2019 Volume 67 Issue 5 Pages 487-492
2019 Volume 67 Issue 5 Pages 487-492
A new mixed metal hydroxide adsorbent (NA11, molar ratioNi–Al = 1 : 1) was prepared and its physicochemical properties (specific surface area, amount of hydroxyl group, scanning electron microscopy images, X-ray diffraction analysis, elemental distribution, and binding energy) were studied. In addition, the amount of borate ion adsorbed using several adsorbents, including NA11, was evaluated in this study. The specific surface area of and amount of hydroxyl group in NA11 was greater than those of the other studied adsorbents. The amount of borate ion adsorbed showed similar trends to those of the specific surface area and number of hyrdroxyl groups, which indicated that the adsorption mechanism of borate ion was related to the specific surface area and the amount of hydroxyl group. After adsorption, the binding energy of boron B(1s) peaked, and the sulfur peak intensity S(2s) and S(2p) reduced. These results suggest that ion exchange between borate and sulfate ions was one of the adsorption mechanisms. Equilibrium adsorption was reached within 6 h in the case of NA11. These data were fitted into a pseudo-second-order model (r = 0.813–0.998). The solution pH affected the capacity of NA11 for adsorbing borate ion from aqueous solution. It was found that adsorbance was greatest at pH 10. Adsorption isotherm data were fitted to both the Freundlich (r = 0.986–0.994) and Langmuir (r = 0.997–0.999) isotherm equations. Collectively, it is suggested that NA11 is prospectively useful for the adsorption of borate ion from aqueous solutions.
Boron dissolved in surface and ground water is present at high level concentrations, and is anthropogenic and geothermal activity dependent.1,2) Boron is one of the most important micro-nutrients for humans, animals, and plants, used to promote the transport and metabolism of carbohydrates, for the synthesis of hemicellulose and related cell wall material, in cell growth and division, to build chloroplast structure, and in the formation of basal grains.3–5) However, previous studies have reported that the ingestion of large amounts of boron can affect the central nervous system and the reproductive system in humans.6,7) Therefore the WHO limited the maximum boron concentration in drinking water to 2.4 ppm in 2011, while some countries set a more stringent standard.8) Thus, there is a need for boron and its derivatives to be eliminated from water with elevated concentrations.9,10)
Currently, there is no effective and economical method to remove boron from aqueous solutions. Among many proposed methods for boron removal, adsorption is the most promising because of its low cost, effective boron uptake, and regeneration capacity.8) Many adsorbents such as metal-organic framework,6) magnesium oxide,11) cobalt(II) deposited chitosan biocomposite,1) diol-functionalized silica particles,12) and Mg–Al layered double hydroxide or oxide13) have been studied for boron removal.
Recently, we demonstrated the capability of metal complex hydroxides to adsorb toxic compounds (heavy metals, inorganic nitrogen, phosphate ions, etc.) from the aqueous phase.14–16) Mixed-metal hydroxide exhibited properties that mimic natural systems more closely than their individual components. Also, multi-component adsorbents demonstrate physicochemical characteristics substantially different from those of their single-component counter parts. The differences in the physicochemical characteristics are considered the major reasons for the changes in adsorption behavior between single- and multi-component systems.15,17) Till date we focused on Fe–Mg, Fe–Al, and Ni–Co complex hydroxides as adsorbents in the purification of wastewater. A previous study reported on the capacity of hydrous metal oxide (single-component including Al, Fe, Mn, and Zr, etc.) to adsorb boron.18) However, no information is available regarding boron adsorption by mixed-metal complex hydroxides.
In this work, nickel–aluminum complex hydroxide was prepared for boron adsorption from an aqueous solution. The physicochemical properties of nickel–aluminum complex hydroxide were investigated. In addition, the kinetic and isothermal behavior, and the parameters influencing boron adsorption, including solution pH and contact time, were also studied.
