2019 Volume 67 Issue 7 Pages 707-712
2019 Volume 67 Issue 7 Pages 707-712
Hypobromous acid (HOBr) is generated not only by eosinophils but also by neutrophils in the presence of Br− at the plasma concentration. Reactivity of HOBr is greatly modulated by coexistent compounds such as amines and amides. In this study, we investigated effects of urea in the reaction of nucleosides with HOBr. When nucleosides were incubated with HOBr without urea in potassium phosphate buffer at pH 7.4 and 37°C, the reactions almost completed within 10 min, with consumptions in the order of 2′-deoxyguanosine > 2′-deoxycytidine > 2′-deoxythymidine > 2′-deoxyadenosine, generating 8-bromo-2′-deoxyguanosine and 5-bromo-2′-deoxycytidine. In the presence of urea, the reaction of nucleosides with HOBr was relatively slow, continuing over several hours. When HOBr was preincubated without urea in potassium phosphate buffer at pH 7.4 and 37°C for 48 h, the preincubated HOBr solution did not react with nucleosides. However, a similar preincubated solution of HOBr with urea reacted with nucleosides to generate 8-bromo-2′-deoxyguanosine and 5-bromo-2′-deoxycytidine. These results imply that a reactive bromine compound with a long life, probably bromourea, is generated by HOBr in neutral urea solution and reacts with nucleosides, resulting in brominated nucleosides.
Eosinophils are a minor component of white blood cells, but are abundant in blood and tissues in various inflammatory disorders.1) Eosinophils secrete eosinophil peroxidase (EPO), which generates a reactive species, hypobromous acid (HOBr), from H2O2 and Br−.2,3) Although the plasma concentrations of Br− and Cl− are 39–84 µM and 100 mM, respectively,4) EPO uses Br−selectively, resulting in HOBr. The formed HOBr plays an important role in defense mechanisms against parasites. Meanwhile, neutrophils are a major component of white blood cells. Neutrophils secrete myeloperoxidase (MPO), which generates hypochlorous acid (HOCl) from H2O2 and Cl−.5–7) HOCl is of central importance in host defense mechanisms. Since HOCl can react with Br− to generate HOBr, a portion of the HOCl formed by the MPO system will react with Br− at the plasma concentration, converting to HOBr.8–11) HOBr can react with nucleic acid bases in nucleosides and DNA. 2′-Deoxyguanosine (dG) and 2′-deoxycytidine (dC) react with reagent HOBr and HOBr formed by EPO–H2O2–Br− systems, MPO–H2O2–Cl−–Br− systems, and other oxidant–Br− systems, generating several products including 8-bromo-2′-deoxyguanosine (8-Br-dG) and 5-bromo-2′-deoxycytidine (5-Br-dC), respectively.8–14) 2′-Deoxythymidine (dT) reacts with HOBr, resulting in thymidine glycol and its phosphate derivative in phosphate buffer.15) Whereas the reactivity of 2′-deoxyadenosine (dA) with HOBr is low, 8-bromoadenine is the major purine oxidation product generated in double-stranded DNA by either reagent HOBr or HOBr generated by an EPO–H2O2–Br− system.10) Recently, 8-Br-dG was reportedly detected in urine from healthy volunteers with a similar concentration of 8-chloro-2′-deoxyguanosine (8-Cl-dG), a product in the reaction of dG with HOCl, while both urinary 8-Br-dG and 8-Cl-dG levels from diabetic patients were 8-fold higher than the levels in healthy volunteers.16) This implies that formation of HOBr as well as HOCl is important occurrence in inflammatory diseases in humans. Generally, HOBr can react with amines (R-NH2) including amides, resulting in bromamines (R-NHBr).17,18) Thus, various amines and amides in humans affect the reactions of various compounds including proteins and nucleic acids with HOBr. Urea (CO(NH2)2), also known as carbamide, is a ubiquitous molecule, showing high concentrations in humans of 5 mM in plasma and 285 mM in urine.19,20) However, there is little information about the effect of urea on the reaction of HOBr with nucleosides. In the present study, we examined the reactions of nucleosides with HOBr in the absence and presence of urea using reversed-phase (RP)-HPLC, and report that HOBr reacts with urea to generate a reactive bromine species that is rather stable under neutral conditions and can react with nucleosides.
