2019 Volume 67 Issue 8 Pages 816-823
2019 Volume 67 Issue 8 Pages 816-823
In this present study a new co-crystals of zoledronic acid with DL-tartaric acid and nicotinamide has been developed with improved solubility. Zoledronic acid is a class III drug with poor oral bioavailability due to its poor permeability and low aqueous solubility; hence an attempt has been made to improve its solubility by co-crystallization technology. Pharmaceutical cocrystals are multi-component crystals with a stoichiometric ratio of active pharmaceutical ingredients (APIs) and cocrystal coformers (CCFs) that are assembled by noncovalent interactions such as hydrogen bonds, π–π packing, and Vander Waals forces. In this study the coformers selected were DL-tartaric acid and nicotinamide based on ease of hydrogen bond formation. The co-crystal of zoledronic acid with DL-tartaric acid were prepared in three ratios (1 : 1, 1 : 2 and 2 : 1) by slow solvent evaporation method and with nicotinamide in 1 : 1 ratio by dry grinding method. The formation of co-crystal was confirmed by powder X-ray diffractometry (PXRD), differential scanning calorimetry (DSC) and Fourier transform (FT)IR. The dynamic solubility of co-crystals with DL-tartaric acid in the ratios 1 : 1, 1 : 2 and 2 : 1 increased by fold as compared to pure drug.
Zoledronic acid is the third generation bisphosphonate which is used predominantly as an anti-resorptive agent in the treatment of osteoporosis, hypocalcaemia, Paget’s disease, and inhibition of bone metastasis.1) Novartis originally developed and marketed zoledronic acid as the monohydrate under the brand names of Zometa® and Reclast®. It is only available in parental dosage forms at a dose of 4 or 5 mg for infusion over at least 15 min.2) Intravenous dosage forms of zoledronic acid leading to renal failure, due to the complex formation with blood calcium, which is being retained in the kidney. Renal deterioration, hypophosphatemia, hypocalcemia and hypomagnesemia are uncommon but can be issues with zoledronic acid due to its high affinity to the bone tissue. Risk of pain, tissue irritation and inflammation at the site of injection were reported. In case of local delivery of zoledronic acid like subcutaneous or intramuscular administration. For these reasons, there is clear need for novel oral dosage form development for zoledronic acid.1)
Oral delivery of zoledronic acid became challenging due to its poor oral bioavailability (<1%) and low gastrointestinal permeation because of formation of insoluble metal complexes. Various attempts have been made to increase the aqueous solubility, permeability and oral bioavailability by crystallization,3) formation of metal salts, amorphous forms of zoledronic acid, and by using amino acids2) and medium chain fatty acids.4)
Cocrystallization technique can be used as one of the technique because of its commercial feasibility and also to increase the diversity of solid-state forms of a drug even for non-ionizable drugs, and alter pharmaceutical properties by modification of stability, mechanical behaviour, solubility, dissolution rate, and bioavailability.5,6) By using the novel approach of generating cocrystals of zoledronic acid with suitable coformers there is an opportunity to improve the solubility and (or) permeability of active pharmaceutical ingredient (API) resulting in novel dosage forms.
The aim of the current study is to develop zoledronic acid cocrystals, by using suitable coformers include DL-tartaric acid, nicotinamide and confirmation of cocrystal formation by solid state characterization (Fourier transform (FT) IR, differential scanning calorimetry (DSC), and X-ray diffraction (XRD)) and analysing the increased solubility of the prepared cocrystal by HPLC method.
Zoledronic acid (ZOL) was obtained as a gift sample from Shilpa Medicare Ltd., Vizag, Andhra pradesh, India. DL-Tartaric acid and nicotinamide are purchased from S.D. FINE CHEM LTD., Boisar. Acetonitrile (HPLC grade) methanol was provided by Finar chemicals, Ahmedabad. Di-potassium hydrogen phosphate and tetra butyl ammonium hydrogen sulphate were purchased from Merck specialities pvt Ltd., Mumbai, India.
MethodsSelection of CoformerOne of the main challenges in cocrystal development is coformer selection that is compatible with specific API. To effectively prioritize coformer for the cocrystal screening approaches like supramolecular synthon approach, Cambridge structural database and Hansen solubility parameter. In the current study, supramolecular synthon approach is used for coformer selection which includes the following steps a) Choosing the target API. b) Knowledge of hydrogen bonding between the API and coformer to find the complementary functional groups for API which is capable of forming a hydrogen bond.7)
Zoledronic acid (target molecule) has 5 hydrogen bond donors and 8 hydrogen bond acceptors. DL-Tartaric acid (coformer-1) has 4 hydrogen bond donors and 6 hydrogen bond acceptors, likewise nicotinamide (coformer-2) 1 hydrogen bond donors and 2 hydrogen bond acceptors (Fig. 1).
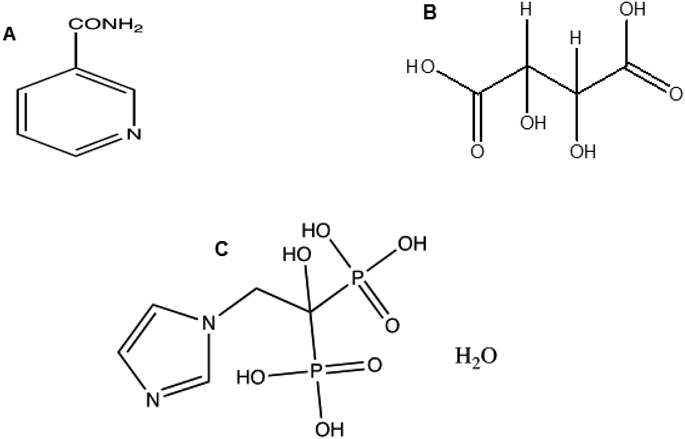
Based on this information, there is high probability of hydrogen bonding for zoledronic acid with coformer-1 and coformer-2.
Preparation of CocrystalCocrystals were prepared by various techniques like slow evaporation, slurry conversion and dry grinding.8) In this study, cocrystals were prepared in the molar ratios 1 : 1, 1 : 2, 2 : 1 of zoledronic caid and DL-tartaric acid, respectively through slow solvent evaporation method. Required amounts of API and coformer are accurately weighed and saturated solutions are prepared separately by adding little amount of solvent (methanol–water 90 : 10% v/v) and few drops of 0.1 N sodium hydroxide solution to increase the solubility of zoledronic acid in the solvent. The two solutions are then mixed and evenly spreaded on a petridish covered by aluminium foil with holes. The solution was allowed to evaporate at room temperature until the sample is completely dry.