Nickel hydroxide (Ni), aluminum hydroxide (Al), and nickel–aluminum complex hydroxide (NA11, molar ratioNi–Al = 1 : 1) were obtained from Kansai Catalyst Co., Ltd. (Japan). Borate ion was prepared using boric acid (Wako Pure Chemical Industries, Ltd., Japan). The morphologies and crystallinities of the adsorbents were measured using scanning electron microscopy (SEM, SU1510, Hitachi, Ltd., Japan) and X-ray diffractometry (XRD, MiniFlex II, Rigaku, Japan). The specific surface area of the adsorbents and the amount of hydroxyl group were measured with a NOVA4200e specific surface analyzer (Yuasa Ionic, Japan) and using the fluoride ion adsorption method.19) Elemental distribution analysis and electron spectroscopy were carried out using an electron microanalyzer (EPMA, JXA-8530F, JEOL, Japan) and an X-ray photoelectron spectroscopy system (AXIS-NOVA, Shimadzu Co., Ltd., Japan), respectively.
Capacity for Borate Ion AdsorptionTo 50 mL of a borate ion solution (200 mg/L at pH 10), 0.1 g of adsorbent was added. The suspension was shaken at 100 rpm for 24 h at 25°C. The sample was then filtered through a 0.45 µm membrane, and the filtrate was analyzed via inductively coupled plasma optical emission spectrometry using an iCAP-7600 (ICP-OES, Thermo Fisher Scientific Inc., Japan). The amount of borate ion adsorbed was calculated using Eq. 1:
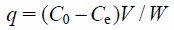 | (1) |
where q is the amount of borate ion adsorbed (mg/g), C0 and Ce are the concentrations before and after adsorption process (mg/L), V is the solvent volume (L), and W is the adsorbent weight (g).
Effect of Contact Time and pH on the Adsorption of Borate IonsFor contact time studies, 0.1 g of adsorbent was added to 50 mL of a borate ion solution (100 mg/L at pH 10). The suspension was shaken at 100 rpm for 1, 3, 5, 8, 12, 16, 20, 24, 36, and 48 h at 25°C. The effect of pH on adsorption was tested by adding 0.1 g of adsorbent to a borate ion solution (50 mL) at 1, 10, 25, 50, 75, and 100 mg/L (pH = 6, 10, and 12). The pH of the solution was adjusted using hydrochloric acid and sodium hydroxide solutions. The suspension was shaken for 24 h at 25°C. The amount of borate ions adsorbed was calculated by Eq. 1. The solution pH was measured using a F-73S digital pH meter (Horiba, Ltd., Japan).
In addition, the concentration of sulfate ions in the filtrate was measured by ion chromatography (DIONEX ICS-900, Thermo Fisher Scientific Inc.). The measurements were performed using the IonPac AS12A system (4 × 200 mm, Thermo Fisher Scientific Inc.). The mobile phase and the regenerant comprised of 2.7 mmol/L Na2CO3 + 0.3 mmol/L NaHCO3 and 12.5 mmol/L H2SO4, respectively. The flow rate was 1.0 mL/min at the ambient temperature. The micro membrane suppressor was an AMMS 300 system (4 mm, Thermo Fisher Scientific Inc.) and the sample volume was 10 µL.
The SEM images of the adsorbents are shown in Fig. 1. Ni consists of spherical particles of various diameters, while NA11 and Al are not spherical. These results are consistent with previous studies.20–22) Figure 2 shows XRD patterns of Al, Ni, and NA11. These demonstrate that Al, Ni, and NA11 have structures similar to gibbsite, β-Ni(OH)2, and nickel hydroxide, respectively. In addition, the NA11 structure exhibits an amorphous pattern. The specific surface area and amount of hydroxyl group in increasing order are NA11 (22.8 m2/g and 1.92 mmol/g) > Ni (17.9 m2/g and 0.80 mmol/g) > Al (4.2 m2/g and 0.17 mmol/g). The amount of borate ion adsorbed on NA11 was greater than that adsorbed on Al or Ni alone (Table 1). These results suggest that amount of borate ion adsorbed is related to the physicochemical properties of the adsorbent. Multi-component adsorbents demonstrate physicochemical properties significantly different from those of their single-component counterparts. The differences in the physicochemical properties are considered as the major reasons for the differences in adsorption behavior between multi- and single-component systems.15,17) A previous study reported that different hydrous metal oxides (Al(III), Al(III)–Fe(III), Fe(III)–Ti(IV), and Al(III)–Ti(IV) etc.) showed different boron adsorption capacities.18) Their material crystal phases have a gibbsite or amorphous structure. The amount of borate ion adsorbed on NA11 was 4.3–25.8 times greater than that on hydrous metal oxides. In addition, this adsorption mechanism was related to the surface hydroxyl groups on hydrous metal oxides. A similar trend was confirmed in this study.