A solution of 100 µM each of dC, dG, dT, and dA without urea was incubated with 400 µM HOBr in 100 mM potassium phosphate buffer at pH 7.4 and 37°C for 2 h. When the reaction mixture was analyzed by RP-HPLC, nucleosides were decreased in the order of dG > dC > dT > dA, and two product peaks (Peaks 1 and 2) appeared in the chromatogram detected at 230 nm (Fig. 1). The two products were isolated by RP-HPLC and identified. Peak 1 at the retention time of 16.1 min showed a UV spectrum with λmax = 285 nm and an electrospray ionization-time-of-flight (ESI-TOF)/MS spectrum with m/z = 304 and 306 (1 : 1) in negative mode (Fig. 2A). Peak 1 was identified as 5-bromo-2′-deoxycytidine (5-Br-dC) (Fig. 2B) by the coincidence of the retention time and the UV and MS spectra of authentic 5-Br-dC. Peak 2 at the retention time of 18.6 min showed a UV spectrum with λmax = 261 nm and an ESI-TOF/MS spectrum with m/z = 344 and 346 (1 : 1) in negative mode (Fig. 2C). Peak 2 was identified as 8-bromo-2′-deoxyguanosine (8-Br-dG) (Fig. 2D) by the coincidence of the retention time and the UV and MS spectra of authentic 8-Br-dG.
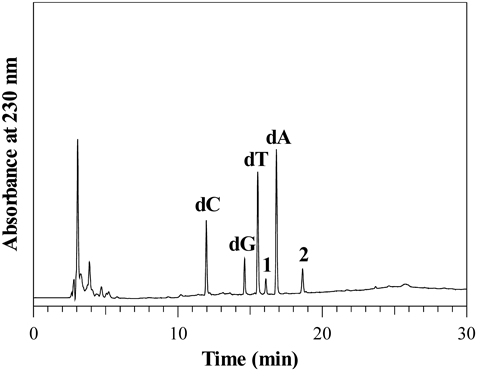
A solution of 100 µM each of dC, dG, dT, and dA without urea was incubated with 400 µM HOBr in 100 mM potassium phosphate buffer at pH 7.4 and 37°C for 2 h. The HPLC system consisted of LC-10ADvp pumps and an SPD-M10Avp UV-vis photodiode-array detector (Shimadzu, Kyoto, Japan). For the RP-HPLC, an Inertsil ODS-3 octadecylsilane column of 4.6 × 250 mm and particle size 5 µm (GL Sciences, Tokyo, Japan) was used. The eluent was 20 mM ammonium acetate (pH 7.0) containing methanol. The methanol concentration was increased from 0 to 50% during 15 min in linear gradient mode, and maintained at 50% from 15 to 30 min. The column temperature was 40°C and the flow rate was 1 mL/min.
