1. Partial enzymatic hydrolysis of 1 using crude β-glucosidase liberated two monoglucosidic compounds (1a and 1b) and an aglycone (1c). The structure of compound 1a was elucidated to be mesembryanthenoidigenic acid 28-O-β-D-glucopyranosyl ester, isolated from Salicornia europaea,9) whereas that of 1b mesembryanthenoidigenic acid 29-O-β-D-glucopyranoide, whose isolation have not yet been reported. The aglycone (1c) was spectroscopically identified with mesembryanthenoidigenic acid, isolated from a South American cactus, Rhipsalis mesembryanthemoides.10,11)
| H | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1 | 0.99 ddd 12.7, 12.7, 4.1 | 1.04 m | 1.28 m | 1.25 m |
| 1.55 m | 1.57 m | 2.24 dd 11.9, 4.2 | 2.24 dd 12.3, 4.0 | |
| 2 | 1.85 2H m | 1.85 m | 4.10 ddd 11.9, 9.2, 4.2 | 4.10 ddd 12.3, 9.3, 4.0 |
| 3 | 3.45 dd 10.2, 4.6 | 3.47 dd 10.2, 5.7 | 3.39 d 9.2 | 3.38 d 9.3 |
| 5 | 0.85 br d 11.8 | 0.86 br d 12.0 | 1.00 m | 0.99 m |
| 6 | 1.37 m | 1.37 m | 1.40 m | 1.38 m |
| 1.55 m | 1.57 m | 1.54 m | 1.52 m | |
| 7 | 1.37 m | 1.32 m | 1.38 m | 1.38 m |
| 1.49 ddd 12.8, 12.8, 3.4 | 1.50 m | 1.49 m | 1.54 m | |
| 9 | 1.64 dd 11.1, 6.7 | 1.66 dd 8.9, 8.9 | 1.74 dd 10.2, 7.3 | 1.70 m |
| 11 | 1.95 2H m | 1.95 m | 1.95 m | 2.02 2H m |
| 2.17 m | 2.09 br dd 13.4, 10.2 | |||
| 12 | 5.43 dd 3.5 3.5 | 5.48 br s | 5.39 br s | 5.42 br s |
| 15 | 1.15 m | 1.19 m | 1.15 m | 1.12 m |
| 2.37 ddd 13.7, 13.7, 3.6 | 2.17 m | 2.35 ddd 13.4, 13.4. 3.5 | 2.43 ddd 13.5, 13.5, 4.7 | |
| 16 | 1.95 m | 1.98 2H m | 1.98 2H m | 1.93 m |
| 2.10 ddd 13.7, 13.7, 3.6 | 2.03 m | |||
| 18 | 3.27 dd 13.8, 4.2 | 3.36 dd 13.7, 3.8 | 3.25 dd 13.6, 3.6 | 2.54 d 11.3 |
| 19 | 1.43 dd 13.6, 4.2 | 1.50 m | 1.41 m | 1.70 m |
| 2.01 dd 13.8, 13.6 | 2.09 m | 2.00 m | — | |
| 20 | — | — | — | 1.26 m |
| 21 | 1.35 m | 1.43 m | 1.33 m | 1.56 m |
| 1.67 ddd 13.7, 13.7, 4.1 | 1.79 m | 1.65 ddd 13.9, 13.9, 3.6 | 1.93 m | |
| 22 | 1.82 m | 1.85 m | 1.79 br d 13.4 | 1.73 m |
| 1.87 m | 2.06 m | 1.87 ddd 13.9, 13.9, 3.6 | 1.96 m | |
| 23 | 1.23 3H s | 1.25 3H s | 1.25 3H s | 1.25 3H s |
| 24 | 1.04 3H s | 1.04 3H s | 1.08 3H s | 1.07 3H s |
| 25 | 0.93 3H s | 0.90 3H s | 1.02 3H s | 1.02 3H s |
| 26 | 1.14 3H, s | 1.03 3H s | 1.12 3H s | 1.15 3H s |
| 27 | 1.21 3H s | 1.27 3H s | 1.19 3H s | 1.12 3H s |
| 29 | 3.40 d 9.2 | 3.41 d 9.2 | 3.39 d 9.0 | 0.93 3H d 6.3 |
| 3.91 d 9.2 | 4.04 d 9.2 | 3.89 d 9.0 | ||
| 30 | 1.10 3H s | 1.22 3H s | 1.09 3H s | 3.84 dd 9.4, 3.1 |
| 4.00 m | ||||
| 1′ | 6.35 d 8.3 | 4.79 d 7.7 | 6.32 d 8.2 | 6.25 d 8.2 |
| 2′ | 4.22 dd 8.8, 8.3 | 4.02 dd 8.6, 7.7 | 4.20 dd 8.8, 8.2 | 4.19 dd 8.6, 8.2 |
| 3′ | 4.38 dd 9.0, 8.8 | 4.21 dd 8.9, 8.6 | 4.28 dd 8.9, 8.8 | 4.27 m |
| 4′ | 4.25 dd 9.2, 9.0 | 4.16 dd 9.2, 8.9 | 4.36 dd 9.1, 8.9 | 4.34 dd 9.3, 9.1 |
| 5′ | 3.99 ddd 9.2, 4.6, 2.3 | 4.21 m | 4.03 m | 4.00 m |
| 6′ | 4.43 dd 12.0, 4.6 | 4.37 dd 11.4, 5.8 | 4.42 dd 11.0, 5.4 | 4.38 dd 12.0, 4.3 |
| 4.48 dd 12.0, 2.3 | 4.88 br d 11.4 | 4.46 br d 11.0 | 4.44 dd 12.0, 2.1 | |
| 1″ | 4.85 d 7.8 | 5.16 d 7.9 | 4.83 d 7.7 | 4.85 d 7.7 |
| 2″ | 4.07 dd 8.2, 7.8 | 4.07 m | 4.05 m | 4.04 dd 8.2. 7.7 |
| 3″ | 4.30 dd 8.6, 8.2 | 4.25 m | 4.24 m | 4.27 m |
| 4″ | 4.42 dd 8.9, 8.6 | 4.26 m | 4.24 m | 4.23 dd 9.1, 8.9 |
| 5″ | 4.05 ddd 8.9, 5.4, 2.3 | 3.95 m | 3.98 m | 3.99 m |
| 6″ | 4.43 dd 11.8, 5.4 | 4.39 dd 11.9, 5.2 | 4.41 dd 11.4, 5.5 | 4.41 dd 12.1, 5.7 |
| 4.59 dd 11.8, 2.3 | 4.53 br d 11.9 | 4.58 br d 11.4 | 4.58 dd 12.1, 2.2 |

Ebenamarioside B (2), [α]D27 –5.6, was isolated as a colorless amorphous powder and its elemental composition was the same as that of 1. NMR spectra were similar to those of 1. Two anomeric protons and carbons (δH 4.79 on δC 105.5 and δH 5.16 on δC 105.9) were also observed in the 1H-, 13C- and heteronuclear single quantum correlation NMR spectra and D-glucose was a sole sugar unit. The distinct difference between 1 and 2 in the NMR signal was the carboxy carbons (C-28), such as δC 176.4 in 1 was shifted down to δC 180.2 in 2 and the anomeric carbon signal from an ester linkage (δC 95.8) appeared in 1 was not observed in 2. While, in the HMBC spectrum, one of the anomeric proton at δH 5.16 was correlated with C-6′ (δC 70.2) and then the other anomeric proton at δH 4.79 with C-29 (δC 81.4). Thus, the structure of 2 was 3β,20-dihydroxyolean-12-en-28-oic acid 29-O-β-D-(6′-O-β-D-glucopyranosyl)glucopyranoside, namely mesembryanthenoidigenic acid 29-O-β-gentiobioside, as shown in Fig. 1.