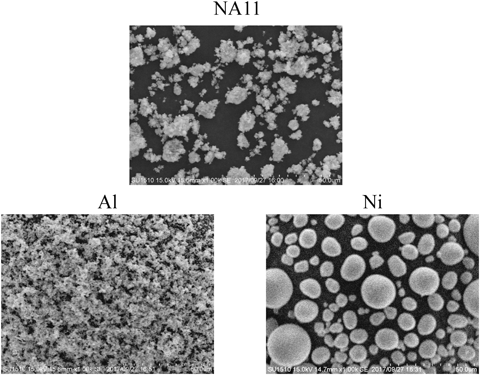
Al, Ni, and NA11 indicate nickel hydroxide, aluminum hydroxide, and nickel–aluminum hydroxide (molar ratioNi–Al = 1 : 1), respectively.
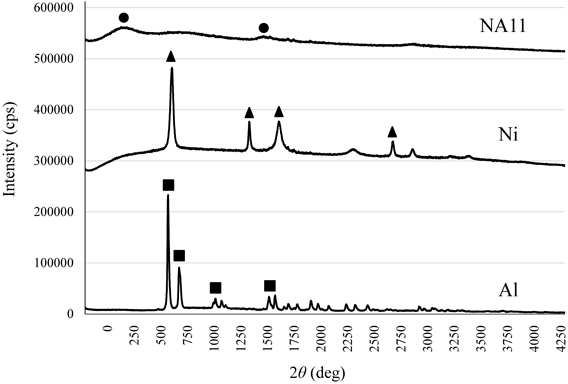
●: Nickel hydroxide, ▲: β-Ni(OH)2, ■: Gibbsite.
| Samples | Specific surface area (m2/g) | Amount of hydroxyl groups (mmol/g) | Amount adsorbed (mg/g) |
|---|---|---|---|
| NA11 | 22.8 | 1.92 | 15.5 |
| Ni | 17.9 | 0.8 | 0.7 |
| Al | 4.2 | 0.17 | 0.2 |
Adsorption condition; initial concentration: 200 mg/L, sample volume: 50 mL, adsorbent: 0.1 g, solution pH: 10, temperature: 25°C, contact time: 24 h, 100 rpm.
Further, the mechanism of borate ion adsorption using NA11 was evaluated. First, SEM images and XRD patterns after borate ion adsorption were measured (Fig. 3). SEM images and XRD patterns confirmed that each adsorbent maintained its original structure. As previously mentioned, the physicochemical properties of the adsorbent are vital factors for the removal of borate ions from aqueous solutions. Particularly, previous studies reported that the specific surface area of or amount of hydroxyl group on an adsorbent affect the adsorption capability of borate ions strongly.11,23) Therefore, the boron distribution on NA11 surface before and after adsorption treatment was measured (Fig. 4, the warm and cold colors indicate high and low concentrations, respectively). The increase in boron on the NA11 surface after adsorption was confirmed, which indicates that adsorbent surface is an important factor for the adsorption of borate ions. Next, the binding energies of boron and sulfur before and after adsorption were evaluated (Fig. 5). After adsorption, the B(1s) peak was detected, and it was found that the S(2s) and S(2p) peaks intensities were reduced, which suggests that borate ions were adsorbed on NA11, and at the same time, sulfur ions were released. In this case, the NA11 was prepared using sulfates and has a layered double hydroxide structure. Finally, we measured the concentration of borate ions and sulfate ions after adsorption (Fig. 6). We confirmed that the amount of borate ion adsorbed on NA11 and the amount of sulfate ion released were positively correlated in this experiment (r = 0.998), supporting the theory that sulfate ions interlayered in NA11 are exchanged with borate ions. These mechanisms are similar to those reported by previous studies.24–27) In addition, the reported adsorption capacities of various adsorbents are shown in Table 2.18,26,28–31) The amount of borate ion adsorbed on NA11 was greater than that of most other adsorbents. Therefore, NA11 would be useful for the adsorption of borate ions from aqueous solutions.