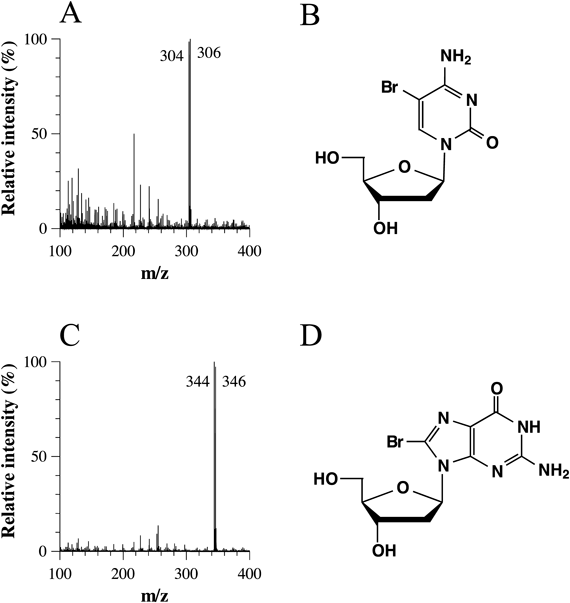
Figure 3A shows the time-dependent changes in concentrations of the nucleosides and the products when 100 µM dC, dG, dT, dA, and 400 µM HOBr without urea were incubated in 100 mM potassium phosphate buffer at pH 7.4 and 37°C for 0–4 h. The concentrations of dG, dC, and dT decreased and those of 5-Br-dC and 8-Br-dG increased with increasing incubation time. The reaction was rather fast, almost completing within 10 min. Figure 3B shows the time-dependent changes in concentrations of the nucleosides and the products when 100 µM dC, dG, dT, dA and 400 µM HOBr in the presence of 100 mM urea were incubated in 100 mM potassium phosphate buffer at pH 7.4 and 37°C for 0–4 h. The concentrations of dG, dC, and dT decreased and those of 5-Br-dC and 8-Br-dG increased with increasing incubation time. The reaction was relatively slow, continuing for several hours. The yield of 8-Br-dG with urea was greater than that without urea, whereas the yield of 5-Br-dC with urea was less than that without urea. Figure 3C shows the urea dose-dependence of the reaction with HOBr when 100 µM dC, dG, dT, dA, and 400 µM HOBr in the presence of 0–100 mM urea were incubated in 100 mM potassium phosphate buffer at pH 7.4 and 37°C for 2 h. With increasing urea concentration, the consumptions of all nucleosides and the yield of 5-Br-dC decreased, whereas the yield of 8-Br-dG increased.
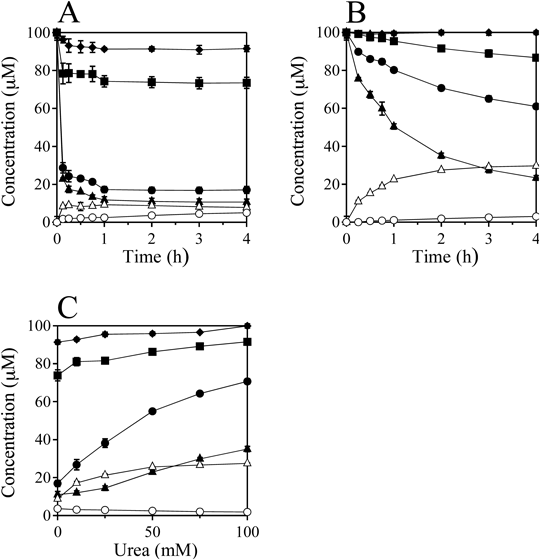
dC (closed circle), dG (closed triangle), dT (closed square), dA (closed rhombus), 5-Br-dC (open circle), 8-Br-dG (open triangle). All the reaction mixtures were analyzed by RP-HPLC. Means ± standard deviation (S.D.) (n = 3) are presented.
To obtain information about the effect of urea on the reactions of nucleosides with HOBr, similar experiments were carried out for urea derivatives and alkylamines instead of urea. Table 1 shows the concentrations of the nucleosides and the products when a solution of 100 µM each of dC, dG, dT, and dA was incubated with 400 µM HOBr in 100 mM potassium phosphate buffer (pH 7.4) at 37°C for 2 h in the presence of the 100 mM additives. The final pH values of the reaction solutions were 7.29–7.41. N-Methylurea and 1,3-dimethylurea showed concentrations of nucleosides and products similar to those with urea, whereas 1,1,3,3-tetramethylurea showed concentrations similar to those without urea. Thiourea inhibited the reactions almost completely. For alkylamines, although methylamine and dimethylamine suppressed the reaction, trimehylamine and tetramethylammounium had little effect.