Zoledronic acid and nicotinamide cocrystals of 1 : 1 molar ratio were prepared by dry grinding method. Desired amounts of zoledronic acid and nicotinamide are weighed and triturated in azite motor and pestle with constant speed. Later the cocrystals were collected for further analysis.
Solid State Characterization Studiesa) FTIRShimadzu FTIR: 8300 system (Kyoto, Japan) was used to obtain the spectra for the developed complexes, physical mixture and pure zoledronic acid. The infrred spectrum was collected over a range of 4000–500 cm−1. (Twenty five scans, resolution 4 cm−1). Preparation of the disc involved dispersing the sample in KBr and then grindingwith applied pressure (1000 psig).
b) DSCShimadzu TA: 60WS thermal analyzer was used to carryout thermal analysis. Required amount of sample (about 5 mg) were placed in aluminium pans (0.1 mm thickness) and then crimped with an aluminium lid. The test ran at the temperature range of 25–300°C with a temperature increase of 10°C/min. All prepared cocrystals and physical mixtures of API and coformer were analysed.
c) XRDRigakuminiflex 600 X-ray diffractometer (Rigaku Co., Tokyo, Japan), operated at 600 W (X-ray tube), with a fixed tube current (15 mA) and a fixed voltage (40 kV) was used to achieve the X-ray powder diffraction pattern. The X-ray beam (diffracted) was monochromated by a graphite monochromator and detection was carried out by a standard scintillation counter. Diffraction intensities were measured over a range of 5–80° (2θ).
Saturated Solubility StudiesShake flask method was used to determine the equilibrium dynamic solubility of pure zoledronic acid, physical mixture and co-crystals of zoledronic acid. Phosphate buffer pH 6.8 (IP) was used to prepare samples by adding excess quantities of each API or physical mixture or cocrystals into injection vials containing 3 mL of the solvent. Thereafter all the prepared samples were shaken in Labtop orbital shaking incubator maintaining the temperature of 37°C at 100 rpm. After 24 h, samples were collected and centrifuged at 10000 rpm maintaining the temperature of 37°C. Then, separate the clear supernatant solution and sufficiently diluted to fall in calibration range before injecting in the HPLC system. The samples were analyzed using the developed HPLC method. The samples were quantified using developed HPLC method with photo diode array (PDA) detector.
HPLC MethodCocrystals were analysed by Shimadzu HPLC (Kyoto, Japan), system controlled by LC solution software and equipped with a LC-10 ADVP (quaternary pump), SIL-10 ADVP (Auto injector), a SPD M-10A VP (photo diode array detector) and SPD-10Avp (UV detector). The separation was carried out on a C18 phenomenex (5.0 micron, 150 × 4.6 mm) column. 25 : 75% (v/v) methanol: buffer (pH 7.00) in the ratio was used as mobile phase (MP). Prepare mobile phase by adding 4.5 g of di-potassium hydrogen phosphate and 2.0 g of tetra butyl ammonium hydrogen sulphate in 1000 mL of milli-Q water, to this add triethyl amine (1 mL) and pH was made to 7.0 ± 0.02 with 10% ortho phosphoric acid. Filter buffer through a membrane (0.45 µ) using a filtration assembly (vacuum) and then degassed by ultrasonication for ten minutes prior to use. A flow rate of 1.0 mL/min was maintained and the column was maintained at 50°C. PDA detector was used to estimate Zoledronic acid. Sample was injected (20 µL) and allowed to run time for 10 min.9–12) Zoledronic acid have a retention time of 2.6 min (Fig. 2).

The difference in the melting pattern of co-crystals was observed when compared to pure Zoledronic acid (210–216°C). In case of zoledronic acid, the existence of several hydration degrees is possible and the existence of drug is affected by properties of solid form and many undesired transitions may occur. Thermogram of zoledronic acid shows two weak endothermic peaks at 71.85 and 133.61°C which indicates the presence of water molecule and anhydrous polymorphic form of the monohydrated zoledronic acid, respectively which is obtained during the process.13) The zoledronic acid and DL-tartaric acid complex of 1 : 1 shown endotherms at 130.04 and 210.81°C which are slightly different from core zoledronic acid and DL-tartaric acid endotherms at 216 and 222°C respectively. Characteristic endotherms are visible in zoledronic acid and nicotinamide complex of 1 : 1 at 131.06 and 156.31°C while they are absent in DSC thermograms of zoledronic acid, physical mixtures and nicotinamide (136.21°C). Thus, it was concluded that zoledronic acid was interacted with the coformers used for cocrystal preparation (Figs. 3, 4 and Table 1).