Ebenamarioside C (3), [α]D27 +14.0, was isolated as a colorless amorphous powder and its elemental composition wad determined to be C42H68O15, with one more oxygen atom than those of 1 and 2. NMR spectroscopic data for the C, D and E-rings were essentially the same as those of 1 and 2. In the 1H-NMR spectrum, two oxymethine protons (δH 3.39 on δC 83.8 and δH 4.10 on δC 68.6) as well as oxymethylene protons (δH 3.39 and 3.89) were observed (Table 1). The position of the oxymethine protons were placed at the vicinal positions from the 1H–1H correlation spectroscopy correlation (COSY) (Fig. 3) and from the evidence of HMBC correlations, namely, H3-23 (δH 1.25) and 24 (δH 1.08) and C-3 (δC 83.8) (Fig. 3). From the PS-NOESY correlations between H-2 (δH 4.10) and H3-25 (axial) (δH 1.02), H-3 (δH 3.39) and H3-23 (axial), and H3-23 and H-5 (axial) (δH 1.00) (Fig. 3), the hydroxy groups at the 2- and 3-positions were placed in equatorial positions, which were further confirmed by the large coupling constant of the vicinal protons (J = 9.2 Hz). Two oxymethines were found to be coupled each other from the 1H–1H-COSY spectroscopic evidence. The positions of the oxymethylene protons and sugar linkages were assigned by the similar manner used for ebenamarioside A (1). Therefore, the structure of 3 was elucidated to be 2α,3β,29-trihydroxyolean-12-en-28-oic acid 28-O-β-D-glucopyranosyl ester 29-O-β-D-glucopyranoside, as shown in Fig. 1. On enzymatic hydrolysis of 3 using crude naringinase, 28-O-β-D-glucopyranosyl ester (3a) was obtained. Glucoside 3a is a known compound, isolated from the stem bark of Terminalia superba (=3a′)12); however, its 13C-NMR data for MeOH-d4 were slightly different from those of 3 to confirm the structure (Table 2). 13C-NMR data of 3a for pyridine-d5 and dimethyl sulfoxide (DMSO)-d6 were also slightly different from those of 3a′ (Table 2). Furthermore, the optical rotation value reported for 3a′ was [α]D −18.1 (c 0.1, MeOH) which showed an opposite sign to that of 3a. In our report, the structure of 3a was carefully elucidated with a highly detailed survey of the one- and two-dimensional NMR spectra. On the other hand, enzymatic hydrolysis of 3 using crude β-glucosidase gave 2α,3β,29-trihydroxyolean-12-en-28-oic acid 29-O-β-D-glucopyranoside (3b) and an aglycone (3c). Glucoside 3b has not been isolated as a natural product and the aglycone (3c) was a known one, isolated from the pericarps of Akebia trifoliata.13)

| C | 1 | 1b | 2 | 3 | 3a | 3aa) | 3a′b) | 3ac) | 3b | 4 | 4a | 4b |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 39.0 | 39.0 | 39.0 | 47.8 | 47.9 | 48.2 | 47.0 | 46.8 | 47.8 | 48.1 | 48.1 | 48.0 |
| 2 | 28.1 | 28.1 | 28.1 | 68.6 | 68.6 | 69.5 | 66.8 | 67.0 | 68.6 | 68.6 | 68.6 | 68.6 |
| 3 | 78.1 | 78.1 | 78.5 | 83.8 | 83.8 | 84.5 | 81.9 | 82.1 | 83.8 | 83.8 | 83.8 | 83.8 |
| 4 | 39.4 | 39.4 | 39.4 | 40.0 | 40.0 | 40.4 | 39.1 | 38.9 | 39.9 | 39.8 | 39.8 | 39.9 |
| 5 | 55.8 | 55.8 | 55.8 | 55.9 | 55.9 | 56.7 | 55.1 | 54.7 | 55.9 | 55.9 | 55.9 | 55.9 |
| 6 | 18.8 | 18.8 | 18.8 | 18.8 | 18.9 | 19.6 | 18.2 | 17.9 | 18.9 | 18.8 | 18.9 | 18.8 |
| 7 | 33.2 | 33.3 | 33.3 | 33.1 | 33.1 | 32.4 | 32.2 | 32.1 | 33.2 | 33.5 | 33.5 | 33.5 |
| 8 | 40.0 | 39.8 | 39.8 | 39.8 | 39.9 | 40.5 | 39.0 | 38.8 | 39.8 | 40.2 | 40.3 | 40.0 |
| 9 | 48.1 | 48.1 | 48.1 | 48.1 | 48.2 | 49.1 | 47.4 | 47.0 | 48.1 | 48.1 | 48.1 | 48.1 |
| 10 | 37.4 | 37.4 | 37.4 | 38.5 | 38.6 | 39.3 | 36.9 | 37.5 | 38.5 | 38.4 | 38.5 | 38.5 |
| 11 | 23.8 | 23.8 | 23.8 | 23.5 | 23.5 | 24.0 | 23.5 | 22.4 | 23.8 | 23.8 | 23.9 | 23.7 |
| 12 | 123.0 | 122.7 | 122.6 | 122.9 | 122.8 | 123.7 | 122.7 | 121.4 | 122.5 | 126.4 | 126.2 | 125.9 |
| 13 | 144.1 | 144.8 | 144.8 | 144.0 | 144.4 | 145.0 | 143.8 | 143.5 | 144.8 | 138.1 | 138.5 | 139.0 |
| 14 | 42.1 | 42.2 | 42.2 | 42.1 | 42.2 | 42.9 | 41.5 | 41.2 | 42.2 | 42.5 | 42.6 | 42.5 |
| 15 | 28.3 | 28.4 | 28.3 | 28.2 | 28.3 | 28.9 | 28.0 | 27.1 | 28.3 | 28.6 | 28.7 | 28.6 |
| 16 | 23.5 | 23.8 | 23.8 | 23.9 | 24.0 | 24.7 | 23.2 | 22.9 | 23.9 | 24.7 | 24.7 | 25.0 |
| 17 | 47.4 | 47.1 | 47.1 | 47.2 | 47.5 | 48.3 | 46.4 | 46.2 | 47.0 | 48.2 | 48.4 | 47.9 |
| 18 | 41.0 | 41.2 | 41.2 | 40.9 | 41.2 | 41.9 | 42.2 | 39.9 | 41.2 | 53.3 | 53.4 | 53.6 |
| 19 | 41.1 | 41.3 | 41.2 | 41.0 | 41.0 | 41.4 | 41.7 | 39.9 | 41.3 | 34.4 | 33.7 | 34.6 |
| 20 | 35.5 | 35.8 | 35.7 | 35.5 | 36.4 | 36.9 | 35.3 | 35.3 | 35.7 | 44.6 | 47.2 | 45.0 |
| 21 | 29.2 | 29.4 | 39.3 | 29.2 | 28.9 | 29.3 | 31.8 | 27.8 | 29.4 | 25.6 | 25.5 | 25.9 |
| 22 | 31.8 | 32.5 | 32.5 | 31.7 | 32.1 | 33.9 | 33.7 | 30.9 | 32.5 | 36.5 | 36.8 | 37.2 |
| 23 | 28.8 | 28.8 | 28.8 | 29.3 | 29.3 | 29.3 | 28.8 | 28.7 | 29.3 | 29.4 | 29.4 | 29.4 |
| 24 | 16.6 | 16.6 | 16.6 | 17.6 | 17.7 | 17.8 | 17.3 | 17.0 | 17.7 | 17.7 | 17.8 | 17.7 |
| 25 | 15.7 | 15.6 | 15.6 | 16.9 | 17.0 | 17.2 | 16.0 | 16.3 | 16.9 | 17.1 | 17.1 | 17.0 |
| 26 | 17.6 | 17.5 | 17.5 | 17.5 | 17.6 | 17.7 | 16.9 | 16.6 | 17.5 | 17.7 | 17.7 | 17.5 |
| 27 | 26.1 | 26.2 | 26.2 | 26.0 | 26.1 | 26.3 | 25.7 | 25.5 | 26.2 | 23.7 | 23.8 | 23.9 |
| 28 | 176.4 | 180.2 | 180.2 | 176.4 | 176.5 | 178.0 | 175.9 | 175.1 | 180.3 | 176.2 | 176.3 | 179.9 |
| 29 | 81.4 | 81.6 | 81.4 | 81.3 | 73.7 | 74.3 | 74.7 | 72.1 | 81.6 | 17.1 | 17.1 | 17.3 |
| 30 | 19.7 | 19.8 | 19.8 | 19.7 | 19.7 | 19.6 | 19.9 | 19.0 | 19.8 | 73.2 | 65.0 | 73.5 |
| 1′ | 95.8 | 105.5 | 95.8 | 95.8 | 95.8 | 95.8 | 94.0 | 95.7 | 95.8 | |||
| 2′ | 74.2 | 75.2 | 74.1 | 74.2 | 74.0 | 74.1 | 72.3 | 74.0 | 74.1 | |||
| 3′ | 79.0 | 78.5 | 78.9 | 79.0 | 78.7 | 79.1 | 77.6 | 78.8 | 79.0 | |||
| 4′ | 71.1 | 71.7 | 71.1 | 71.2 | 71.1 | 71.3 | 69.4 | 71.2 | 71.2 | |||
| 5′ | 79.4 | 77.3 | 79.3 | 79.4 | 78.3 | 78.6 | 76.6 | 79.1 | 79.3 | |||
| 6′ | 62.2 | 70.2 | 62.2 | 62.2 | 62.4 | 62.2 | 60.6 | 62.3 | 62.3 | |||
| 1″ | 105.5 | 105.5 | 105.9 | 105.4 | 105.5 | 104.8 | 105.1 | |||||
| 2″ | 75.3 | 75.4 | 75.2 | 75.2 | 75.4 | 75.2 | 75.3 | |||||
| 3″ | 78.7 | 78.7 | 78.6 | 78.6 | 78.7 | 78.6 | 78.7 | |||||
| 4″ | 71.7 | 71.8 | 71.7 | 71.7 | 71.8 | 71.7 | 71.8 | |||||
| 5″ | 78.6 | 78.6 | 78.1 | 78.5 | 78.6 | 78.5 | 78.6 | |||||
| 6″ | 62.9 | 62.9 | 62.7 | 62.9 | 62.9 | 62.9 | 63.0 |
a) Data for CD3OD. b) Data were taken from ref. 12 (CD3OD). c) Data for DMSO-d6.
