2020 Volume 68 Issue 4 Pages 369-379
2020 Volume 68 Issue 4 Pages 369-379
Tyrosinase plays important roles in many different disease related processes, and the development of its inhibitors is particularly important in biotechnology. In this study, thirty-nine 3-/4-alkoxyphenylethylidenethiosemicarbazides were synthesized as novel tyrosinase inhibitors based on structure-based molecular design. Our experimental results demonstrated that thirty-one of them possess remarkable tyrosinase inhibitory activities with IC50 value below 1 µM, and 5a, 6e, 6g and 6t did not display any toxicity to 293T cell line at the concentration of 1000 µmol/L. According to the inhibitory activities, several compounds were selected for detail investigation on the structure–activity relationships (SARs), mechanisms of enzyme inhibition, inhibitory kinetics and cytotoxicity. In particular, the interaction between the selected inhibitors and the active center of tyrosinase was considered and discussed in detail based on their structural characteristics. Taken together, the results presented here demonstrated that the newly designed compounds are promising candidates for the treatment of tyrosinase-related disorders and further development of them may have significant contribution in biomedical science.
Tyrosinase (EC 1.14.18.1) is a multifunctional oxygenase widely distributed in nature, structurally belonging to the type-3 copper protein family.1–3) It catalyzes the hydroxylation of L-tyrosine to L-3,4-dihydroxyphenylalanine (L-DOPA) and the oxidation of L-DOPA to dopaquinone. Dopaquinone is highly reactive and can be oxidized spontaneously to form melanin, which is responsible for the color of mammal-skin and -hair.4–8) Recent studies showed that tyrosinase is involved in the molting process of insects9–12) and the browning of fruits and vegetables.13–15) More importantly, tyrosinase also plays important roles in many human diseases, including Parkinson's and other neurodegenerative diseases,16–18) and the formation of some dermatological disorders, such as hyperpigmentation, freckles, melasma, ephelide, and senile lentigines.19–21) Therefore, tyrosinase inhibitors have become increasingly important in food industry, agriculture, cosmetics, and biomedical science.
Over the past decade, many efforts have been spent on searching for new and effective tyrosinase inhibitors, and a tremendously large number of naturally occurring and synthetic tyrosinase inhibitors have been reported.22–35) Unfortunately, only few of them can be used in practice due to the lack of their individual activities or safety concerns. Therefore, more efforts are urgently needed to search and develop new, potent and safe tyrosinase inhibitors with improved therapeutic profiles. Among those reported tyrosinase inhibitors, thiosemicarbazide derivatives occupied a particularly prominent position36–56) because of their remarkable inhibitory activities against tyrosinase. However, the structural modification of 1-(1-phenyl-ethylidene)thiosemicarbazides for functional optimization has not been reported.
Although the crystallographic structure of tyrosinase revealed that there is a lipophilic long-narrow gorge near to the binuclear copper active center,57–59) which may be related the enzyme inhibitory mechanism, and the specific mode of the interaction between the thiosemicarbazone molecule with these two copper ions in the active center of tyrosinase has been reported,45) the detailed binding process of inhibitors to these two copper ions is still not known. According to the information provided in-house51–53) (Fig. 1) and from other groups,42,54–56) thirty-nine novel 3-/4-alkoxy substituted phenylethylidenethiosemicarbazide derivatives bearing both a thiosemicarbazone moiety and a proper alkoxy lipophilic group were designed and synthesized, and their inhibitory effects on mushroom tyrosinase were evaluated. Furthermore, the structure–activity relationships (SARs) were discussed, and the inhibition mechanisms, the inhibitory kinetics and the cytotoxicity of selected compounds were investigated in the present study. Based on the results, the interaction process between the thiosemicarbazone molecule of the inhibitors and the two copper ions in active center of tyrosinase was speculated. We hope that this study can lead to the development of highly potent and safe pharmacological agents for the treatment of tyrosinase-related disorders.

The synthesis of 5a–m, 6a–v, 11 and 12a–c was summarized in Chart 1, and the structures of the corresponding alkoxy substituent at the phenyl ring were given in Table 1. Briefly, the alkoxy-acetophenones 3a–m, 4a–n, 4p–v and the alkoxy-benzaldehydes 9, 10a–c were synthesized through the alkylation procedure. Subsequently, the condensation of the corresponding 3a–m, 4a–v, 9, 10a–c with thiosemicarbazide was carried out in anhydrous alcohol using acetic acid as catalyst to provide the desired target compounds 5a–m, 6a–v, 11 and 12a–c in good to excellent yields, and their structures were characterized by MS, 1H-NMR, 13C-NMR spectra and element analysis.