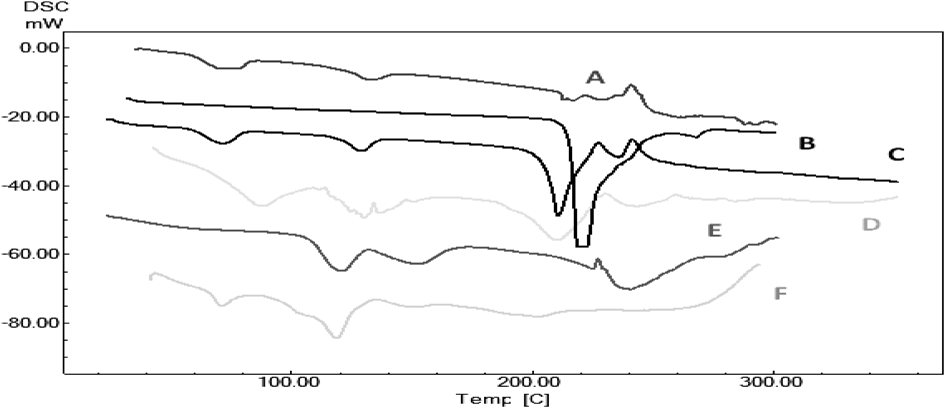
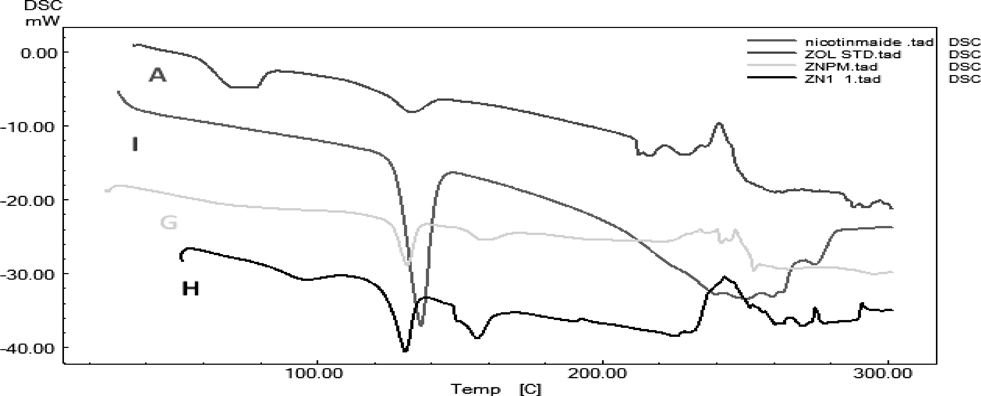
| Sample | Melting endotherm |
|---|---|
| ZOL | 216.94°C |
| DL-Tartaric acid | 222.46°C |
| ZDPM | 210.88°C |
| ZD11 | 130.04 and 210.81°C |
| ZD12 | 121.14°C |
| ZD21 | 118.83°C |
| Nicotinamide | 136.21°C |
| ZNPM | 131.76 and 158.93°C |
| ZN11 | 131.06 and 156.31°C |
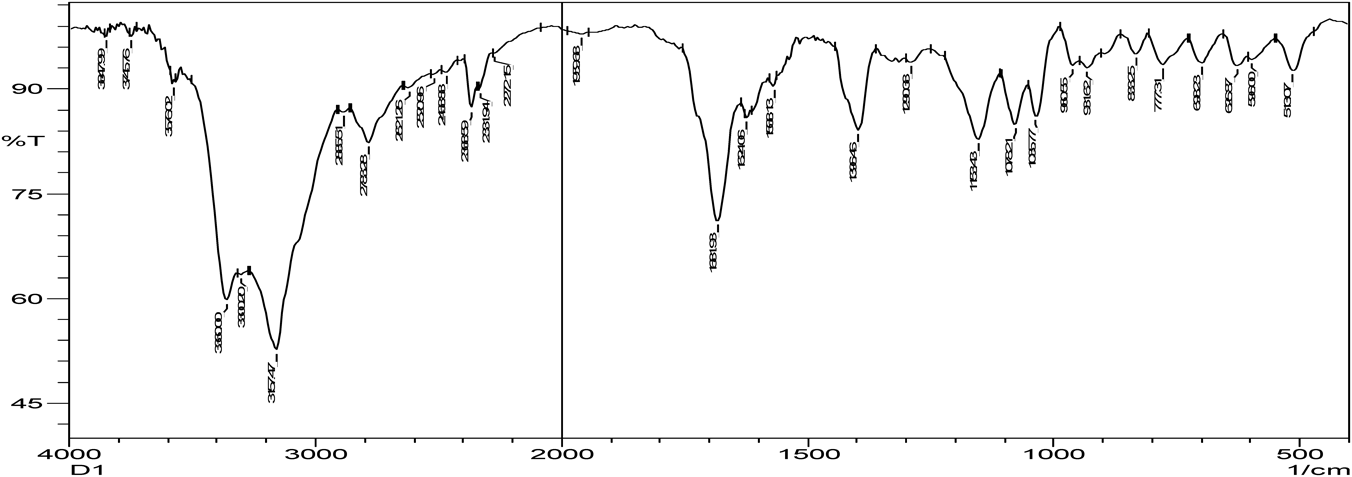
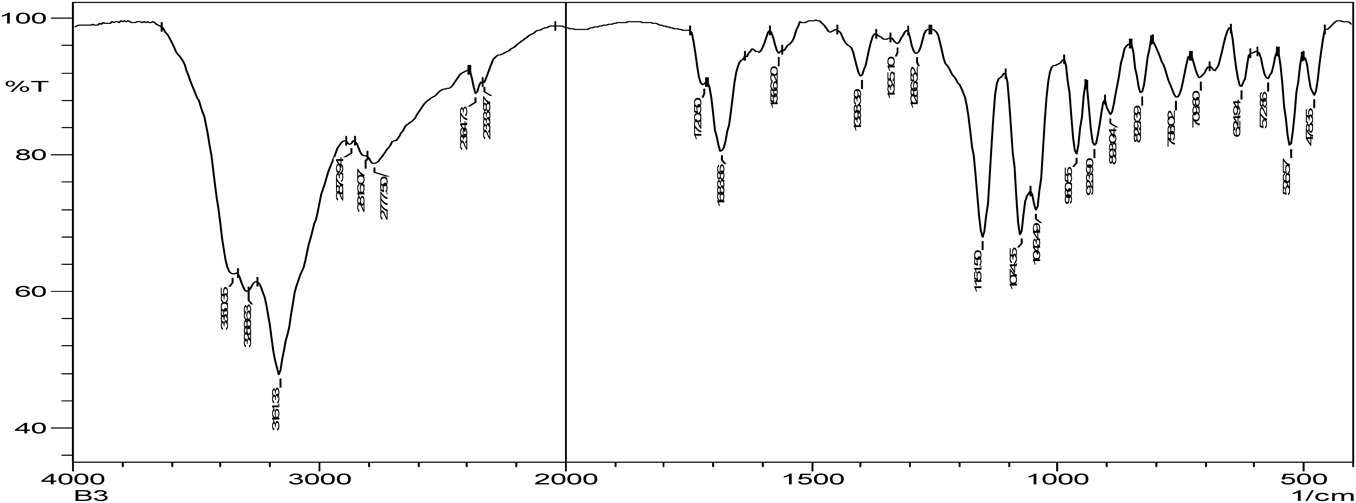
Hydrogen bonding is the characteristic of cocrystal formation. FTIR spectra revealed that co-crystals were formed as the spectra was different with broadening at frequency range of OH group (3410.15–3145.90 cm−1) when compared to that of pure zoledronic acid. The ketone group (C=O) in DL-tartaric acid shows a peak at 1739 cm−1. On forming co-crystals of zoledronic acid with DL-tartaric acid in 1 : 1 the intensity of C=O ketone group (1739 cm−1) peak in DL-tartaric acid decreased in the co-crystal and the peak broadening of OH group indicates intermolecular hydrogen bond formation as C=O is an acceptor and OH is a donor. In case of zoledronic acid and DL-tartaric acid of 1 : 2 and 2 : 1, the C=O ketone (1739 cm−1) and OH group at (3574.10 cm−1) were disappeared which are present in DL-tartaric acid and zoledronic acid, respectively, indicates formation of hydrogen bonding (Figs. 5–9).
