2019 Volume 67 Issue 9 Pages 1019-1022
2019 Volume 67 Issue 9 Pages 1019-1022
A novel catalyst system—a combination of the readily available 2,2′-biphenol with the inexpensive, nontoxic, and eco-friendly B(OH)3—promoted the Nazarov cyclization of activated and inactivated divinyl ketones to afford the corresponding cyclopentenones up to 96% yield under, in a cis-selective manner. Compared with the conventional harsh conditions with hazardous reagents, user-friendly method was established with bench-stable and easy-to-handle reagents.
Following its first report in the 1940s, the Nazarov cyclization reaction has been identified as a valuable method for the formation of cyclopentenones from divinyl ketones via the 4π electrocyclization of pentadienyl cation intermediates.1–3) Because of its synthetic utility, the Nazarov cyclization has been employed as the key steps in the total syntheses of natural products having cyclopentane rings.4) Recently, further studies on domino reactions by a manipulation of interrupted reactive intermediates,5–10) enantioselective cyclizations,11–16) and the use of new catalysts17–39) have been explored extensively. In particular, there is much focus on organocatalyzed systems as environmentally benign, easy-to-handle alternatives to the hazardous reagents employed in conventional Nazarov cyclization conditions.27–39) For examples, Tius and colleagues reported an elegant cooperative asymmetric organocatalysis via imine intermediates as activated carbonyl groups.30–33) As another case, Matlin’s group demonstrated a hydroxylamine-catalyzed reaction based on density functional theory (DFT) analysis of the reaction pathway.37) Quite recently, an enantioselective Nazarov reaction catalyzed by an axially chiral boron reagent was established by Oestreich and colleagues.38) Our group also attempted to develop a novel catalytic system for the Nazarov cyclization with amine reagents, aiming at efficient activation of the carbonyl group. Unexpectedly, we found that a novel combination of the bench-stable 2,2′-biphenol with the inexpensive, nontoxic, and eco-friendly reagent B(OH)3 promoted the cyclization of not only activated α-alkoxy divinyl ketones but also inactivated divinyl ketones. We describe herein our preliminary studies toward this novel catalyst system for the Nazarov cyclization (Chart 1).
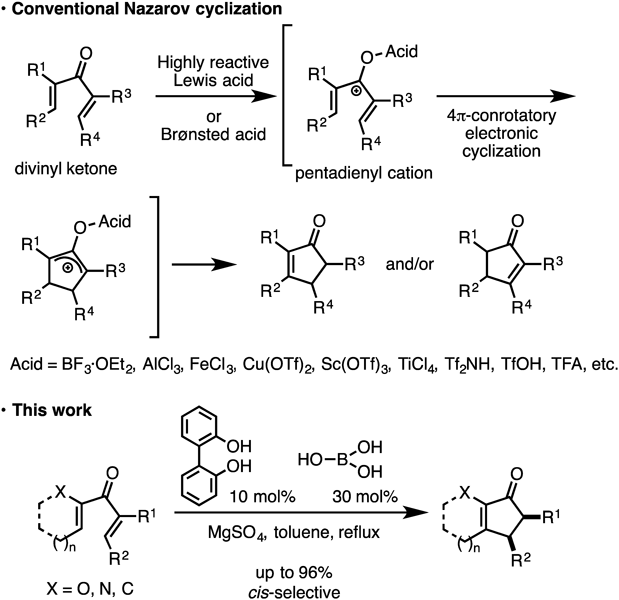
We initially attempted the organomediated Nazarov reaction with a stoichiometric amount of 2-aminophenylboronic acid in an attempt to activate the carbonyl group through imine formation with a simultaneous inductive effect of the vacant orbital on the adjacent boron atom. The reaction afforded the desired cyclopentenone in 27% yield from divinyl ketone 1 (Table 1, entry 1); however, further investigation revealed that phenylboronic acid was essential for promoting the Nazarov cyclization (entries 2 and 3). While the Nazarov cyclization of 1 proceeded in refluxing toluene without any activators (entry 4), phenylboronic acid accelerated the electrocyclization within a short time.