●: Nickel hydroxide, ▲: β-Ni(OH)2, ■: Gibbsite.

Adsorbent sample (NA11) is collected before and after adsorption of borate ion, adsorption condition; initial concentration: 200 mg/L, sample volume: 50 mL, adsorbent: 0.1 g, solution pH: 10, temperature: 25°C, contact time: 24 h, 100 rpm, the warm and cold colors indicate high and low concentrations, respectively. (Color figure can be accessed in the online version.)

Adsorbent sample (NA11) is collected before and after adsorption of borate ion, adsorption condition; initial concentration: 200 mg/L, sample volume: 50 mL, adsorbent: 0.1 g, solution pH: 10, temperature: 25°C, contact time: 24 h, 100 rpm.

Sample solution was obtained after adsorption of borate ion, adsorption condition; initial concentration: 1, 10, 25, 50, 75, and 100 mg/L, sample volume: 50 mL, adsorbent: 0.1 g, temperature: 25°C, contact time: 24 h, 100 rpm.
| Adsorbents | Adsorption capacity (mg/g) | Reference |
|---|---|---|
| Al(III)–Fe(III) | 3.6 | 18 |
| Fe(III)–Ti(IV) | 2.2 | 18 |
| Al(III)–Ti(IV) | 2.7 | 18 |
| Waste calcite | 1.6 | 28 |
| Rice residues | 4 | 29 |
| Calcined alunite | 3.4 | 30 |
| Chitosan | 3.9 | 31 |
| Mg–Al LDH | 13.7 | 26 |
| Al | 0.2 | This study |
| Ni | 0.7 | This study |
| NA11 | 15.5 | This study |
To evaluate the adsorption kinetics, the adsorption of borate ions was measured as a function of contact time. The effect of contact time on the adsorption of borate ion is shown in Fig. 7. In this experimental condition, the amount adsorbed increased rapidly from time t = 0 h until t = 6 h and then plateaued at 24 h. In order to investigate the mechanism of borate ion removal, the constants of sorption were determined in terms of the pseudo-first-order and pseudo-second-order models. The rate equation for the adsorption of solutes can be determined for a liquid solution. This pseudo-first-order rate equation is following Eq. 2.
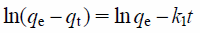 | (2) |
where qe and qt are the amounts (mg/g) of borate ion adsorbed at equilibrium and at time t (h), respectively; k1 is the rate constant of the pseudo-first-order model adsorption (1/h).
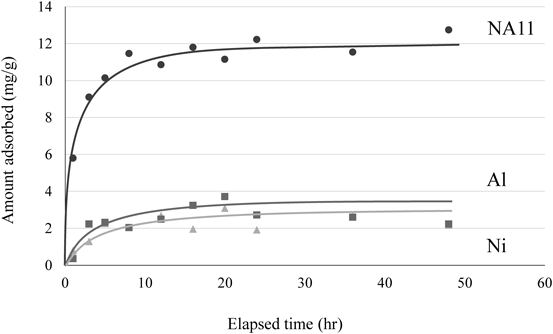
Sample solution was obtained after adsorption of borate ion with elapsed time, adsorption condition; initial concentration: 100 mg/L, sample volume: 50 mL, adsorbent: 0.1 g, solution pH: 10, temperature: 25°C, contact time: 1, 3, 5, 8, 12, 16, 20, 24, 36, and 48 h, 100 rpm.
If the rate of adsorption is a second-order mechanism, the pseudo-second-rate equation is as follows Eq. 3.