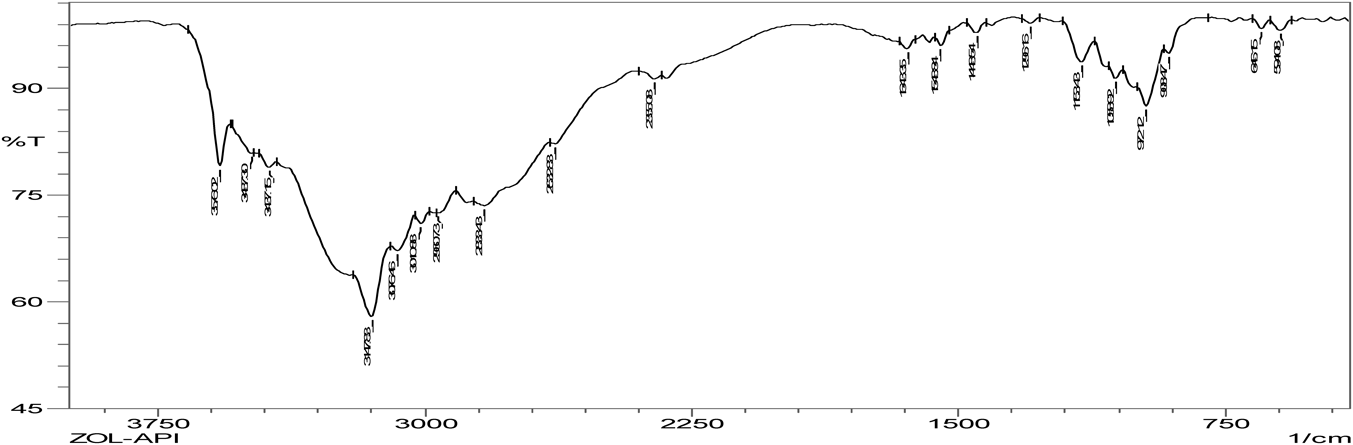
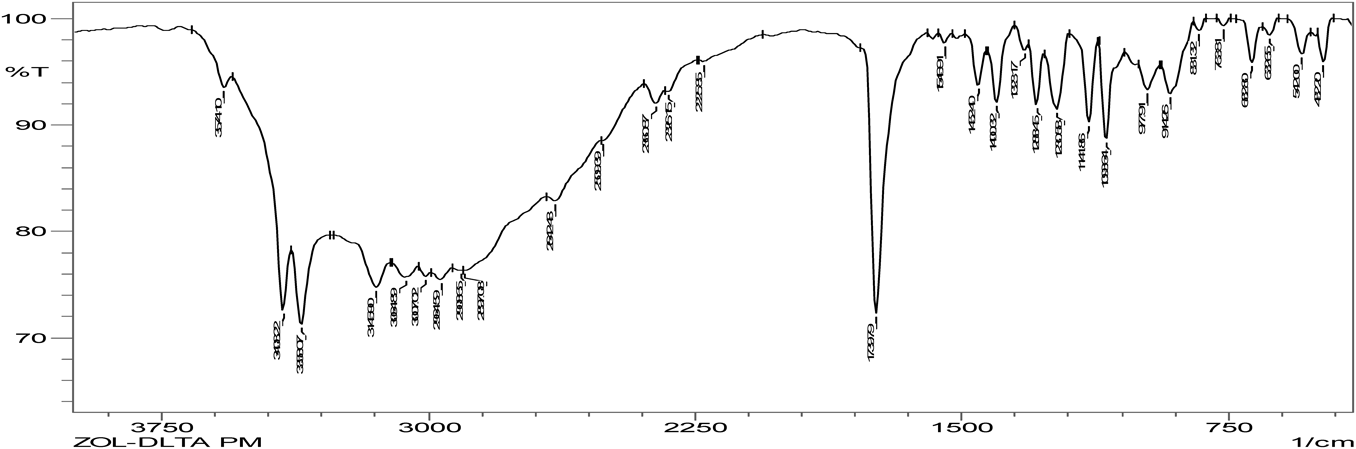
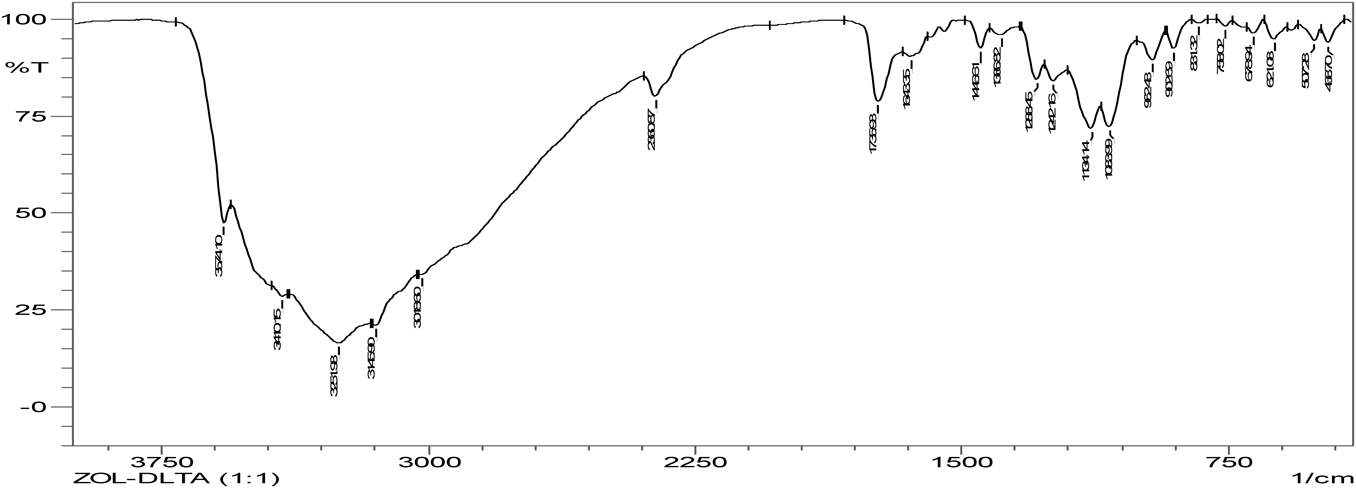
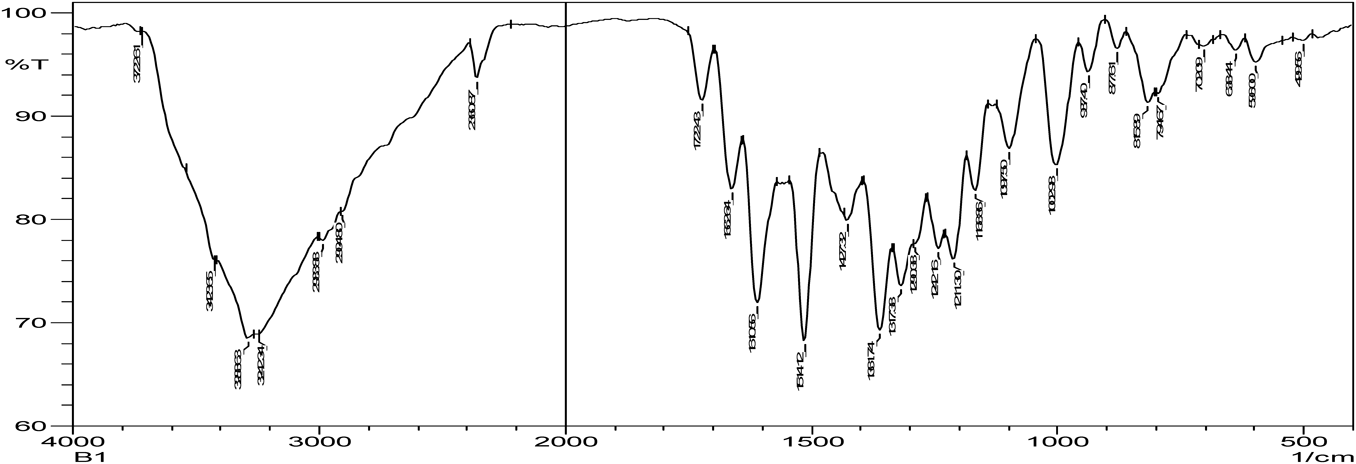
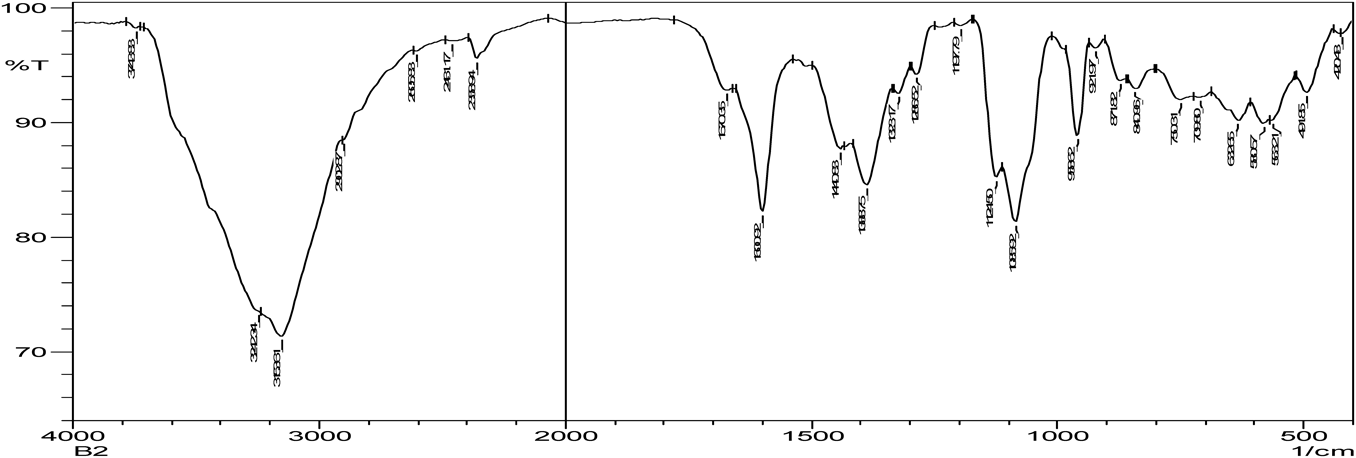
The C=O amide group in nicotinamide shows a peak at 1681.93 cm−1. In the zoledronic acid and nicotinamide in 1 : 1 ratio,. the intensity of C=O amide group (1681.93 cm−1) was decreased and the OH group at (3574.10 cm−1) was disappeared indicating hydrogen bond formation. (Figs. 5, 10, 11) Thus the results of IR spectrum represent the formation of hydrogen bonding which is characteristic of cocrystals.
The intensity of PXRD pattern for zoledronic acid API at 2θ angle of 25.3652 was found to be 100%. Nicotinamide and DL-tartaric acid shown 100% intensity at 25.8724 and 21.9581 over 2θ angle where as, cocrystals of zoledronic acid with DL-tartaric acid of 1 : 1, 1 : 2 and with nicotinamide of 1 : 1 showed 100% at 37.5004, 20.1388 and 34.7537 over 2θ angle. The shifting of 100% intensity in comparision with pure drug is mainly due to interplanar distance (d angle) indicating different arrangement of molecules. it confirms the development of new crystalline phase (Figs. 12–17 and Table 2).
