2019 Volume 67 Issue 7 Pages 717-720
2019 Volume 67 Issue 7 Pages 717-720
This study demonstrates the relation between the redox properties and cytotoxicity of anthraquinone derivatives with a hydroxyl and methoxy group. The redox behavior of the anthraquinone derivatives was initially observed via cyclic voltammetry and their characteristics were investigated using molecular orbital calculations. The cytotoxicity of the anthraquinone derivatives was then evaluated using human leukemia HL-60 and H2O2 resistant HP100 cells, and its correlation with the redox properties of these compounds was investigated. Therefore, it was suggested that the anthraquinone derivatives express cytotoxicity through H2O2 production, and that generation of the oxidized radical form influences their cytotoxicity.
Anthracycline antibiotics such as doxorubicin (DOX) are important antitumor agents in clinical applications, and much research is devoted to studying their derivatives. It is known that DNA damage occurs by active oxygen generation in the mechanism of the antitumor activity of DOX. Two pathways were proposed for this mechanism of DNA damage. In one, reactive oxygen species (ROS) are generated by reducing DOX using reduced nicotinamide adenine dinucleotide phosphate (NADPH)-P450 reductase to produce reduced semiquinone radicals and oxygen. In the other, DOX is oxidized using metal ions to form oxidized semiquinone radicals, and ROS are generated using a complex of a metal ion reductant and oxygen (Fig. 1).
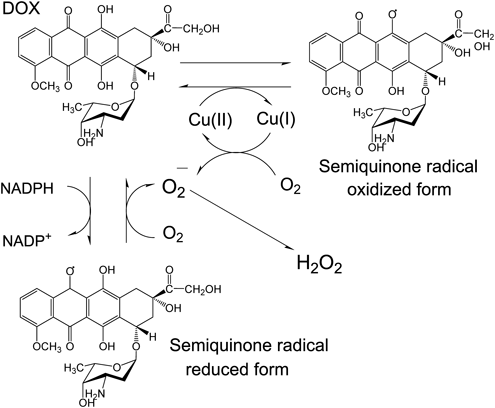
Based on previous reports, some anthracycline antibiotics including DOX, produce oxidized semiquinone radicals in the presence of Cu(II), oxidatively damaging DNA.1–3) The active species in this damaging mechanism are believed to be Cu(I) and H2O2 complexes produced by redox reactions.1–3) Since this oxidative mechanism occurs at a lower concentration than that of the reduced semiquinone radical in the presence of NADPH-P450 reductase,1) an injury mechanism occurs in the presence of Cu(II); so, it is suggested that production of oxidized radical forms might be key to the DNA damage effect.
Conversely, the redox activity exhibited by the anthracycline antitumor agent originates from the basic anthraquinone structure. Many studies on the redox properties of this anthraquinone derivative have been reported.4–8) Most of these relate to anion radicals and include changes in reduction potential because of intramolecular and intermolecular hydrogen bonds,5) and disproportionation/comproportionation reactions of reductants and dianions of anthraquinone7); there are no reports on the properties of oxidized radicals.
In this study, we focused on substances that have an 9,10-anthraquinone (AQ) structure as similar as DOX and would be considered to generate both reduced and oxidized radicals. Specifically, the redox behavior of the six types of anthraquinone derivatives shown in Table 1 were observed, and their properties were evaluated using molecular orbital calculations. The cytotoxicity of the anthraquinone derivatives was then evaluated using human leukemia HL-60 and H2O2 resistant HP100 cells, and we subsequently investigated the correlation with the redox properties of these compounds.
| Compounds | R1 | R2 | R3 | R4 | |
|---|---|---|---|---|---|
| AQ | H | H | H | H |  |
| 1H | OH | H | H | H | |
| 1M | OCH3 | H | H | H | |
| 1H4M | OH | OCH3 | H | H | |
| 1H5M | OH | H | OCH3 | H | |
| 1H8M | OH | H | H | OCH3 |
The AQ, 1-hydroxyanthraquinone (1H), 1,4-, 1,5-, and 1,8-dihydroxyanthraquinone (1H4H, 1H5H, and 1H8H) were purchased from Tokyo Chemical Industry Co., Ltd. (Japan) and purified by repeated sublimation under reduced pressure immediately before use. The 1-methoxyanthraquinone (1M), 1-hydroxy-4-methoxyanthraquinone (1H4M), 1-hydroxy-5-methoxyanthraquinone (1H5M), and 1-hydroxy-8-methoxyanthraquinone (1H8M) were obtained by methylating 1H, 1H4H, 1H5H, and 1H8H, respectively. The methylations were performed using dimethyl sulfate as an electrophilic methyl source, and the obtained filtrate was concentrated and separated using a TLC plate (benzene, silica gel 60 F254, 2 mm), recrystallized from benzene, and dried sufficiently under reduced pressure before use. The products were confirmed via elemental analysis.