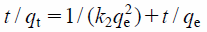 | (3) |
where k2 is the rate constant of the pseudo-second-order model adsorption (g/mg/h). The plot of t/qt versus t yields a straight line with a slope of 1/qe and an intercept of k2qe2.11,12,32,33)
The resulting parameters of these models and their correlation coefficients, r, as well as the experimental value of qe,exp, are listed in Table 3. The results showed that the pseudo-first-order rate expression is not fully valid as reflected in the correlation coefficients of 0.718, 0.558, and 0.462 for NA11, Ni, and Al, respectively. For the pseudo-second-order rate model, the correlation coefficients of NA11, Ni, and Al are 0.998, 0.813, and 0.983. The amount of adsorbent calculated, qe,cal (12.7 mg/g for NA11), in the pseudo-second-order model was closer to the actual experimental value, qe,exp (12.7 mg/g for N11).1,11) These results indicate that chemisorption is possibly the dominant mechanism in this adsorption reaction.34,35)
| Samples | qe,exp (mg/g) | PFOM | PSOM | ||||
|---|---|---|---|---|---|---|---|
| k1 (1/h) | qe,cal (mg/g) | r | k2 (g/mg/h) | qe,cal (mg/g) | r | ||
| NA11 | 12.7 | 4.9 × 10−2 | 3.4 | 0.718 | 7.1 × 104 | 12.7 | 0.998 |
| Ni | 3.7 | 2.9 × 10−2 | 2.3 | 0.588 | −9.3 | 0.89 | 0.813 |
| Al | 3.7 | 2.0 × 10−2 | 1.7 | 0.462 | −0.14 | 2.35 | 0.983 |
The values of k1 (1/h) and k2 (g/mg/h) were obtained from the pseudo-first-order model (PFOM) and the pseudo-second-order model (PSOM), respectively. The values of qe,exp (mg/g) or qe,cal (mg/g) were the actual experimental or the calculated amount of borate ion adsorbed.
The effect of pH on the adsorption of borate ions on NA11 is shown in Fig. 8. The amount adsorbed decreases from pH 10 to pH 12 to pH 6. Many researchers have reported the following: boron is mainly present as boric acid B(OH)3, and only partially as borate ions BO33−. This relationship is a function of pH according to dissociation reaction shown in Eq. 4.9,36)
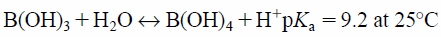 | (4) |

Sample solution was obtained after adsorption of borate ion at pH 6, 10, and 12, adsorption condition; initial concentration: 1, 10, 25, 50, 75, and 100 mg/L, sample volume: 50 mL, adsorbent: 0.1 g, temperature: 25°C, contact time: 24 h, 100 rpm.
Boric acid is a highly weak acid (pKa ca. 9.2). At pH <7, boron exists as boric acid (non-ionized form), while at pH >10, it exists as borate (ionized form). The pHpzc of NA11 used in this study is 6.57. Therefore, hydroxyl functional groups on the NA11 become partially protonated, resulting in positively charged surfaces on the adsorbent. This indicates that amount adsorbed is lower at the pH 6 condition.37) On the contrary, ion exchanges between borate and the hydroxyl groups on NA11 occurred readily at pH 10. However, hydroxide ion increased with increasing pH (pH 12), therefore, the competition between hydroxyl and borate ions occurred. These phenomena indicate that the amount of borate ion adsorbed is decreased at the pH 12 condition.
In addition, the amount adsorbed increased with increasing initial concentration of borate ion. The Langmuir model assumes that the surface of the adsorbent is uniform and adsorption is monolayer, namely, the adsorption process takes place at specific sites and that once a borate ion molecule occupies a site, no further adsorption can take place at that site. The general expression of the Langmuir isotherm is as follows8):
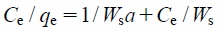 | (5) |
where Ce is the equilibrium concentration (mg/L), qe is the amount adsorbed at equilibrium (mg/g), and Ws and a are Langmuir constants relating to the monolayer adsorption capacity and energy of sorption, respectively.
Freundlich adsorption, which is widely used to describe the adsorption process on heterogeneous surfaces caused by the presence of different functional groups on the surface and several adsorbent–adsorbate interactions, was employed to explain borate ion adsorption isotherms.