Ebenamarioside D (4), [α]D26 −5.6, was isolated as a colorless amorphous powder and its elemental composition was determined to be C42H68O15, which was the same as that of 3. The 1H-NMR spectrum showed resonances for five singlet methyls and one doublet methyl, two oxygenated methines, two anomeric protons and one olefinic proton along with two oxymethylene protons coupled in a geminal system, but also coupled with one more proton [δH 3.84 (dd, J = 9.4, 3.1 Hz) and 4.00 (m)]. Although six quaternary carbons were observed in the 13C-NMR spectra of aforementioned oleanane-type aglycones, only five quaternary ones were present in the molecule, and two more methine (C-19 and -20) and one less methylene carbons were observed in 4, when the functionality was compared with that of 3. 13C-NMR chemical shifts of the A and B rings were essentially the same as those of 3 and the presence of a double bond between C-12 and C-13 was also similar to aforementioned triterpene aglycones. The proton spin-spin coupling sequences from the doublet methyl signal (δH 0.93) to oxymethylene protons via two methine signals (δH 1.70 and 1.26) were observed in the 1H–1H COSY spectrum (Fig. 4). The HMBC correlations between the doublet methyl proton and C-18 (δC 53.3), C-19 (δC 34.4) and C-20 (δC 44.6), and the oxymethylene protons and C-19, C-20 and C-21 (δC 25.6) established the scaffold of the ring E to possess the ursane-type carbon frame work (Fig. 4). This was also supported by the significant PS-NOESY correlations H-18 (δH 2.54) and H3-29 (δH 0.93), and H-18 and H-20 (δH 1.26). The sugar linkages were assigned by the similar manner used for ebenamariosides A and C (1, 3). Therefore, the structure of ebenamarioside D (4) was elucidated to be 2α,3β,30-trihydroxyursan-12-en-28-oic acid 28-O-β-D-glucopyranosyl ester 30-O-β-D-glucopyranoside, as shown in Fig. 1. Enzymatic hydrolysis using crude β-glucosidase gave a mixture of monoglucosidic compounds (4a and 4b) and 2α,3β,30-trihydroxyurs-12-en-28-oic acid as an aglycone (4c), which is known as a microbial transformation product from corosolic acid by Streptomyces asparaginoviolaceus.14) The mixture of monoglucosidic compounds was separated by silica gel CC and HPLC to give 2α,3β,30-trihydroxyurs-12-en-28-oic acid 28-O-β-D-glucopyranosyl ester (4a) and 2α,3β,30-trihydroxyurs-12-en-28-oic acid 30-O-β-D-glucopyranoside (4b). These two monoglucosidic compounds were first described in this experiment.

Compound 5, [α]D26 +10.2, was isolated as a colorless powder and its elemental composition was determined to be C13H22O4. In the 1H-NMR spectrum, two singlet (δH 0.88 and 1.12) and one doublet (δH 1.28, J = 6.4 Hz) methyls, two olefinic protons [δH 6.03 (dd, J = 15.5, 4.5 Hz) and 6.06 (d, J = 15.5 Hz)] coupled in a trans geometry, two sets of methylene protons (δH 1.64 and 1.80, and 1.75 and 2.00), two oxygenated methylene protons (δH 3.68 and 3.77) and two oxygenated methine (δH 4.09 and 4.35) protons were found (Table 3). The 13C-NMR spectrum displayed 13 signals including three methyls, three methylenes, two methines with oxygen atoms, two olefinic carbons, two oxygenated tertiary and one quaternary carbon (Table 3). The number of carbons and degrees of unsaturation suggested that compound 5 was a megastigmane with a bicyclic scaffold. Two 1H–1H COSY correlations [–C(2)H2-C(3)HOH-C(4)H2– and –C(7)H = C(8)H-C(9)HOH-C(10)H3] and HMBC correlations between H2-11 (δH 3.68 and 3.77) and C-5 (δC 87.5) and other diagnostic correlations shown in Fig. 5a suggested 5 was 5,11-eopxy-3,6,9-trihydroxymegastigman-7-ene. The relative stereochemistry was established by the PS-NOESY spectrum. Correlations between H-7 (δH 6.06) and H-2ax (δH 1.64), H-4ax (δH 1.75), H3-12 (δH 0.88) and H3-13 (δH 1.12) suggested these substituents were in the same face and those between H-11b (δH 3.68) and H-2 eq (δH 1.80), H-3 (δH 4.09) and H-4 eq (δH 2.00) these were in the same face and the opposite face to the side chain (Fig. 5b). A related compound (9) which showed superimposable NMR spectra with those of 5 was isolated from Asclepias fruticosa and the absolute structure of 9 was determined by the modified Mosher’s method15) to have 1R,3S,5R,6S,9R configurations.16) Compound 5 was also subjected to the modified Mosher method and, as a result, 5 was found to have 1R,3S,5R,6S,9S configurations (Fig. 6). Therefore, 5 has the opposite configuration at the 9-position to that of 9 and thus it was found to be a new compound in nature.
| 5 | 6 | |||
|---|---|---|---|---|
| C | H | C | H | |
| 1 | 48.8 | — | 49.5 | — |
| 2 | 44.4 | 1.64 (ddd, 13.6, 10.5, 2.1) | 53.2 | 2.35 (dd, 18.1, 2.6) |
| 1.80 (ddd, 13.6, 7.2, 1.4) | 2.65 (dd, 18.1, 2.9) | |||
| 3 | 66.0 | 4.09 (dddd, 10.5, 10.5, 7.2, 7.2) | 211.4 | — |
| 4 | 45.8 | 1.75 (dd, 13.6, 10.5) | 53.9 | 2.43 (dd, 18.1, 2.6) |
| 2.00 (ddd, 13.6, 7.2, 1.4) | 2.78 (d, 18.1) | |||
| 5 | 87.5 | — | 87.5 | — |
| 6 | 82.6 | — | 82.4 | — |
| 7 | 127.0 | 6.06 (d, 15.5) | 125.7 | 6.02 (dd, 15.4, 1.5) |
| 8 | 139.6 | 6.03 (d, 15.5, 4.5) | 140.7 | 6.17 (dd, 15.4, 5.5) |
| 9 | 69.2 | 4.35 (qd, 6.4, 4.5) | 68.9 | 4.38 (dqd, 6.4, 5.5, 1.5) |
| 10 | 24.0 | 1.28 (d, 6.4) | 24.0 | 1.28 (3H, d, 6.4) |
| 11 | 16.3 | 0.88 (3H, s) | 19.2 | 1.18 (3H, s) |
| 12 | 77.1 | 3.68 (d, 7.4) | 78.4 | 3.65 (d, 7.5) |
| 3.77 (dd, 7.4, 2.1) | 3.91 (dd, 7.5, 2.9) | |||
| 13 | 19.5 | 1.12 (3H, s) | 19.2 | 1.18 (3H, s) |
Multiplicities and coupling constants in Hz are in the parentheses.