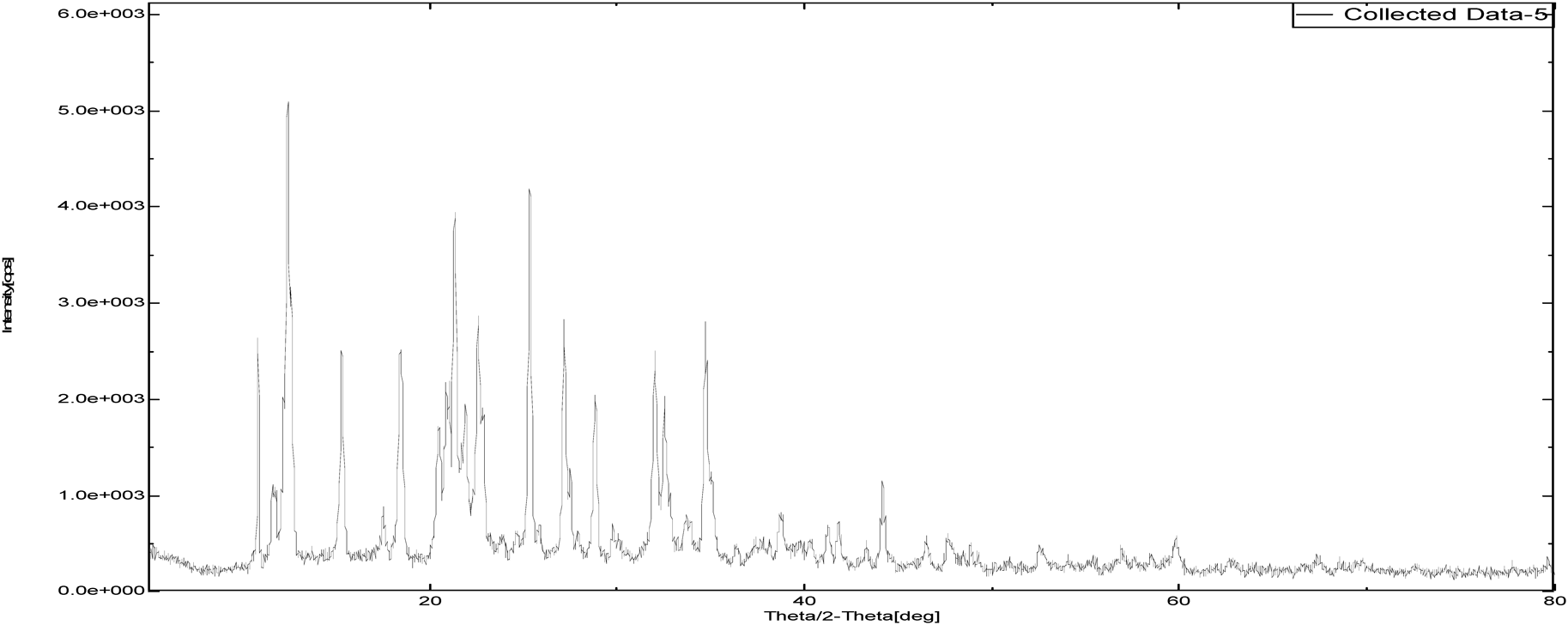
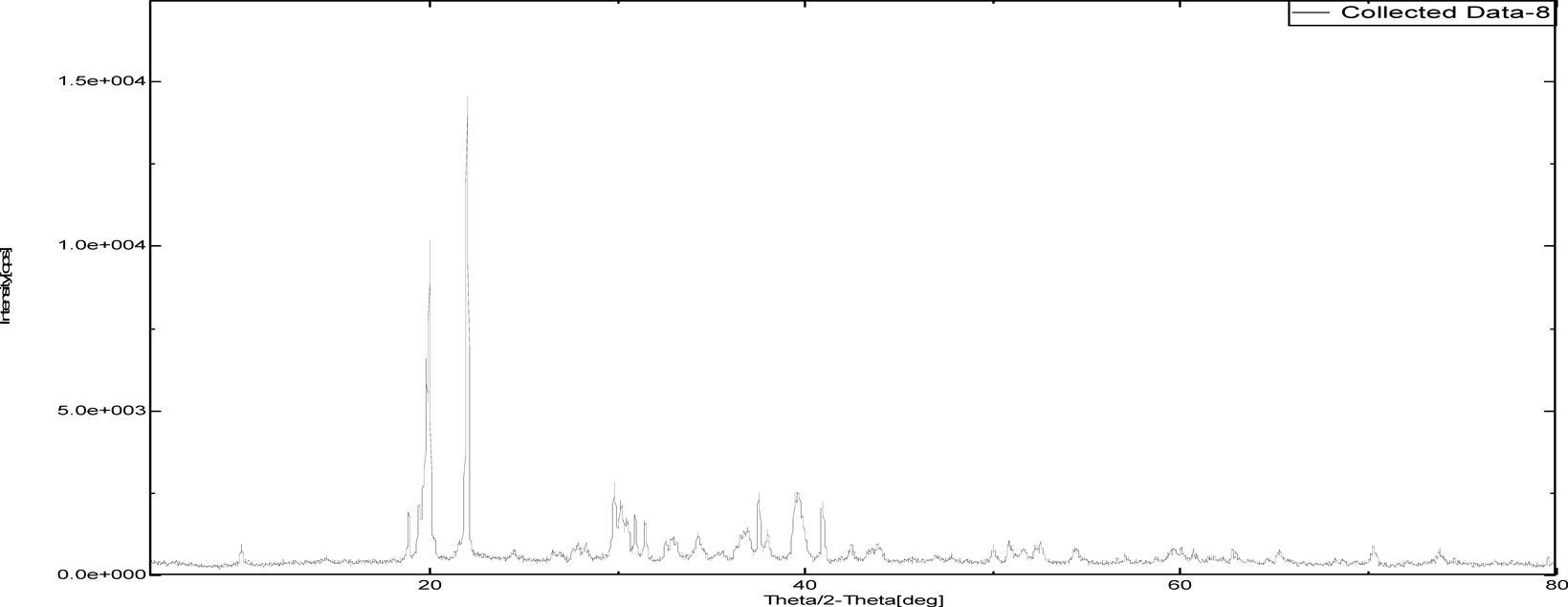
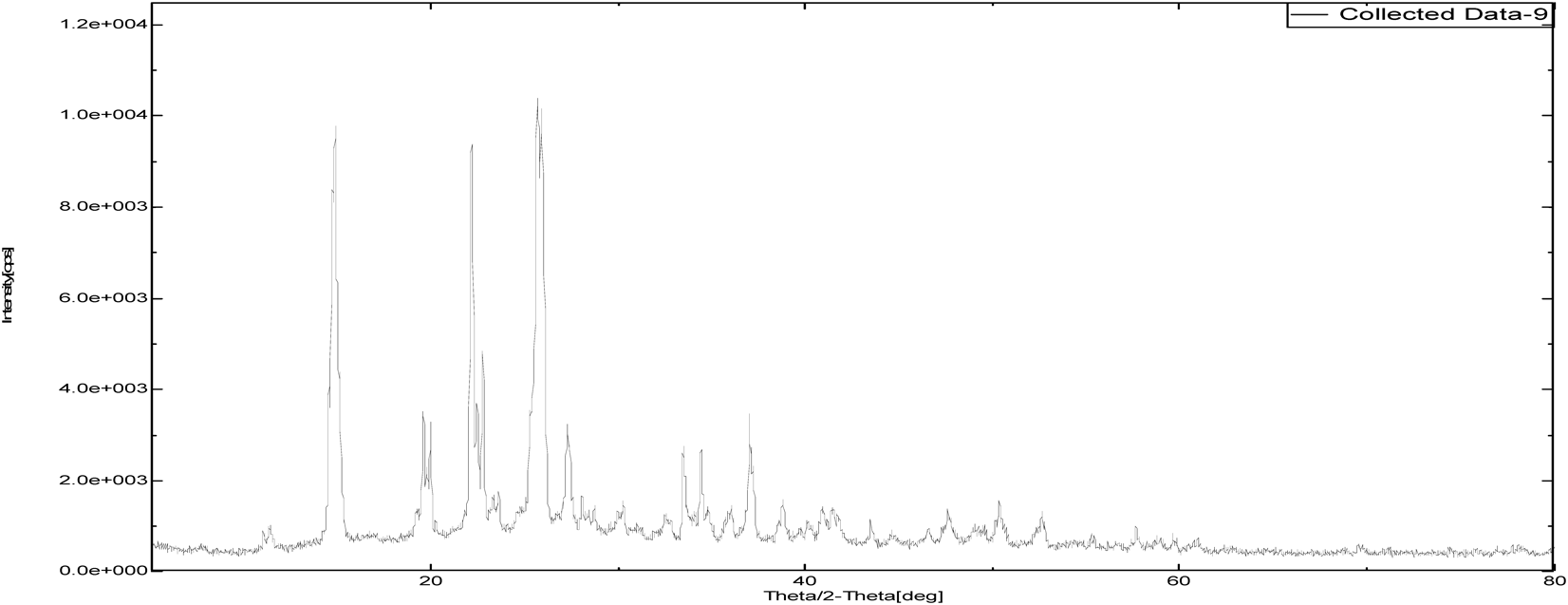

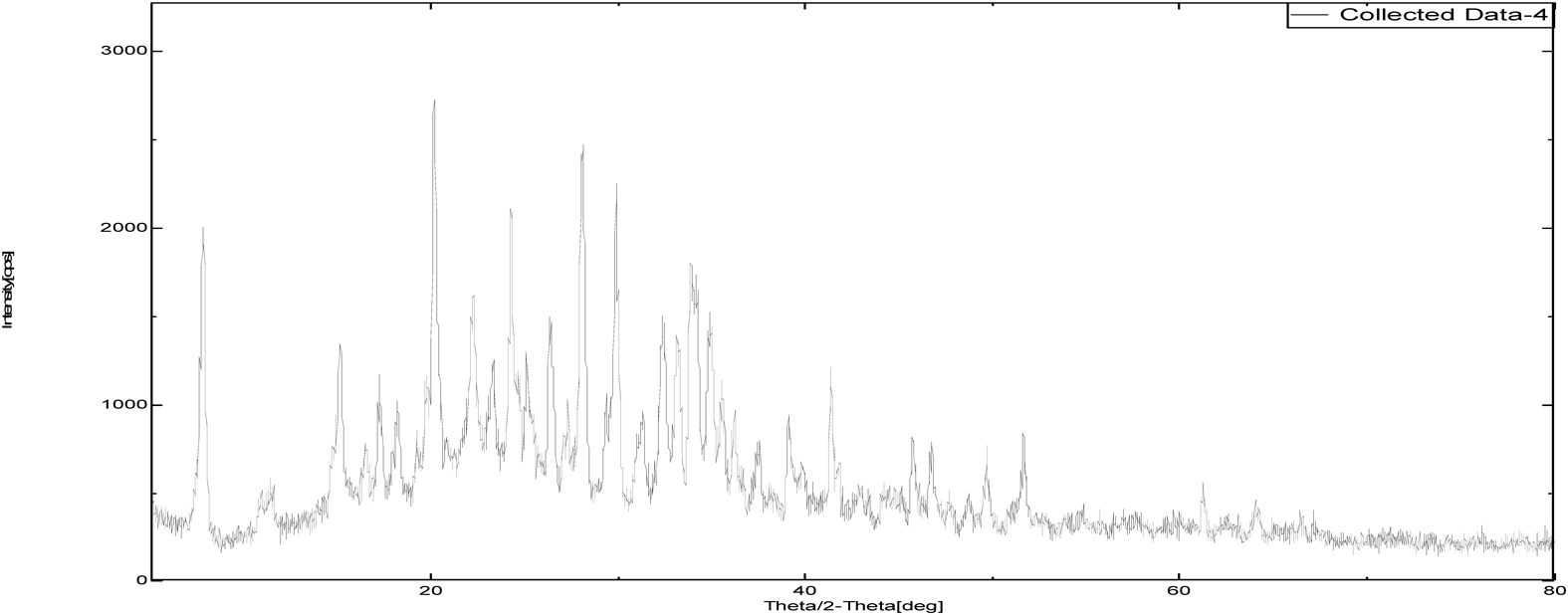
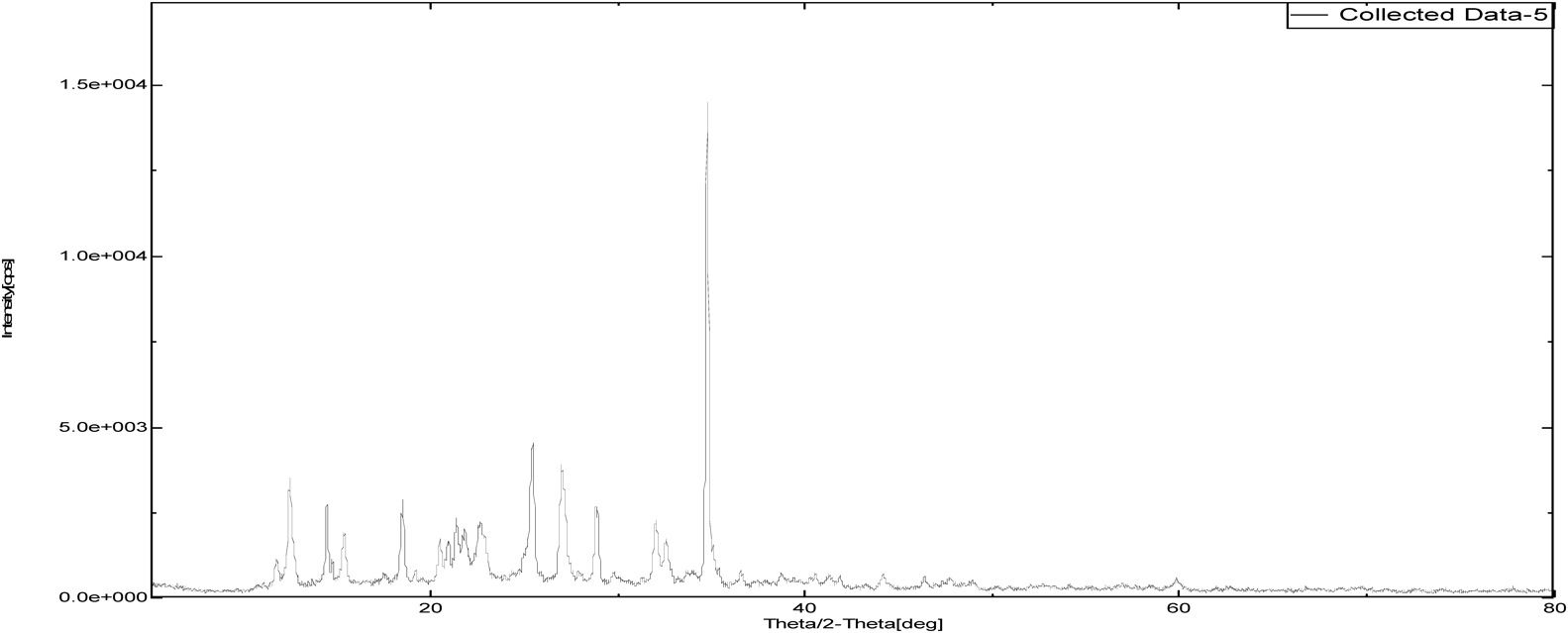
| Sample Id | 2θ (degree) | d (angle) | Intensity |
|---|---|---|---|
| Zoledronic acid API | 25.3652 | 3.50 | 649.98 |
| DL-Tartaric acid | 21.9581 | 4.044 | 1833.7 |
| Nicotinamide | 25.8724 | 3.44 | 1915.67 |
| Zoledronic acid + DL-Tartaric acid 1 : 1 | 37.5004 | 2.39 | 386.87 |
| Zoledronic acid + DL-Tartaric acid 1 : 2 | 20.1388 | 4.40 | 567.01 |
| Zoledronic acid + Nicotinamide 1 : 1 | 34.7537 | 2.58 | 2001.88 |
The saturation solubility of pure zoledronic acid in phosphate buffer (pH 6.8) IP, at 37°C was found to be 4.83 mg/mL after 24 h. Whereas the co-crystal form of zoledronic acid with DL-tartaric acid in the ratio 1 : 1, 1 : 2 and 2 : 1 prepared by slow evaporation showed dynamic solubility of 6.74, 8.03 and 29.06 mg/mL after 24 h in phosphate buffer (pH 6.8) IP, at 37°C, respectively.