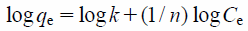 | (6) |
where qe is the adsorption capacity (mg/g) of the adsorbate, Ce is the equilibrium concentration (mg/L) of the adsorbate, and k and n are the Freundlich constants related to the adsorption of the adsorbent and the intensity of adsorption, respectively.
Table 4 shows the Freundlich and Langmuir constants for the adsorption of borate ions. The correlation coefficients indicate that both the Freundlich (0.986–0.994) and Langmuir (0.997–0.999) isotherm equations are good fits for the data corresponding to borate ion adsorption on NA11. The value of maximum adsorption capacity (Ws) of NA11 calculated from the Langmuir isotherm model is 87.7 mg/g at pH 10. It is apparent that the borate ion adsorption capacity of NA11 is high. In addition, borate ions were easily adsorbed on NA11 when 1/n was in the range of 0.1–0.5, but not when 1/n > 2.38) These findings are also consistent with those of previous studies, in which borate ion adsorption readily occurred when 1/n < 2 (0.79–1.03). Taken together, in this study, the adsorption of borate ion on NA11 is attributed to monolayer adsorption.
| Samples | Freundlich constants | Langmuir constants | |||||
|---|---|---|---|---|---|---|---|
| 1/n | log K | r | Ws (mg/g) | a (L/mg) | r | ||
| NA11 | pH 6 | 0.89 | −0.98 | 0.986 | 21.8 | 4.2 × 10−3 | 0.999 |
| pH 10 | 1.03 | −0.64 | 0.986 | 87.7 | 3.4 × 10−3 | 0.997 | |
| pH 12 | 0.79 | −0.44 | 0.994 | 11.2 | 3.2 × 10−2 | 0.998 | |
The values of Ws and a were obtained from the Langmuir equation, and those of log K and 1/n were obtained from the Freundlich equation. The Ws and a are the maximum adsorption capacity (mg/g) and the equilibrium constant (L/mg), respectively. In addition, K is a constant inductive of the relative adsorption capacity of the adsorbent, and n is a constant indicative of the intensity of the adsorption and varies with surfaced heterogeneity.
Finally, the adsorption capability of borate ion in complex solution system was not evaluated in this study. Therefore, further studies are needed to elucidate the application of NA11 in the fields. Additionally, the pH in solution affects the adsorption capability of borate ion from aqueous solution (Fig. 8). The pH in environmental water is usually neutral, indicating that adsorption treatment using NA11 is more suitable for wastewater from factories or industries compared to environmental water. Collectively, NA11 could be used for wastewater pretreatment in factories or industries.
In this study, NA11 was demonstrated to be a better borate ion adsorbent than Ni and Al. The specific surface area of and amount of hydroxyl group on NA11 (22.8 m2/g and 1.92 mmol/g, respectively) were greater than those of Ni and Al adsorbents alone. In addition, the amount of borate ion adsorbed increased from Al to Ni to NA11. These results suggest that the amount adsorbed is related to the physicochemical properties of the adsorbent (particularly, the specific surface area and the amount of hydroxyl group). In addition, the binding energy of boron was observed to be at its peak after adsorption treatment, while the sulfur peak S(2s) and S(2p) intensities had sharply decreased. Therefore, the ion exchange between the borate and sulfate ions is thought to be a good mechanism for the adsorption of borate ions from aqueous solutions. In the case of NA11, the adsorption equilibrium was reached within 6 h. The data were fitted to the pseudo-second-order model, which indicated that the adsorption was related to chemisorption. Moreover, the amount of borate ion adsorbed was affected by the solution pH, where the amount adsorbed at pH 10 was the greatest. Adsorption isotherm data were fitted to both the Freundlich (r = 0.986–0.994) and Langmuir (r = 0.997–0.999) equation models. Thus, NA11 is a promising candidate for the adsorption of borate ions from aqueous solutions.
Ministry of Education, Culture, Sports, Science and Technology (MEXT)-supported Program for the Strategic Research Foundation at Private Universities, 2014–2018 (S1411037).
The authors declare no conflict of interest.