a) Significant long range 1H–1H correlations due to formation of W-figure were observed between H-2ax and H-12a and H-2 eq and H-4 eq

Figures are δ5b–δ5a in ppm.
Compound 6, [α]D25 −0.74, was isolated as a colorless syrup and its elemental composition was determined to be C13H20O4 which was two hydrogen fewer than that of 5. 13C-NMR spectrum also displayed 13 signals including three methyls, three methylenes, one oxygenated methine, two oxygenated tertiary carbons, one quaternary carbon, one ketone, instead of oxygenated methine and one disubstituted double bond, whose NMR chemical shifts for CDCl3 were almost superimposable to those of drummondol [6a, [α]D23 −21.0 (MeOH)] isolated from Sesbania drummondii by Powell and Smith, Jr.,17) whose geometry at the 9-position and the absolute configuration of bicyclo[3,2,1]octane region remains to be determined. Meanwhile, Çaliş et al. isolated drummondol 9-O-β-D-glucopyranoside from Capparis spinosa and the absolute configuration of the aglycone (6b) of the 9-position was determined to be 9S by the modified Mosher method.16,18) That of the ring region was discussed using the Cotton effects in the CD spectrum of 6b, compared with those of (+)-(S)-abscisic acid metabolites. Compound 6 showed similar Cotton effects [∆ε (nm): +0.64 (241), –0.30 (296)] to those of 6b18) and hence the absolute stereochemistries of 6 and 6b were expected to be the same at the 6-position. NaBH4 reduction of 6 gave two products (6c and 6d). Hydride was introduced from the less hindered 3si face to form the major compound 6d and the minor compound 6c was obtained by the reduction of 6 from the 3re face. The 1H-NMR signal of the H-3 proton of 6d was appeared as a doublet of a doublet, J = 5.7, 5.7 Hz, indicating that the α-hydroxy group formed at the 3-position was in the pseudo axial orientation due to steric hindrance toward the epoxide ring.19,20) Meanwhile, the NMR spectroscopic data of the minor one (6c) were identical with those of 5 and similarly 6c was subjected to the modified Mosher’s method to give (R)- and (S)-α-methoxy-α-trifluoromethylphenylacetic acid (MTPA) esters of 6c, which were the identical compounds with 5a and 5b from 5. Therefore, the absolute configurations of drummondol (6) isolated in this experiment was confirmed to be 1R,5R,6S,9S, which was the same as the aglycone of (9S)-drummondol 9-O-β-D-glucopyranoside from C. spinosa (Fig. 1) and 6 is expected to be a new compound as a non-glucosidic form; however, a direct correlation with the original drummondol (6a) was precluded.
The cytotoxic activity of isolated ebenamariosides (1–4), their derivatives (1a, 1b, 1c, 3a, 3b, 3c, 4a 4b and 4c), and compounds 5 and 6 was assayed using the human lung adenocarcinoma cell line A549. Compounds 1c and 4c showed slight activity with IC50 values of 174 ± 16 µM and 107 ± 8 µM, respectively, where that of the positive control etoposide was 23.3 ± 4.3 µM, while other compounds did not show any activity at 100 µg/mL. Unfortunately, none of compounds were active toward L. major at 100 µg/mL.
Closing RemarksFrom the leaves of Diospyros maritima, four triterpene saponins, named ebenamariosides A–D (1–4) and two megastigmanes (5, 6) were isolated. The structures of ebenamariosides were carefully elucidated by interpretation of one- and two-dimensional NMR spectroscopies and enzymatic hydrolysis of 1, 3 and 4 using crude β-glucosidase and naringinase gave corresponding monoglucosides and aglycones. The structures of megastigmanes were confirmed by the modified Mosher’s method and the Cotton effect in the CD spectrum. Assays of inhibitory activities for triterpene derivatives toward human lung adenocarcinoma cell line, A549 and Leishmania major did not show any significant activity.
Optical rotations were measured on a JASCO P-2200 digital polarimeter. IR spectra were measured on JASCO FT/IR-6100 spectrophotometers. 1H- and 13C-NMR spectra were taken on a Bruker Avance III at 600 MHz and 150 MHz, respectively, with tetramethylsilane as an internal standard. CD spectra were obtained with a JASCO J-720 spectropolarimeter. Positive and negative-ion HR-ESI-MS were performed with a Thermo Fisher Scientific LTQ Orbitrap XL. Silica gel column chromatography (CC) was performed on silica gel 60 (70–230 mesh) (E. Merck, Darmstadt, Germany) and reversed-phase octadecylsilanized (ODS) open CC on Cosmosil 75C18-OPN (Nacalai Tesque, Kyoto, Japan) (Φ = 50 mm, L = 25 cm). HPLC was performed on an ODS column [Inertsil ODS-3 (GL Science Inc., Tokyo, Japan; Φ = 10 mm, L = 25 cm, 4.0 mL/min), Cosmosil πNAP (Nacalai Tesque; Φ = 10 mm, L = 25 cm, 4.0 mL/min) and Cosmosil PBr (Nacalai Tesque; Φ = 10 mm, L = 25 cm, 4.0 mL/min)], and the eluate was monitored with photodiode arrtay (200–400 nm) and refractive index monitors. Crude β-glucosidase (Sumizyme BGA) was a generous gift from Shin Nihon Chemical Co., Ltd. (Anjo, Aichi, Japan) (Lot No. 0930708-03). Crude naringinase was from Amano Enzyme Inc. (Nagoya, Aichi, Japan) as a gift (Lot No. NAG1252306). MTPAs were purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan).
Plant MaterialLeaves of D. maritima were collected in Taketomi-cho, Yaeyama-gun, Okinawa, Japan, in November, 2003 and a voucher specimen was deposited in the Herbarium of Pharmaceutical Sciences, Graduate School of Biomedical and Health Sciences, Hiroshima University (03-DM-Okinawa-1105). The plant was identified by one of the authors (M.A.).
Extraction and IsolationAir-dried leaves of D. maritima (7.80 kg) were extracted with MeOH (45 L) three times. The MeOH extract was concentrated to 6 L and then washed with n-hexane (6 L, 245 g). The methanolic layer was concentrated to a viscous gum. The gummy mass was suspended in H2O (6 L), and then partitioned with ethyl acetate (EtOAc) (6 L) and 1-butanol (1-BuOH) (6 L), successively, to give 397 g and 216 g of EtOAc and 1-BuOH-soluble fractions. The remaining water-layer was concentrated to give a H2O-soluble fraction (245 g). The 1-BuOH-soluble fraction was subjected to a Diaion HP-20 CC (Φ = 80 mm, L = 50 cm), and eluted with H2O–MeOH (4 : 1, 5 L), (3 : 2, 5 L), (2 : 3, 5 L), and (1 : 4, 5 L), and MeOH (5 L), 1 L-fractions being collected.
The residue (17.5 g) in fractions 4–7 of a Diaion HP-20 CC was subjected to silica gel CC (Φ = 40 mm, L = 55 cm), and eluted with CHCl3 (3 L), CHCl3–MeOH (99 : 1, 3 L), (49 : 1, 3 L), (97 : 3, 3 L), (19 : 1, 3 L), (37 : 3, 3 L), (9 : 1, 3 L), (7 : 1, 3 L), (17 : 3, 3 L), (33 : 7, 3 L), (4 : 1, 3 L), (3 : 1, 3 L), and (7 : 3, 3 L), 500 mL-fractions being collected. Compounds 6 (266 mg) and 5 (437 mg) were obtained in fractions 18–19 and 23–26, respectively.