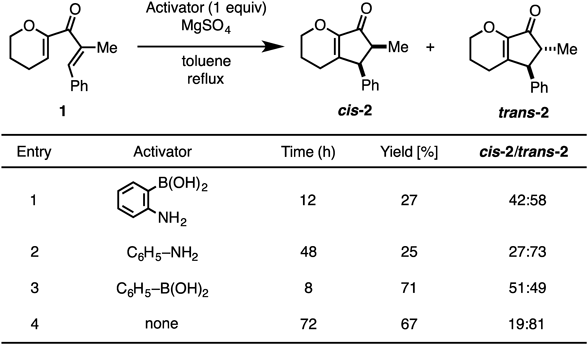 |
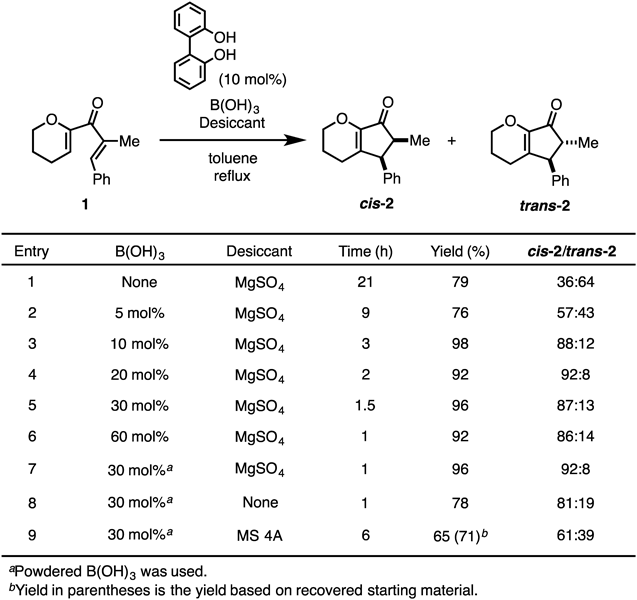
Thus, we turned our attention to the catalytic use of aryl boronic acid derivatives (Table 2). Aryl boronic acids with electron-withdrawing or electron-donating substituents were examined for this reaction. Among them, 4-methoxyphenyl boronic acid was the most efficient, and the desired cyclopentenone was obtained in 83% yield (entries 2–6). On the other hand, the steric bulkiness around the boron atom decreased the catalytic activity (entry 7). Boronic acids having an electron-rich or basic heteroaromatic ring also showed low catalytic activities (entries 8–11). For increasing the catalytic activity of the boron species, we attempted to enhance the Lewis acidity of the boron atom by avoiding the efficient overlap of the oxygen lone pair with the p-orbitals on boron. For examples, using optically active 1,1′-bi-2-naphthols (BINOLs), Chong and colleagues succeeded in the asymmetric 1,4-addition of an alkynyl group to chalcones via activation of B-1-alkynyldiisopropylboronates, leading to B-1-alkynyl(BINOL)boronates.40,41) Yasuda et al. established a hetero-Diels–Alder reaction between Danishefsky’s diene and benzaldehyde under the Lewis acid catalysis of an originally developed cage-shaped borate ester.42) In both cases, electron donation from the oxygen lone pair of the ligands into the vacant orbitals of the boron atoms was efficiently circumvented by the structurally strained frameworks of the ligand backbones. Therefore, we examined an easily accessible combination of biphenols and B(OH)3. In the presence of BINOL with boric acid, the desired cyclization proceeded smoothly to yield cyclopentanones 2 in good yields (entry 12), with 2,2′-biphenol furnishing 2 in 98% yields (cis–trans = 88 : 12) within 3 h (entry 13). The result obtained with benzo[d][1,3,2]dioxaborol-2-ol43) supported the importance of such a “twisted” diol backbone (entry 14). While prolonged heating decreased the cis–trans selectivity of the product, enhancement of the reaction rate by our catalytic system was highly meaningful to obtain the desired product in a cis-selective manner.