Cocrystal form of zoledronic acid and nicotinamide in the ratio 1 : 1 prepared by dry grinding method showed dynamic solubility of 97.19 mg/mL after 24 h in phosphate buffer (pH 6.8) IP, at 37°C, respectively (Fig. 18 and Table 3).
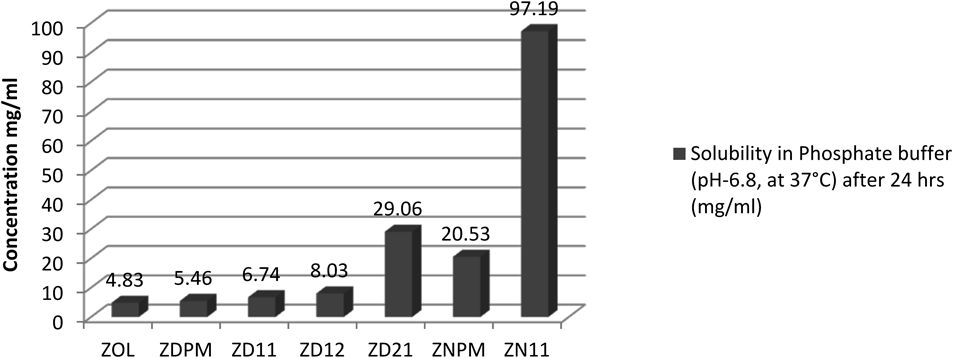
Whereas ZOL, pure zoledronic acid; ZDPM, zoledronic acid + DL-tartaric acid physical mixture; ZD11, zoledronic acid + DL-tartaric acid 1 : 1; ZD12, zoledronic acid + DL-tartaric acid 1 : 2; ZD21, zoledronic acid + DL-tartaric acid 2 : 1; ZNPM, zoledronic acid + nicotinamide physical mixture; ZN11, zoledronic acid + nicotinamide 1 : 1.
| Sample | Solubility in phosphate buffer (pH 6.8, at 37°C) (mg/mL) | Solubility fold increase in comparision with pure zoledronic acid |
|---|---|---|
| ZOL API | 4.83 | — |
| ZDPM | 5.46 | 1.13 |
| ZD11 | 6.74 | 1.39 |
| ZD12 | 8.03 | 1.66 |
| ZD21 | 29.06 | 6.01 |
| ZNPM | 20.53 | 4.25 |
| ZN11 | 97.19 | 20.12 |
Zoledronic acid was able to form stable cocrystals with DL-tartaric acid and nicotinamide in different ratios. The cocrystals formed were characterized and the analysis for saturation solubility was carried out. The formation of the cocrystals was confirmed by solid-state characterization which included PXRD, DSC and FTIR, the results of which proved the structural modifications in the cocrystal. PXRD results exhibited changes in the peak locations and patterns indicating the development of new crystalline phase. DSC thermogram showed different melting patterns for cocrystals when compared to the pure drug. These results were also supported by FTIR spectrum that confirmed the hydrogen bond formation. A 20.12 fold increase in the saturation solubility of cocrystals zoledronic acid with nicotinamide of 1 : 1 ratio was observed in its co-crystallized form which is very high compared to the solubility of cocrystals with DL-tartaric acid of 1 : 1, 1 : 2 and 2 : 1 having fold increase of 1.39, 1.66 and 6.01 respectively. Thus, the cocrystallization with nicotinamide improved the solubility of zoledronic acid, and the good permeability is expected which have to been studied furtherly.
The authors declare no conflict of interest.