| Additives | dC (µM) | dG (µM) | dT (µM) | dA (µM) | 5-Br-dC (µM) | 8-Br-dG (µM) |
|---|---|---|---|---|---|---|
| None | 16.9 ± 1.6 | 11.0 ± 1.7 | 73.9 ± 2.9 | 91.3 ± 1.2 | 3.6 ± 0.3 | 8.8 ± 0.6 |
| Urea | 70.7 ± 1.4 | 35.0 ± 1.4 | 91.5 ± 1.5 | 100.0 ± 1.1 | 1.9 ± 0.0 | 27.5 ± 0.2 |
| N-Methylurea | 64.0 ± 4.1 | 27.4 ± 1.7 | 88.2 ± 3.9 | 97.4 ± 2.5 | 1.8 ± 0.1 | 26.8 ± 0.3 |
| 1,3-Dimethylurea | 66.3 ± 3.0 | 28.6 ± 0.5 | 92.3 ± 0.3 | 100.1 ± 0.4 | 2.0 ± 0.2 | 27.8 ± 1.6 |
| 1,1,3,3-Tetramethylurea | 27.9 ± 1.1 | 16.8 ± 0.7 | 83.0 ± 1.7 | 94.9 ± 0.2 | 3.6 ± 0.1 | 9.5 ± 0.3 |
| Thiourea | 100.5 ± 1.3 | 100.2 ± 1.1 | 100.8 ± 1.1 | 100.2 ± 1.3 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Methylamine–HCl | 95.2 ± 0.8 | 93.4 ± 1.4 | 95.5 ± 0.9 | 98.1 ± 0.8 | 0.2 ± 0.1 | 2.2 ± 0.3 |
| Dimethylamine–HCl | 100.0 ± 0.5 | 100.4 ± 0.8 | 101.0 ± 0.8 | 100.1 ± 0.8 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Trimethylamine–HCl | 26.6 ± 2.3 | 24.3 ± 2.1 | 76.0 ± 6.1 | 97.8 ± 0.2 | 4.6 ± 0.1 | 15.9 ± 0.6 |
| Tetramethylammonium–Cl− | 17.2 ± 4.0 | 8.3 ± 2.3 | 81.3 ± 3.2 | 95.1 ± 0.5 | 5.4 ± 0.2 | 5.4 ± 0.9 |
a) Concentrations of the nucleosides and products when a solution of 100 µM each of dC, dG, dT, and dA were incubated with 400 µM HOBr in 100 mM potassium phosphate buffer (pH 7.4) at 37°C for 2 h in the presence of the 100 mM additives. All the reaction mixtures were analyzed by RP-HPLC. Means ± S.D. (n = 3) are presented.
These results suggest that HOBr reacts with urea and its derivatives, resulting in another reactive bromine compound that can react slowly with the nucleosides. To confirm the existence of this long-life reactive species in urea/HOBr solution, HOBr was preincubated without or with urea. Solutions of 400 µM HOBr with or without 100 mM urea were preincubated in 100 mM potassium phosphate buffer (pH 7.4) at 37°C for 48 h in a capped tube. Each preincubated solution was then incubated with 100 µM each of dC, dG, dT, and dA at 37°C for 0–4 h. All the concentrations were determined after the addition of the nucleosides solution (see Experimental). Figure 4A shows the time-dependent changes in concentrations of the nucleosides and the products in the preincubation reaction without urea. Consumption of the nucleosides and the formation of 5-Br-dC and 8-Br-dG were not observed at the detection limit of 0.1 µM. Figure 4B shows the time-dependent changes in the preincubation reaction with 100 mM urea. The concentrations of dG, dC, and dT decreased and those of 5-Br-dC and 8-Br-dG increased with increasing incubation time. Figure 4C shows the urea dose-dependence of the preincubated reaction when a solution of 400 µM HOBr was preincubated in the presence of 0–100 mM urea at pH 7.4 and 37°C for 48 h followed by incubation with nucleosides at 37°C for 24 h. The consumption of nucleosides and the yields of the products increased with increasing urea concentration. Similar preincubation experiments were carried out for the urea derivatives and alkylamines instead of urea. Table 2 shows the concentrations of the nucleosides and the products when a solution of 400 µM HOBr was preincubated with 100 mM additives in 100 mM potassium phosphate buffer at pH 7.4 and 37°C for 48 h, and then incubated with 100 µM each of dC, dG, dT, and dA at 37°C for 24 h. The final pH values of the reaction solutions were 7.26–7.45. N-Methylurea and 1,3-dimethylurea showed higher yields of 5-Br-dC and 8-Br-dG than urea, whereas 1,1,3,3-tetramethylurea and thiourea showed no reactions. Dimethylamine and trimethylamine showed no reaction, although methylamine and tetramethylammonium generated small amounts of the products.