Electrochemical Measurements and CalculationsCyclic voltammograms (CVs) were recorded at room temperature (approx. 25°C) under nitrogen atmosphere using an ALS 600C model electroanalytical system (BAS Co., JAPAN) via a three-electrode system comprising a 3 mm diameter glassy carbon electrode (GC) as a working electrode, a platinum wire counter electrode, and a Ag/Ag+ reference electrode (BAS Co., JAPAN). The best available anhydrous grade CH3CN from Aldrich Chemical Co. (U.S.A.) was used as a solvent. Further details of our electrochemical measurements are given in a previous paper.5,9) Quantum chemical calculations were performed at Hartree–Fock (HF) and density functional theory (DFT) levels as implemented in the Gaussian 03 program. Geometry optimization for AQ and its derivatives are performed by employing the standard split-valence double-ζ 6–31G basis sets augmented by the polarization d and diffusion orbitals 6–31 + G(d).10,11)
Cell Culture and Treatment with AqsHP100 cells were derived from human leukemia HL-60 cells by repeated exposure to H2O2, followed by outgrowth of viable cells. HP100 cells were approximately 340 times more resistant to H2O2 than HL-60 cells.12) The catalase activity of HP100 cells is 18 times that of HL-60 cells.13) HL-60 and HP100 cells were grown in RPMI 1640 supplemented with 6% fetal bovine serum (FBS) at 37°C under 5% CO2 in a humidified atmosphere. The cells (0.5 × 106 cells/mL) were then treated with the indicated concentrations of AQs (0.5% dimethyl sulfoxide (DMSO)).
Determination of Cytotoxicity Induced by AQs in HL-60 and HP100 CellsA lactate dehydrogenase (LDH) activity assay kit (CytoTox-ONE™ Homogeneous Membrane Integrity Assay, Promega Co., U.S.A.) was used to measure cytotoxicity in cultures: 5.0 × 104 cells were seeded in each well of 96-well culture plates. After treating with AQs for 24 h, the cytotoxicity was determined according to the manufacturer’s instructions.
CVs of AQ, 1H, 1M, and 1HIM are shown in Figs. 2a–d, respectively. These CVs (Fig. 2) were scanned in the negative and positive directions (with the initial potential set to 0 V). When scanning in the negative direction, AQ and its derivatives were reduced and displayed two cathodic peaks corresponding to the generation of anion radicals and dianions, typical of quinones. Comparing the negative-scan CV of 1H with that of AQ indicated that 1H receives electrons more readily than AQ, generating anion radicals and dianions. (see Table 2 for details of the results.) The difference between AQ and 1H is explained by the formation of intramolecular hydrogen bonds between OH and C=O, which stabilizes the anion radicals and dianions produced. However, a negative-scan CV of 1M showed two reduction waves, which were more negative than those for AQ. This negative shift is presumed to be because of the substituent effect of the methoxy group causing the π electron density of the entire molecule to increase, so that 1M hardly reduces than AQ. Additionally, the negative-scan CV of 1H4M was similar to that of 1H; thus, the effect of intramolecular hydrogen bonding of the hydroxyl group during the reduction of 1H4M was greater than the influence of the methoxy group.

| Negative-scan CV | LUMO | Positive-scan CV | HOMO | |
|---|---|---|---|---|
| Ecp1 | /a.u. | Eap1 | /a.u. | |
| E vs. Fc2+/Fc3+ | E vs. Fc2+/Fc3+ | |||
| 1H4M | −1.252 | −0.11540 | 0.998 | −0.22801 |
| 1H5M | −1.241 | −0.11563 | —a) | −0.24338 |
| 1H8M | −1.251 | −0.11558 | —a) | −0.24148 |
| 1M | −1.406 | −0.10930 | 1.635 | −0.24585 |
a) Not observed.
In the positive-scan CVs, no electrochemical responses were observed for AQ and 1H, but large oxidation peaks were observed for 1M and 1H4M. This appears to be caused by the π electron density of the entire molecule increasing because of the methoxy group, making it easier to oxidize.14) Also, for 1H4M, the oxidation peak potential is negative by as much as 150 mV than that of 1M, indicating that 1H4M is more easily oxidized than 1M. Therefore, to clarify the synergistic effect of the methoxy and hydroxyl groups in the oxidation process, CVs of 1H5M and 1H8M were recorded and compared with the result for 1H4M.