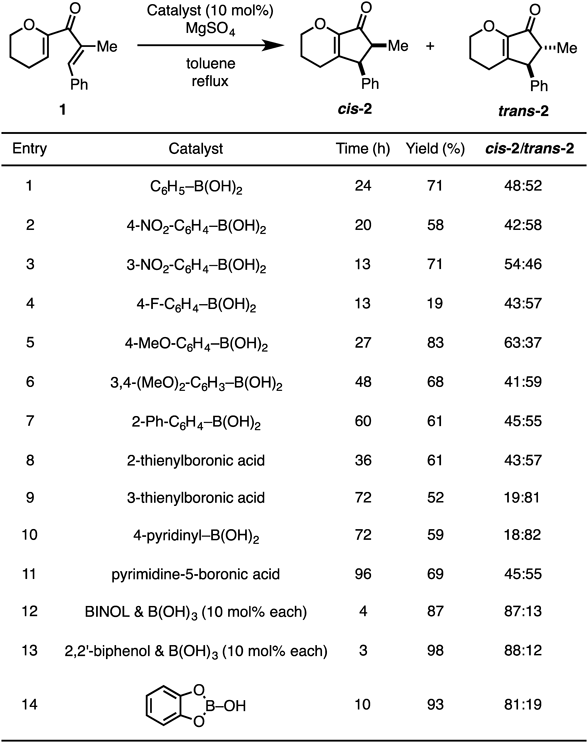 |
As shown in Chart 2, B(OH)3 by itself did not catalyze the Nazarov cyclization before the addition of 2,2′-biphenol. Furthermore, because the result obtained in the absence of B(OH)3 was comparable to that in refluxing toluene (Table 1, entry 4), we could confirm that 2,2′-biphenol did not exhibit any catalytic activity. We assumed that some active species were generated in situ from these reagents and hence attempted further optimization of the reaction conditions.
Accordingly, the reaction conditions were optimized by using a combination of 2,2′-biphenol and B(OH)3 (Table 3). A high yield, good cis–trans selectivity, and reaction rate enhancement were simultaneously realized in the presence of 10 mol% 2,2′-biphenol with 30 mol% B(OH)3, and the desired product was obtained in 96% yield (cis–trans = 87 : 13) within 1.5 h (entry 5). The use of powdered B(OH)3 slightly increased the reaction rate to improve the cis–trans ratio (entry 7). The use of an appropriate desiccant (MgSO4) enhanced the catalytic activity in our system (entries 7–9).
 |
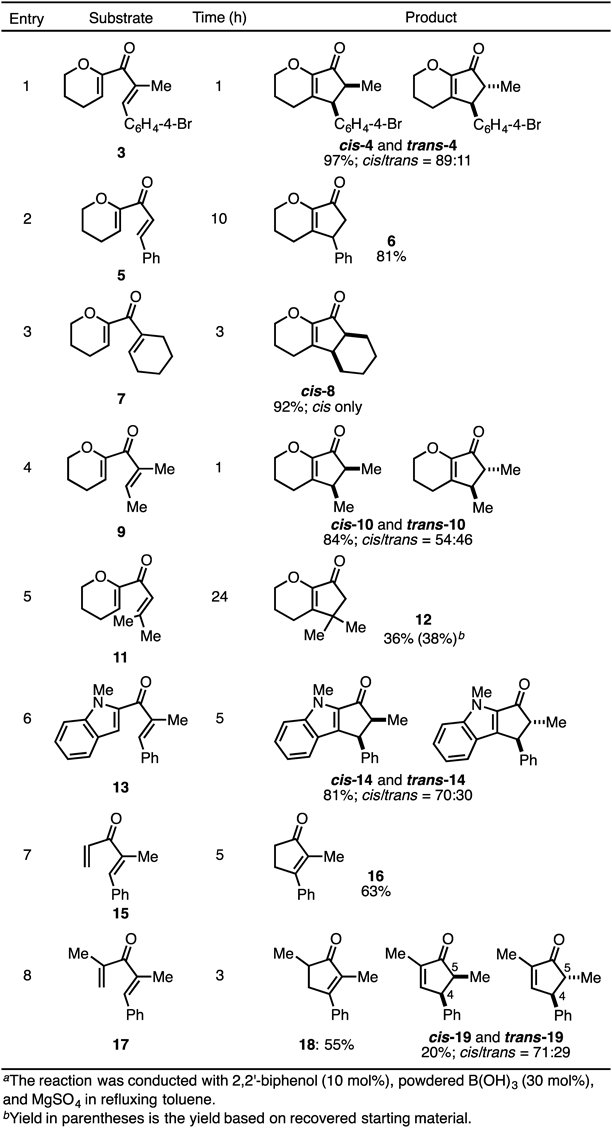
With the optimal conditions in hands, we next explored the substrate scope of this reaction (Table 4). The reaction with bromoaryl substrate 3 proceeded smoothly to give the corresponding product 4 in high yield, in a cis-selective manner (entry 1). Since the reaction rate with 5 was decreased (entry 2), the substituent at the α-position would allow the olefin to adopt a suitable conformation for the electrocyclization. Substrate 7 possessing a carbocycle was also applicable to this reaction and a cis-fused ring system was produced in good yield (entry 3). On the other hand, an acyclic alkyl side chain at the β-position of the product accelerated the isomerization from cis-10 to trans-10 without steric repulsion (entry 4). A quaternary carbon center could be induced in the reaction starting from β-disubstituted enone 11 (entry 5). The indole nitrogen could also work as an activator of the dienone system under our Nazarov cyclization conditions (entry 6). Additionally, we extended our reaction conditions to inactivated dienones (entries 7 and 8). Compound 