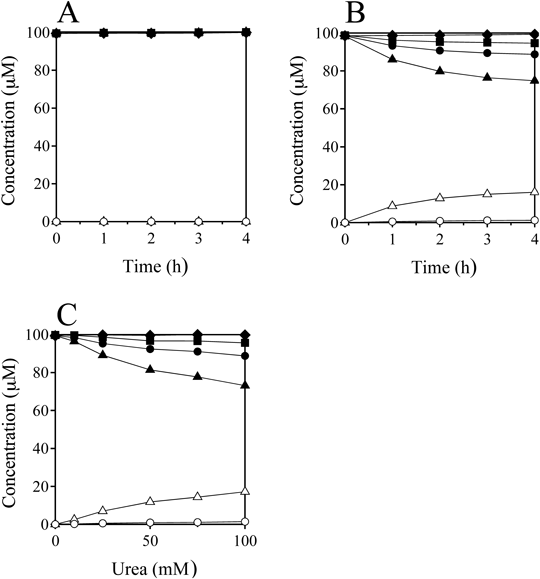
dC (closed circle), dG (closed triangle), dT (closed square), dA (closed rhombus), 5-Br-dC (open circle), 8-Br-dG (open triangle). All the reaction mixtures were analyzed by RP-HPLC. Means ± S.D. (n = 3) are presented.
| Additives | dC (µM) | dG (µM) | dT (µM) | dA (µM) | 5-Br-dC (µM) | 8-Br-dG (µM) |
|---|---|---|---|---|---|---|
| None | 99.5 ± 0.5 | 99.5 ± 0.5 | 99.8 ± 0.1 | 99.4 ± 0.3 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Urea | 88.9 ± 0.2 | 73.1 ± 0.4 | 95.6 ± 0.2 | 99.8 ± 0.3 | 1.4 ± 0.0 | 17.2 ± 0.2 |
| N-Methylurea | 85.6 ± 2.6 | 60.0 ± 3.8 | 95.4 ± 1.6 | 101.4 ± 0.8 | 1.9 ± 0.1 | 25.1 ± 1.6 |
| 1,3-Dimethylurea | 58.6 ± 3.2 | 23.5 ± 2.2 | 85.4 ± 1.3 | 100.1 ± 0.4 | 3.9 ± 0.2 | 32.1 ± 0.5 |
| 1,1,3,3-Tetramethylurea | 101.4 ± 0.7 | 101.3 ± 0.8 | 101.7 ± 0.6 | 101.9 ± 0.7 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Thiourea | 100.1 ± 0.1 | 99.6 ± 0.8 | 100.7 ± 0.1 | 99.4 ± 0.9 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Methylamine–HCl | 99.5 ± 0.1 | 99.1 ± 0.3 | 99.6 ± 0.2 | 100.4 ± 0.4 | 0.1 ± 0.0 | 0.4 ± 0.0 |
| Dimethylamine–HCl | 99.8 ± 0.3 | 99.3 ± 0.2 | 100.3 ± 0.4 | 100.0 ± 0.3 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Trimethylamine–HCl | 100.6 ± 1.0 | 100.9 ± 0.7 | 101.0 ± 0.8 | 101.1 ± 0.9 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Tetramethylammonium–Cl− | 97.2 ± 1.2 | 97.2 ± 1.2 | 98.6 ± 0.7 | 99.1 ± 1.2 | 0.2 ± 0.0 | 1.4 ± 0.0 |
a) Concentrations of the nucleosides and products when a solution of 400 µM HOBr was preincubated with 100 mM additives in 100 mM potassium phosphate buffer at pH 7.4 and 37°C for 48 h in a capped tube, and then incubated with 100 µM each of dC, dG, dT, and dA at 37°C for 24 h. All the reaction mixtures were analyzed by RP-HPLC. Means ± S.D. (n = 3) are presented.