The residue (29.2 g) in fractions 11–14 of a Diaion HP-20 CC was subjected to silica gel CC (Φ = 50 mm, L = 54.5 cm), and eluted with CHCl3 (3 L), CHCl3–MeOH (99 : 1, 3 L), (49 : 1, 3 L), (97 : 3, 3 L), (19 : 1, 3 L), (37 : 3, 3 L), (9 : 1, 3 L), (7 : 1, 3 L), (17 : 3, 3 L), (33 : 7, 3 L), (4 : 1, 3 L), (3 : 1, 3 L), and (7 : 3, 3 L), 500 mL-fractions being collected. The residue (3.00 g out of 6.74 g) in fractions 57–65 of silica gel CC was separated by ODS CC (Φ = 50 mm, L = 25 cm), and eluted with a linear gradient solvent system from MeOH–H2O (1 : 9, 2 L) to MeOH–H2O (9 : 1, 2 L), 10 g-fractions being collected. The residue (160 mg) in fractions 223–234 was purified by HPLC (Inertsil ODS-3, H2O–MeOH, 1 : 1) to give 36.4 mg of 3 from the peak at 10.2 min. The residue (88.1 mg) in fractions 240–249 was purified by HPLC (Cosmosil πNAP, H2O–MeOH, 2 : 3) to give 32.8 mg of 4 from the peak at 10.2 min. The residue (2.81 g) in fractions 66–71 of silica gel CC was separated by ODS CC (Φ = 50 mm, L = 25 cm), and eluted with a linear gradient solvent system from MeOH–H2O (1 : 9, 2 L) to MeOH–H2O (9 : 1, 2 L), 10 g-fractions being collected. The residue (136 mg) in fractions 106–129 was purified by HPLC (Cosmosil PBr, H2O–MeOH, 3 : 2) to give 15.0 mg of 7 and 19.9 mg of 8 from the peaks at 25.0 min and 26.4 min, respectively.
The residue (64.5 g) in fractions 5–19 of a Diaion HP-20 CC was subjected to silica gel CC (Φ = 80 mm, L = 40 cm), and eluted with CHCl3 (6 L), CHCl3–MeOH (99 : 1, 6 L), (49 : 1, 6 L), (97 : 3, 6 L), (19 : 1, 6 L), (37 : 3, 6 L), (9 : 1, 6 L), (7 : 1, 6 L), (17 : 3, 6 L), (33 : 7, 6 L), (4 : 1, 6 L), (3 : 1, 6 L), and (7 : 3, 6 L), 1 L-fractions being collected. The residue (3.00 g out of 12.3 g) in fractions 53–62 was separated by ODS CC (Φ = 50 mm, L = 25 cm), and eluted with a linear gradient solvent system from MeOH–H2O (1 : 9, 2 L) to MeOH–H2O (9 : 1, 2 L), 10 g-fractions being collected. The residue (63.0 mg) in fractions 243–246 was purified by HPLC (Inertsil ODS-3, H2O–MeOH, 3 : 7) to give 10.8 mg of 1 from the peak at 5.9 min. The residue (196 mg) in fractions 247–263 was purified by HPLC (Inertsil ODS-3, H2O–MeOH–CH3COOH, 7 : 13 : 0.1) to give 3.8 mg of 2 from the peak at 18.4 min.
Ebenamarioside A (1)Colorless amorphous powder, [α]D25 +15.1 (c = 0.72, MeOH); IR νmax (film) cm−1: 3439, 2928, 2871, 1745, 1636, 1458, 1072; 1H-NMR (600 MHz, pyridine-d5): Table 1; 13C-NMR (150 MHz, pyridine-d5): Table 2; HR-ESI-MS (positive-ion mode): m/z: 819.4499 [M + Na]+ (Calcd for C42H68O14Na: 819.4501).
Ebenamarioside B (2)Colorless amorphous powder, [α]D27 −5.6 (c = 0.43, MeOH); IR νmax (film) cm−1: 3393, 2936, 2872, 1686, 1043; 1H-NMR (600 MHz, pyridine-d5): Table 1; 13C-NMR (150 MHz, pyridine-d5): Table 2; HR-ESI-MS (negative-ion mode): m/z: 795.4529 [M − H]− (Calcd for C42H67O14: 795.4525).
Ebenamarioside C (3)Colorless amorphous powder, [α]D27 +14.0 (c = 0.10, pyridine); IR νmax (film) cm−1: 3353, 2927, 2876, 1705, 1045; 1H-NMR (600 MHz, pyridine-d5): Table 1; 13C-NMR (150 MHz, pyridine-d5): Table 2; HR-ESI-MS (positive-ion mode): m/z: 835.4447 [M + Na]+ (Calcd for C42H68O15Na: 835.4450).
Ebenamarioside D (4)Colorless amorphous powder, [α]D26 −5.6 (c = 0.86, MeOH); IR νmax (film) cm−1: 3400, 2932, 2877, 1740, 1071; 1H-NMR (600 MHz, pyridine-d5): Table 1; 13C-NMR (150 MHz, pyridine-d5): Table 2; HR-ESI-MS (positive-ion mode): m/z: 835.4449 [M + Na]+ (Calcd C42H68O15Na: 835.4450).
Compound 5Colorless amorphous powder, [α]D26 +10.2 (c = 0.33, MeOH); IR νmax (film) cm−1: 3379, 2930, 2876, 1450, 1375, 1135, 1043; 1H-NMR (600 MHz, CD3OD): Table 3; 13C-NMR (150 MHz, CD3OD): Table 3; HR-ESI-MS (positive-ion mode): m/z: 265.1411 [M + Na]+ (Calcd C13H22O4Na: 265.1410).
Compound 6Colorless syrup, [α]D26 approx. 0.00 (c = 0.81, MeOH); IR νmax (film) cm−1: 3414, 2932, 2877, 1715, 1455, 1242, 1042; 1H-NMR (600 MHz, CD3OD): Table 3; (CDCl3) δ: 6.22 (1H, dd, J = 15.4, 5.4 Hz, H-8), 5.91 (1H, dd, J = 15.4, 1.2 Hz, H-7), 4.45 (1H, qd, J = 6.4, 5.4 Hz, H-9), 3.91 (1H, dd, J = 8.3, 3.0 Hz, H-12a), 3.75 (1H, d, J = 8.3 Hz, H-12b), 2.65 (1H, d, J = 18.3 Hz, H-4a), 2.60 (1H, dd, J = 18.3, 2.0 Hz. H-4b), 2.55 (1H, dd, J = 18.1, 3.0 Hz, H-2a), 2.42 (1H, dd, J = 18.1, 2.0 Hz, H-2b), 1.33 (3H, d, J = 6.4 Hz, H3-10), 1.21 (3H, s, H3-13); 0.99 (3H, s, H3-11); 13C-NMR (150 MHz, CD3OD): Table 3; (CDCl3) δ: 208.6 (C-3), 139.8 (C-8), 123.7 (C-7), 85.5 (C-5), 81.6 (C-6), 77.2 (C-12), 68.1 (C-9), 52.6 (C-4), 52.5 (C-2), 47.7 (C-1), 24.0 (C-10), 18.7 (C-13), 15.6 (C-11); CD (c = 6.46 × 10−5 M, MeOH) ∆ε (nm): +0.64 (241), −0.30 (296); HR-ESI-MS (positive-ion mode): m/z: 263.1254 [M + Na]+ (Calcd C13H20O4Na: 263.1254).
Sugar AnalysisAbout 500 µg each of 1–4 was hydrolyzed with 1 M HCl (0.1 mL) at 90°C for 2 h. The reaction mixtures were partitioned with an equal amount of EtOAc (0.1 mL), and the water layers were analyzed by HPLC with a chiral detector (JASCO OR-4090) on an amino column [InertSustain NH2, 4.6 × 250 mm (GL Science Inc.), CH3CN–H2O (4 : 1), flow rate: 1 mL/min]. All the hydrolyzates gave a peak for D-glucose at 10.9 min with positive optical rotation signs. The peaks were identified by co-chromatography with an authentic sample.