15 furnished the corresponding cyclopentenone as the sole product (entry 7). However, α,α′-dimethyl substrate 17 gave an isomeric mixture of Nazarov products, 18, cis-19, and trans-19 in 75% yield (entry 8). Cyclopentenone 18 bearing a tetrasubstituted olefin was the major product. Similar to the case of cis-2 and trans-2, the relative stereochemistry of cis-19 and trans-19 was determined from the upfield shift of the signals attributed to the C5-methyl protons in cis-19 (0.68 ppm) relative to that in trans-19 (1.28 ppm), because of the shielding effect of the adjacent phenyl group.
 |
Finally, we investigated the active species in our catalyst system, as shown in Chart 3. When monohydroxy biphenol derivative 20 was used instead of 2,2′-biphenol, the reaction was not accelerated at all. This indicated that one biphenol and three boron atoms would generate a complex, in which the biphenol would chelate onto the boron atom. Furthermore, substrates 21a or 21b equipped with tert-butyldimethylsilyl- (TBS-) or tert-butyldiphenylsilyl-(TBDPS-)ethers, which are susceptible to highly acidic conditions, were consumed predominantly via unproductive pathways. This result indicated that a powerful Lewis or Brønsted acid was generated in situ upon mixing the weak 2,2′-biphenol (pKa1 7.6) and B(OH)3 (pKa 9.2),44) promoting undesired side reactions. Similar to past studies45–48) in which a cyclic boroxinate Brønsted acid was isolated from a mixture of 1 equiv. of the biphenol derivative and 3 equiv. of the boron reagent, an analogous cyclic boroxinate might be generated as the active species in our reaction system.49)

In summary, we have developed a novel organocatalytic Nazarov cyclization by using a combination of easy-to-handle, unhazardous, and bench-stable reagents, 2,2′-biphenol and B(OH)3. Further investigations of the actual catalytic species in our reaction, as well as the application of this system to an asymmetric cyclization process and extension to other acid-catalyzed reactions, are ongoing in our laboratory.
This work is supported in part by JSPS Core-to-Core Program, B. Asia-Africa Science Platforms (for Y.M.).
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.