Comparison of Redox Behavior of 1H4M, 1H5M, and 1H8MBoth negative and positive-scan CVs of 1H4M, 1H5M, and 1H8M are shown in Figs. 3a–c, respectively. In the negative scans, CVs of 1H4M, 1H5M, and 1H8M were observed at almost the same potential, and altering the substitution position of the methoxy group produced no observable effect. However, the positive scan indicated that only 1H4M showed an oxidation peak, and 1H5M showed a large oxidation response but no peak current. In the case of 1H5M, it is considered that the peak waveform of the oxidation current was not obtained because the generated oxidation radical is unstable and is not saturated on the electrode surface. Here, the current observed on the positive direction of ca. 1.9 V is the oxidation current of the solvent itself, which is the upper limit of the measurable range. It was suggested that the effect of the methoxy group, which is an electron donor, greatly contributed to the oxidation response based on the results shown in Fig. 1; however, this was found to be significantly influenced by the coexisting OH group. To determine why oxidative behaviors were different among 1H4M, 1H5M and 1H8M, molecular orbital calculations were performed, and the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energies obtained are listed in Table 2. In order to make discussion, CVs and the molecular orbital calculation results of 1M are also shown in Table 2. As shown in Table 2, the LUMO energies of 1H4M, 1H5M, and 1H8M are almost identical, and almost unaffected by differences in the position of the substituent. This is consistent with the similarity of the first half-wave reduction potentials. Conversely, for the HOMO energy, that of 1H4M is larger than those of 1H5M and 1H8M; i.e., the HOMO is shallow. This indicates that 1H4M is more readily oxidized than the other compounds, consistent with the experimental result showing that only 1H4M exhibits an oxidation peak. These results showed that, even for compounds with the same substituent, the HOMO energy was influenced by the substitution position. On the other hand, as shown in Table 2, the HOMO energies of 1M, 1H5M and 1H8M were found to be almost the same, but the oxidative behavior was quite different. These results suggested that the electrochemical measurement is one of the important way to understand the oxidative behavior of those.

The cytotoxicity of AQs was evaluated by cell death using LDH as an indicator, with human myeloid leukemia cells HL-60 and HL-60-derived HP100 cells exhibiting high catalase activity.15) Figure 4 shows the cytotoxicity results for 1H4M (a), 1H5M (b), 1H8M (c), and 1M (d). As shown in Fig. 4a, the toxicity to HL-60 increased with the increase in 1H4M concentration. However, the toxicity to HP100 was less than half the toxicity to HL-60, indicating that H2O2 is involved in the process leading to cell death. Specifically, the cytotoxicity seen in HL-60 is thought to be due to ROS originating from electron transfer to oxygen accompanying the formation of reduced or oxidized 1H4M radicals.

HL-60 and HP100 cells (0.5 × 106 cells/mL) were treated with AQs at 37°C for 24 h. After treating with AQ derivatives, the cytotoxicity was analyzed using the LDH activity assay kit (CytoTox-ONE™ Homogeneous Membrane Integrity Assay, Promega) according to the manufacturer’s instructions. The data were expressed as mean ± standard deviation (S.D.) (n = 3). * p < 0.05 and ** p < 0.01, vs. control; # p < 0.05 and ## p < 0.01, HL-60 vs. HP100 by Student’s t-test.
Conversely, when the same experiment was performed using 1H8M, as shown in Fig. 4c, the toxicity to HL-60 was lower than with 1H4M, and no significant 1H8M concentration dependence was observed. Figures 4b and d showed the results of cytotoxicity experiments with 1H5M and 1M, respectively. Although 1H5M and 1M showed a similar concentration dependence to 1H4M, the cytotoxicity of 1H5M and 1M were lower than that of 1H4M. Additionally, as shown in Fig. 4, the toxicity to HP100 was less than half the toxicity to HL-60, suggesting that H2O2 is involved in cell death with 1H4M, 1H5M, and 1M. As shown in Fig. 1, there are two pathways by which anthraquinone derivatives generate H2O2 through the formation of reduced and oxidized radicals. However, in the case of DOX, it was reported that, relatively high concentration of that was required for the expression of cytotoxicity via reduction radical formation, while the effect via oxidative radical formation was appeared at low concentration.1) Therefore, under this experimental condition, it is reasonable that very low cytotoxicity was observed in 1H8M which shows no oxidative response.
CV measurements, molecular orbital calculations, and cytotoxicity tests were performed on 1H4M, 1H5M, and 1H8M, as anthraquinone derivatives. Although the reductive behavior of these compounds were identical, the oxidative properties were different depending on the position of the substituent. And 1H4M which showed the oxidation peak and 1H5M which showed the oxidation response showed the higher cytotoxicity by H2O2 production than 1H8M. In addition, 1 M which showed oxidation peak current, that means oxidized radical formation, also showed cytotoxicity. From these results, it was suggested that the generation of oxidized radicals plays an important role in the cytotoxic effect.
The authors declare no conflict of interest.