Enzymatic Hydrolysis of 1, 3 and 4 to 1a, 1b and 1c, 3a, 3b and 3c, and 4a, 4b and 4c, RespectivelyEbenamarioside A (1) (9.8 mg) in 1 mL of H2O hydrolyzed with 15 mg of crude glucosidase at 37°C for 72 h. The reaction mixture was subjected to silica gel CC (Φ = 2 cm, L = 15 cm) with increasing amounts of MeOH in CHCl3 [CHCl3–MeOH (9 : 1, 50 mL), (9 : 1, 100 mL to 7 : 3, 100 mL, linear gradient), (7 : 3, 50 mL) and (1 : 1, 100 mL)], 10-mL fractions being collected. An aglycone (1c) (2.8 mg) was obtained in fractions 5–6 and the monosaccharide mixture (1a and 1b) (5.5 mg) in fractions 9–12. The mixture fraction was purified by HPLC (ODS-3, H2O–MeOH, 4 : 1) to give 1.9 mg of 1a and 3.0 mg of 1b from the peaks at 4.8 min and 8.7 min, respectively. Mesembryanthenoidigenic acid 28-O-β-D-glucopyranosyl ester (1a): Amorphous powder, [α]D25 +31.4 (c = 0.09, MeOH), HR-ESI-MS (positive-ion mode) m/z: 657.3975 [M + Na]+ (Calcd for C36H58O9Na: 657.3973)9); mesembryanthenoidigenic acid 30-O-β-D-glucopyranoside (1b): Amorphous powder, [α]D25 +14.5 (c = 0.15, MeOH), IR νmax (film) cm−1: 3370, 2929, 2867, 1686, 1636, 1457, 1077; 1H-NMR (600 MHz, pyridine-d5) δ: 5.47 (1H, dd, J = 3.3, 3.3 Hz, H-12), 4.61 (1H, dd, J = 11.7, 2.1 Hz, H-6″a), 4.44 (1H, dd, J = 11.7, 5.3 Hz, H-6″b), 4.87 (1H, d, J = 7.7 Hz, H-1″), 4.28 (1H, dd, J = 8.7, 8.4 Hz, H-4″), 4.27 (1H, dd, J = 8.4, 8.3 Hz, H-3″), 4.10 (1H, dd, J = 8.3, 7.7 Hz, H-2″), 4.00 (1H, m, H-5″), 3.95 (1H, d, J = 9.3 Hz, H-29a), 3.46 (1H, dd, J = 10.2, 5.6 Hz, H-3), 3.44 (1H, d, J = 9.3 Hz, H-29b), 3.37 (1h, dd, J = 13.6, 3.9 Hz, H-18), 2.19 (1H, ddd, J = 13.2, 13.0, 3.6 Hz, H-15a), 2.12 (1H, ddd, J = 13.0, 13.0, 3.0 Hz, H-16a), 2.08 (1H, ddd, J = 14.0, 14.0, 4.0 Hz, H-22a), 2.04 (1H, dd, J = 13.9, 13.8 Hz, H-19a), 1.93 (3H, m, H2-11 and H-16b), 1.84 (3H, m, H2-2 and H-22b), 1.75 (1H, ddd, J = 13.6, 13.3, 3.6 Hz, H-21a), 1.66 (1H, dd, J = 11.0, 6.7 Hz, H-9), 1.57 (1H, m. H-6a), 1.56 (1H, m, H-1a), 1.51 (1H, ddd, J = 12.6, 12.6, 3.5 Hz, H-7a), 1.45 (1H, dd, J = 13.6, 4.2 Hz, H-19b), 1.43 (1H, m, H-21b), 1.38 (1H, m, H-6b), 1.33 (1H, m, H-7b), 1.26 (3H, s, H3-27), 1.25 (3H, s, H3-23), 1.21 (3H, s, H3-30), 1.18 (1H, m, H-15b), 1.04 (3H, s, H3-24), 1.03 (3H, s, H3-26), 1.01 (1H, m, H-1b), 0.91 (3H, s, H3-25), 0.87 (1H, br d, J = 10.4 Hz, H-5); 13C-NMR (150 MHz, pyridine-d5): Table 2, HR-ESI-MS (positive-ion mode) m/z: 633.4000 [M − H]− (Calcd for C36H57O9: 633.3997); mesembryanthenoidigenic acid (1c): [α]D25 +24.9 (c = 0.14, MeOH), HR-ESI-MS (positive-ion mode) m/z: 471.3473 [M − H]− (Calcd for C30H47O4: 471.3469).10,11)
Ebenamarioside C (3) (9.5 mg) in 1 mL of H2O was hydrolyzed with crude naringinase (15 mg) at 37°C for 72 h. The reaction mixture was subjected silica gel CC (Φ = 2 cm, L = 15 cm) with increasing amounts of MeOH in CHCl3 [CHCl3-MeOH (9 : 1, 50 mL), (9 : 1, 100 mL to 7 : 3, 100 mL, linear gradient), (7 : 3, 50 mL) and (1 : 1, 100 mL)], 10-mL fractions being collected. 29-O-β-D-glucopyranosyl estre (3a) was obtained in fractions 11–13. Similarly ebenamarioside C (3) (15 mg) in 1 mL of H2O was hydrolyzed with crude β-glucosidase and silica gel CC with the same condition as above gave 5.2 mg of an aglycone (3c) and 5.6 mg of 29-O-β-D-glucopyranoside (3b) in fractions 5–8 and 11–13, respectively. 2α,3β,29-Trihydroxyolean-12-en-28-oic acid 28-O-β-D-glucopyranosyl ester (3a): Amorphous powder, [α]D24 +18.7 (c = 0.08, MeOH), 13C-NMR (150 MHz, pyridine-d5, MeOH-d4 and DMSO-d6): Table 1, HR-ESI-MS (positive-ion mode) m/z: 673.3922 [M + Na]+ (Calcd for C36H58O10Na: 673.3922). 2α,3β,29-Trihydroxyolean-12-en-28-oic acid 29-O-β-D-glucopyranoside (3b): Amorphous powder; [α]D24 +9.6 (c = 0.28, MeOH); IR νmax (film) cm−1: 3379, 2934, 2872, 1687, 1459, 1050; 1H-NMR (600 MHz, pyridine-d5) δ: 5.43 (1H, dd, J = 3.3, 3.3 Hz, H-12), 4.87 (1H, d, J = 7.7 Hz, H-1″), 4.60 (1H, dd, J = 11.8, 2.2 Hz, H-6″a), 4.44 (1H, dd, J = 11.8, 5.3 Hz, H-6″b), 4.28 (2H, m, H-3″ and 4″), 4.10 (2H, m, H-2 and 2″), 4.00 (1H, m, H-5″), 3.94 (1H, d, J = 9.1 Hz, H-29a), 3.43 (1H, d, J = 9.1 Hz, H-29b), 3.40 (1H, d, J = 9.6 Hz, H-3), 3.35 (1H, dd, J = 13.3, 3.1 Hz, H-18), 2.25 (1H, dd, J = 12.4, 4.2 Hz, H-1a), 2.18 (1H, ddd, J = 13.3, 13.0, 3.1 Hz, H-15a), 2.10 (1H, m, H-11a), 2.05 (1H, m, H-22a), 2.01 (1H, m, H-19a), 1.98 (2H, m, H2-16), 1.95 (1H, m, H-11b), 1.84 (1H, br d, J = 13.6 Hz, H-22b), 1.76 (1H, m, H-9), 1.75 (1H, m, H-21a), 1.56 (1H, m, H-6a), 1.51 (1H, m, H-7a), 1.43 (2H, m, H-19b and 21b), 1.38 (1H, m, H-6b), 1.31 (1H, m, H-7b), 1.28 (1H, m, H-1b), 1.27 (3H, s, H3-23), 1.23 (3H, s, H3-27), 1.21 (3H, s, H3-30), 1.20 (1H, m, H-15b), 1.08 (3H, s, H3-24), 1.02 (3H, s, H3-26), 1.01 (1H, m, H-5), 0.99 (3H, s, H3-25, 13C-NMR (150 MHz, pyridine-d5): Table 2; HR-ESI-MS (positive-ion mode) m/z: 649.3951 [M − H]− (Calcd for C36H57O10: 649.3946). 2α,3β,29-Trihydroxyolean-12-en-28-oic acid (3c): Amorphous powder; [α]D24 +34.6 (c = 0.26, MeOH); HR-ESI-MS (positive-ion mode) m/z: 487.3422 [M − H]− (Calcd for C30H47O5: 487.3418).