In the presence of urea, nucleosides reacted with HOBr relatively slowly, generating brominated nucleosides (Fig. 3B). The preincubated urea/HOBr solution also reacted with nucleosides, generating brominated nucleosides (Fig. 4B). This implies that urea reacted with HOBr to generate a reactive bromine compound that is considerably stable in a neutral solution. It has been reported that urea reacts with bromine to generate a bromine compound reactive to sugar derivatives, designated as bromocarbamide (bromourea).21) Urea also reacts with N-bromosuccinimide to generate a bromine compound that reacts specifically with tryptophan residues in a protein.22,23) This bromine compound, showing a UV spectrum with λmax 275 nm (ε 265 M−1 cm−1), is also designated as bromourea. In the present study, a similar UV spectrum with λmax 277 nm was observed when the UV spectrum of a mixture of 400 µM HOBr and 100 mM urea in 100 mM potassium phosphate buffer (pH 7.4) at room temperature was measured by UV-Vis spectrometer (data not shown). Bromourea may also form in the present urea/HOBr solution as the reactive bromine compound, which can then react with nucleosides resulting in their bromination.
The present data shown in Tables 1 (the direct reactions) and 2 (the preincubation reactions) suggest the following: For urea and its derivatives, urea, N-methylurea, and 1,3-dimethylurea react with HOBr generating corresponding bromoureas, which have a strong oxidative state (+1) of bromine atoms and can cause nucleoside bromination by electrophilic substitution. Since in the preincubation reactions, the order of consumption of nucleosides and yields of brominated nucleosides was urea < N-methylurea < 1,3-dimethylurea, the order of half-lives of their bromoureas would be urea < N-methylurea < 1,3-dimethylurea. In contrast, 1,1,3,3-tetramethylurea does not react with HOBr, forming no brominated derivatives. Thus, at least one hydrogen atom on the nitrogen atom of urea is required to form the bromoureas, and the substitution of a hydrogen atom on the nitrogen atom of urea by a methyl group increases the stability of bromoureas. For alkylamines, since methyamine and dimethylamine suppress both the direct reaction and the preincubation reaction of HOBr, they should react with HOBr to generate corresponding bromamines that cannot react with nucleosides under the present conditions, since they are too stable to react with nucleosides. It has been reported that the reactivity of bromamines of methylamine and dimethylamine to phenol is much smaller than that of HOBr at neutral pH.24) Since trimethylamine and tetramethylammonium showed no or little effect on both the direct and preincubation reactions, their reactivities to HOBr are low.
In recent years, research on the relationship between chronic inflammation and cancer has increased significantly.25,26) Chronic inflammation can cause cancer, since it leads to modifications of DNA as a consequence of oxidative stress. Inflammatory cells including eosinophils and neutrophils are increased at the sites of inflammation. HOBr was generated by both eosinophils and neutrophils, which protect against invading microorganisms and materials. Excessive or misplaced generation of HOBr can cause host tissue damage, leading to various diseases including asthma, arthritis, and cancer. 8-Br-dG was reportedly detected in urine from healthy volunteers, while urinary 8-Br-dG levels from diabetic patients were 8-fold the levels in healthy volunteers.16) An in vitro study showed that human DNA polymerases incorporated 2′-deoxyguanosine 5′-monophosphate (dGMP), 2′-deoxyadenosine 5′-monophosphate (dAMP), and 2′-deoxythymidine 5′-monophosphate (dTMP) in addition to a one-base deletion opposite an 8-Br-dG residue in an oligodeoxynucleotide, suggesting that 8-Br-dG in DNA is a mutagenic lesion.27)
Although urea at a high concentration (100 mM) significantly affected the reaction of HOBr with nucleosides to form brominated nucleosides under the present reaction conditions, urea at plasma concentration (5 mM) showed little effect (Figs. 3C, 4C). Thus, HOBr generated by MPO and EPO at inflammation sites mainly react with peripheral materials by direct reaction. Since methylamine and dimethylamine efficiently depressed the reaction of HOBr with nucleosides (Tables 1, 2), amines such as the lysine residues of proteins would suppress the reaction of HOBr with DNA. However, a small portion of HOBr may react with urea at inflammation sites, generating bromourea. The formed bromourea, which is more stable than HOBr itself under neutral conditions, can move to distant sites or other organs and may cause damage to their DNA, including the formation of brominated bases. This effect should be particularly likely in the urinary system including the kidney and bladder, since the urea concentration in urine is high (285 mM).