Ebenamarioside C (4) (16.6 mg) in 1 mL of H2O was hydrolyzed with crude β-glucosidase 37°C for 72 h. The reaction mixture was separated by silica gel CC with the same condition as above to give 4.1 mg of an aglycone (4c) and 9.4 mg of a mixture of two monoglucosidic compounds (4a and 4b) in fractions 5–8 and 10–15, respectively. The mixture was purified by HPLC (ODS-3, H2O–MeOH, 4 : 1) to afford 6.3 mg of 28-O-β-D-glucopyranosyl ester (4a) and 1.9 mg of 30-O-β-D-glucopyranoside (4b) from the peaks at 3.9 min and 9.2 min, respectively. 2α,3β,30-Trihydroxyurs-12-en-28-oic acid 28-O-β-D-glucopyranosyl ester (4a): Amorphous powder; [α]D24 +17.9 (c 0.31, MeOH); IR νmax (film) cm−1: 3373, 2925, 2877, 1732, 1456, 1073; 1H-NMR (600 MHz, pyridine-d5) δ: 6.31 (1H, d, J = 8.1 Hz, H-1′), 5.48 (1H, dd, J = 3.4, 3.4 Hz, H-12), 4.47 (1H, dd, J = 11.8, 2.4 Hz, H-6′a), 4.41 (1H, dd, J = 11.8, 4.4 Hz, H-6′b), 4.39 (1H, dd, J = 9.5, 8.9 Hz, H-4′), 4.31 (1H, dd, J = 8.9, 8.7 Hz, H-3′), 4.23 (1H, dd, J = 8.7, 8.1 Hz, H-2′), 4.11 (1H, ddd, J = 11.1, 9.6, 4.3 Hz, H-2), 4.04 (1H, ddd, J = 9.5, 4.4, 2.4 Hz, H-5′), 3.93 (1H, dd, J = 10.7, 2.9 Hz, H-30a), 3.88 (1H, dd, J = 10.7, 5.6 Hz, H-30b), 3.39 (1H, d, J = 9.4 Hz, H-3), 2.65 (1H, d, J = 11.5 Hz, H-18), 2.49 (1H, ddd, J = 13.8, 13.7, 4.4 Hz, H-15a), 2.26 (1H, dd, J = 12.5, 4.4 Hz, H-1a), 2.21 (1H, ddd, J = 13.5, 13.4, 4.2 Hz, H-16a), 2.07 (1H, m, H-22a), 2.06 (1H, m, H-16b), 2.05 (2H, m, H2-11), 2.01 (1H, m, H-19), 1.86 (2H, m, H2-21), 1.84 (1H, m, H-22b), 1.74 (1H, dd, J = 10.0, 7.4 Hz, H-9), 1.54 (1H, m, H-7a), 1.52 (1H, m, H-6a), 1.40 (1H, m, H-7b), 1.38 (1H, m, H-6b), 1.27 (1H, m, H-1b), 1.26 (3H, s, H3-23), 1.20 (3H, s, H3-27), 1.19 (3H, s, H3-26), 1.18 (1H, m, H-15b), 1.16 (1H, m, H-20), 1.11 (3H, d, J = 6.4 Hz, H3-29), 1.09 (3H, s, H3-24), 1.04 (3H, s, H3-25), 1.01 (1H, d, J = 12.1 Hz, H-5); 13C-NMR (150 MHz, pyridine-d5): Table 2; HR-ESI-MS (positive-ion mode) m/z: 673.3925 [M + Na]+ (Calcd for C36H58O10Na: 673.3922).
2α,3β,30-Trihydroxyurs-12-en-28-oic acid 30-O-β-D-glucopyranoside (4b): Amorphous powder; [α]D25 +5.5 (c = 0.10, MeOH); IR νmax (film) cm−1: 3370, 2930, 2871, 1686, 1457, 1050; 1H-NMR (600 MHz, pyridine-d5) δ: 5.45 (1H, dd, J = 3.1, 3.1 Hz, H-12), 4.90 (1H, d, J = 7.8 Hz, H-1″), 4.62 (1H, dd, J = 11.7, 2.2 Hz, H-6″a), 4.45 (1H, dd, J = 11.7, 5.3 Hz, H-6″b), 4.30 (1H, dd, J = 8.8, 8.9 Hz, H-3″), 4.27 (1H, dd, J = 8.9, 8.9 Hz, H-4″), 4.10 (1H, m, H-2), 4.09 (1H, m, H-2″), 4.07 (1H, m, H-30a), 4.03 (1H, m, H-5″), 3.89 (1H, dd, J = 9.5, 3.5 Hz, H-30b), 3.40 (1H, d, J = 9.4 Hz, H-3), 2.65 (1H, d, J = 11.3 Hz, H-18), 2.32 (1H, ddd, J = 13.8, 13.8, 4.4 Hz, H-15a), 2.24 (1H, dd, J = 12.5, 4.4 Hz, H-1a), 2.07 (1H, m, H-16a), 2.04 (1H, m, H-21a), 2.03 (1H, m, H-22a), 2.02 (2H, m, H2-11), 1.96 (1H, m, H-16b), 1.94 (1H, m, H-22b), 1.75 (2H, m, H-9 and 19), 1.66 (1H, m, H-21b), 1.55 (1H, m, H-6a), 1.54 (1H, m, H-7a), 1.39 (1H, m, H-20), 1.38 (1H, m, H-6b), 1.37 (1H, m, H-7b), 1.28 (3H, s, H3-23), 1.28 (1H, m, H-1b), 1.17 (3H, s, H3-27), 1.16 (1H, m, H-15b), 1.08 (3H, s, H3-24), 1.04 (3H, s, H3-26), 1.03 (1H, m, H-5), 1.01 (3H, d, J = 6.4 Hz, H3-29), 0.98 (3H, s, H3-25); 13C-NMR (150 MHz, pyridine-d5): Table 2; HR-ESI-MS (positive-ion mode) m/z: 649.3948 [M − Na]− (Calcd for C30H57O10: 649.3946).
2α,3β,30-Trihydroxyurs-12-en-28-oic acid (4c): Amorphous powder; [α]D23 +17.7 (c = 0.13, EtOH); HR-ESI-MS (positive-ion mode) m/z: 487.3423 [M − H]− (Calcd for C30H47O5: 487.3418).
Preparation of (R)- and (S)-MTPA Esters (5a and 5b) from 5A solution of 5 (1.0 mg) in 0.5 mL of dry CH2Cl2 were reacted with (R)-MTPA (21.5 mg) in the presence of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC) (13.4 mg) and N,N-dimethyl-4-aminopyridine (4-DMAP) (16.1 mg). The mixture was then occasionally stirred at 37°C for 24 h. After the addition of CHCl3 (1.5 mL), the reaction mixture was successively washed with H2O (1 mL), 1 M HCl (1 mL), NaHCO3-saturated H2O (1 mL), and brine (1 mL). The organic layer was dried with Na2SO4 and evaporated under reduced pressure. The residue was purified by preparative TLC [silica gel (0.25 mm thickness), being applied for 8 cm width, with development with CHCl3–MeOH (19 : 1) for 9 cm and then eluting with CHCl3–MeOH (1 : 1)] to furnish an ester 5a (0.5 mg) from the band at Rf = 0.67. Through the same procedure, 5b (0.6 mg, Rf = 0.59) were prepared from 5 (1.0 mg) using (S)-MTPA (25.4 mg), EDC (15.8 mg), and 4-DMAP (15.5 mg), respectively.
(R)-MTPA ester of 5 (5a): amorphous powder; 1H-NMR (600 MHz, CDCl3) δ: 7.35–7.55 (5H, m, aromatic protons), 6.05 (1H, d, J = 15.2 Hz, H-7), 6.02 (1H, dd, J = 15.2, 4.9 Hz, H-8), 5.66 (1H, qd, J = 6.5, 4.9 Hz, H-9), 5.44 (1H, dddd, J = 10.5, 10.5, 7.2, 7.2 Hz, H-3), 3.87 (1H, d, J = 8.2 Hz, H-12a), 3.77 (1H, dd, J = 8.2, 2.0 Hz, H-12b), 3.53 (3H, s, –OMe), 3.51 (3H, s, –OMe), 2.18 (1H, ddd, J = 13.6, 7.2, 1.5 Hz, H-4), 2.03 (1H, ddd, J = 13.6, 7.2, 1.5 Hz, H-2), 1.73 (1H, dd, J = 13.6, 10.5 Hz, H-4), 1.70 (1H, ddd, J = 13.6, 10.5, 2.0 Hz, H-2), 1.45 (3H, d, J = 6.5 Hz, H3-10), 1.08 (3H, s, H3-13), 0.90 (3H, s, H3-11); HR-ESI-MS (positive-ion mode) m/z: 697.2206 [M + Na]+ (Calcd for C33H36O8F6Na: 697.2207).
(S)-MTPA ester of 5 (5b): amorphous powder; 1H-NMR (600 MHz, CDCl3) δ: 7.35–7.55 (10H, m, aromatic protons), 6.11 (1H, d, J = 15.5 Hz, H-7), 6.08 (1H, dd, J = 15.5, 5.5 Hz, H-8), 5.63 (1H, qd, J = 6.5, 5.5 Hz, H-9), 5.44 (1H, dddd, J = 10.7, 10.7, 7.1, 7.1 Hz, H-3), 3.88 (1H, d, J = 8.3 Hz, H-12a), 3.78 (1H, dd, J = 8.3, 2.1 Hz, H-11b), 3.52 (3H, s, –OMe), 3.49 (3H, s, –OMe), 2.25 (1H, ddd, J = 13.6, 7.1, 1.5 Hz, H-4), 1.99 (1H, ddd, J = 13.6, 7.1, 1.5 Hz, H-2), 1.85 (1H, ddd, J = 13.6, 10.7 Hz, H-4), 1.62 (1H, ddd, J = 13.6, 10.7, 2.1 Hz, H-2), 1.40 (3H, d, J = 6.5 Hz, H3-10), 1.10 (3H, s, H3-13), 0.91 (3H, s, H3-11); HR-ESI-MS (positive-ion mode) m/z: 697.2206 [M + Na]+ (Calcd for C33H36O8F6Na: 697.2207).
NaBH4 Reduction of 6To a solution of 6 (11.0 mg) in MeOH (1 mL) was added 8.2 mg of NaBH4 and the reaction mixture was stirred for 5 min at 25°C. Excess NaBH4 was quenched by the addition of 1 mL of acetone and then the reaction mixture was evaporated to dryness. The resultant residue was purified by HPLC [Inertsil ODS-3, 6 × 250 mm, H2O–MeOH (1 : 4), flow rate: 1.6 mL/min] to give 3.1 mg of 6c (=5) and 5.8 mg of 6d from the peaks at 10.9 min and 13.3 min. respectively.
Compound 6c: amorphous powder; [α]D24 +7.1 (c = 0.31, MeOH); HR-ESI-MS (positive-ion mode) m/z: 265.1409 [M + Na]+ (Calcd for C13H22O4Na: 265.1410).
Compound 6d: amorphous powder; [α]D24 −5.5 (c = 0.29, MeOH); IR νmax (film) cm−1: 3402, 2929, 2889, 1604, 1453, 1381, 1101, 1053; 1H-NMR (600 MHz, CD3OD) δ: 6.02 (1H, dd, J = 15.5, 5.6 Hz, H-8), 5.82 (1H, dd, J = 15.5, 1.0 Hz, H-7), 4.33 (1H, qd, J = 6.4, 5.6 Hz, H-9), 4.12 (1H, d, J = 6.9 Hz, H-12a), 4.02 (1H, dd, J = 5.7, 5.7 Hz, H-3), 3.76 (1H, dd, J = 6.9, 2.3 Hz, H-12b), 2.13 (1H, dd, J = 15.5, 5.7 Hz, H-4a), 2.02 (1H, ddd, J = 15.3, 5.7, 2.2 Hz, H-2a), 1.84 (1H, dd, J = 15.5, 2.2 Hz, H-4b), 1.73 (1H, dd, J = 15.3, 2.3 Hz, H-2b), 1.25 (3H, d, J = 6.4 Hz, H3-10), 1.13 (3H, s, H3-13), 0.86 (3H, s, H3-11); 13C-NMR (150 MHz, CD3OD) δ: 139.4 (C-8), 126.8 (C-7), 87.2 (C-5), 82.5 (C-6), 76.3 (C-12), 69.1 (C-9), 66.0 (C-3), 48.0 (C-1), 45.1 (C-4), 44.9 (C-2), 24.1 (C-10), 19.7 (C-13), 16.3 (C-11); HR-ESI-MS (positive-ion mode) m/z: 265.1408 [M + Na]+ (Calcd for C13H22O4Na: 265.1410).
Cytotoxic Activity toward Human Lung Adenocarcinoma, A549 CellsCytotoxic activity toward lung adenocarcinoma cells was determined by colorimetric cell viability assay using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). Lung adenocarcinoma cell line A549 was purchased from the JCRB Cell Bank, Japan. A549 cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% heat inactivated FCS, and kanamycin (100 µg/mL) and amphotericin B (5.6 µg/mL). In a 96-well plate, 1 µL aliquots of sample solutions and the cancer cells (5 × 103 cells/well) in 100 µL medium were added to each well, and then the plate incubated at 37°C under a 5% CO2 atmosphere for 72 h. A solution (100 µL) of MTT (0.5 mg/mL) was then added to each well and the incubation was continued for a further 1 h. The absorbance of each well was measured at 540 nm using a Molecular Devices Versamax tunable microplate reader. DMSO was used as a negative control and doxorubicin as a positive control. The cytotoxic activity was calculated as:
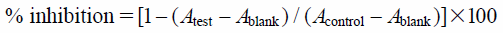 |
where Acontrol is the absorbance of the control DMSO well, Atest the absorbance of the test wells, and Ablank the absorbance of the cell-free wells.
Anti-Leishmania ActivityThe anti-Leishmania major activity toward promastigotes was determined by the colorimetric cell viability MTT assay. The promastigotes at the logarithmic growth phase were cultured in M199 medium supplemented with 10% heat-inactivated fetal bovine serum and 100 µg/mL of kanamycin. In a 96-well plate, 1 µL aliquot of sample solutions and L. major cells (1 × 105 cells/well) in 100 µL medium were added to each well, and then the plate was incubated at 27°C under an ambient atmosphere for 72 h. A solution of MTT (100 µL) was then added to each well and the incubation was continued overnight. The formazan product of MTT reduction was then dissolved in DMSO and then the absorbance was measured using a Molecular Devices Versamax tunable microplate reader. DMSO was used as a negative control and amphotericin B as a positive control. The experiment was performed in triplicate. The anti-Leishmania major activity was quantified as the percentage of the control absorbance of reduced dye at 540 nm. The inhibitory activity was calculated as:
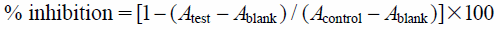 |
where Acontrol is the absorbance of the control (DMSO) well, Atest the absorbance of the test wells, and Ablank the absorbance of the cell-free wells.
The measurements of HR-ESI-MS were performed with LTQ Orbitrap XL spectrometer at the Natural Science Center for Basic Research and Development (N-BARD), Hiroshima University. This work was supported in part by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and the Japan Society for the Promotion of Science (Nos. 22590006, 23590130, 25860078, 15H04651, 17K08336 and 18K06740).
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.