HOBr is generated not only by eosinophils but also neutrophils, which are of central importance in host defense, in the presence of Br− at the plasma concentration. The present study showed that urea can react with HOBr to generate a reactive bromine compound, probably bromourea, which may brominate nucleosides and nucleic acids at sites distant from its generation. This suggests that urea may be of importance in elucidating the genotoxicity of HOBr in humans.
dC, dG, dT, and dA were purchased from Sigma-Aldrich (MO, U.S.A.). 5-Br-dC and 8-Br-dG were obtained from Nacalai Tesque (Kyoto, Japan) and TCI (Tokyo, Japan), respectively. Bromide-free hypobromous acid (HOBr) was prepared by the addition of silver nitrate and subsequent distillation, as previously reported.8,28) The concentration of HOBr was determined spectrophotometrically at 331 nm in 10 mM NaOH using a molar extinction coefficient of 315 M−1 cm−1.28) Water was purified with a Millipore Milli-Q deionizer.
Reaction ConditionsFor the reaction of nucleosides with HOBr (Fig. 1), 1 mL solutions of 100 µM each of dC, dG, dT, and dA were incubated with 400 µM HOBr in the presence or absence of 100 mM urea in 100 mM potassium phosphate buffer at pH 7.4 and 37°C for 2 h. For time-dependence experiments of the reaction of nucleosides with HOBr (Figs. 3A, B), 1 mL solutions of 100 µM each of dC, dG, dT, and dA were incubated with 400 µM HOBr in the absence or presence of 100 mM urea in 100 mM potassium phosphate buffer at pH 7.4 and 37°C for 0–4 h. For urea dose-dependence experiments of the reaction of nucleosides with HOBr (Fig. 3C), 1 mL solutions of 100 µM each of dC, dG, dT, and dA were incubated with 400 µM HOBr in 0–100 mM urea in 100 mM potassium phosphate buffer at pH 7.4 and 37°C for 2 h. For time-dependence experiments of the reactions of preincubated HOBr (Figs. 4A, B), a 900 µL solution including 5 µL of 80 mM HOBr (444 µM) and 200 µL of 500 mM potassium phosphate buffer (111 mM, pH 7.4) with or without 100 µL of 1000 mM urea (111 mM) was incubated at 37°C for 48 h in a 2 mL capped tube (Bio-Bik 2200, Ina Optika, Osaka, Japan). A 100 µL solution of 1 mM each of dC, dG, dT, and dA was then added to the preincubated 900 µL solution, followed by incubation at 37°C for 0–4 h. The total concentrations added to the solution were 100 µM each for dC, dG, dT, and dA, 400 µM HOBr, and 0 or 100 mM urea in 100 mM potassium phosphate buffer. For urea dose-dependent preincubation experiments (Fig. 4C), HOBr was similarly preincubated in the presence of 0–100 mM urea and then the preincubated solution was incubated with nucleosides for 24 h.
HPLC and MS ConditionsThe HPLC system consisted of LC-10ADvp pumps and an SPD-M10Avp UV-Vis photodiode-array detector (Shimadzu, Kyoto, Japan). For the RP-HPLC, an Inertsil ODS-3 octadecylsilane column of 4.6 × 250 mm and particle size 5 µm (GL Sciences, Tokyo, Japan) was used. The eluent was 20 mM ammonium acetate (pH 7.0) containing methanol. The methanol concentration was increased from 0 to 50% during 15 min in linear gradient mode, and maintained at 50% from 15 to 30 min. The column temperature was 40°C and the flow rate was 1 mL/min. The ESI-TOF/MS measurements were performed on a MicroTOF spectrometer (Bruker, Bremen, Germany) in negative mode. The sample isolated by RP-HPLC was directly infused into the MS system by a syringe pump without a column.
Quantitative ProceduresThe concentrations of the products were evaluated from integrated peak areas on RP-HPLC chromatograms detected at 230 or 260 nm, and compared with those for standard solutions of dC, dG, dT, dA, 5-Br-dC, and 8-Br-dG.
The authors declare no conflict of interest.