Reagents and reaction conditions: (a) RX (X = Br or I), K2CO3, anhydrous acetone, RT, 4–8 h; (b) thiosemicarbazide, anhydrous ethanol, acetic acid, 50–80°C, 3 h.
| Entry | Alkoxy group structure | IC50 (µM) | Entry | Alkoxy group structure | IC50 (µM) |
|---|---|---|---|---|---|
| 5a | C2H5 | 0.848 | 6f | (CH2)3OH | 0.334 |
| 5b | n-C3H7 | 0.748 | 6g | n-C4H9 | 0.408 |
| 5c | n-C4H9 | 0.620 | 6h | iso-C4H9 | 0.353 |
| 5d | (CH2)4Cl | 0.525 | 6i | (CH2)4Cl | 0.432 |
| 5e | n-C5H11 | 0.768 | 6j | n-C5H11 | 0.269 |
| 5f | iso-C5H11 | 1.040 | 6k | n-C6H13 | 0.404 |
| 5g | n-C6H13 | 0.707 | 6l | Cyclohexyl | 0.251 |
| 5h | n-C8H17 | 1.061 | 6m | n-C7H15 | 0.790 |
| 5i | CH2Ph | 0.690 | 6n | n-C8H17 | 1.091 |
| 5j | CH2PhBr-4 | 0.353 | 6o | Phenyl | 0.237 |
| 5k | (CH2)3Ph | 1.152 | 6p | CH2PhBr-4 | 0.566 |
| 5l | CH2CO2C2H5 | 0.590 | 6q | (CH2)3Ph | 0.699 |
| 5m | (CH2)3CO2C2H5 | 0.828 | 6r | CH2CO2C2H5 | 0.439 |
| Ref. | Acetophenones | >100 | 6s | CH2CO2H | 0.686 |
| Ref. | Thiosemicarbazide | >100 | 6t | (CH2)3CO2C2H5 | 0.182 |
| Ref. | Kojic acid | 28.5 | 6u | (CH2)3CO2H | 0.323 |
| 6a | C2H5 | 0.461 | 6v | CH2CH(OC2H5)2 | 0.747 |
| 6b | C2H4OH | 0.260 | 11 | (CH2)3OH | 1.017 |
| 6c | n-C3H7 | 0.365 | 12a | C2H5 | 2.250 |
| 6d | CH2CH = CH2 | 0.424 | 12b | n-C3H7 | 1.361 |
| 6e | CH2C ≡ CH | 0.564 | 12c | n-C4H9 | 1.150 |
1-(1-Phenylethylidene)thiosemicarbazide had a potent inhibitory activity on mushroom tyrosinase with IC50 value of 0.34 µM.45) Inspired by this and with the established three-dimensional structure57–59) of tyrosinase in mind, the inhibitory effects of our synthetic thiosemicarbazide derivatives, 5a–m, 6a–v, 11 and 12a–c, on mushroom tyrosinase were investigated, in which the well-known tyrosinase inhibitor, kojic acid, and its parental compounds acetophenone and thiosemicarbazide were employed as the standard references. The corresponding results were listed in Table 1. As predicted, all the structured-based condensation products displayed effective tyrosinase inhibitory activities, which was in good agreement with our speculation.
From Table 1, the following SARs results could be derived:
a) 5a–m, 6a–v, 11 and 12a–c, a total of thirty-nine compounds were synthesized, and thirty-one of them showed more potent tyrosinase inhibitory activities than the well-known tyrosinase inhibitor kojic acid with IC50 values below 1 µM.
b) From Fig. 2 we find that for 1-phenylethylidenethiosemicarbazide compounds, the introduction of hydroxy or amino group could not promote their inhibitory activity comparing with unsubstituted 1-(1-phenylethylidene)thiosemicarbazide (IC50 value, 0.34 µM),45) indicating that hydrophilic group is not favorable substituent, even is disadvantageous one, such as in 1-(2,4,6-tyihydroxyphenyl)ethylidenethiosemi-carbazide (IC50 value, 22.0 µM).45)
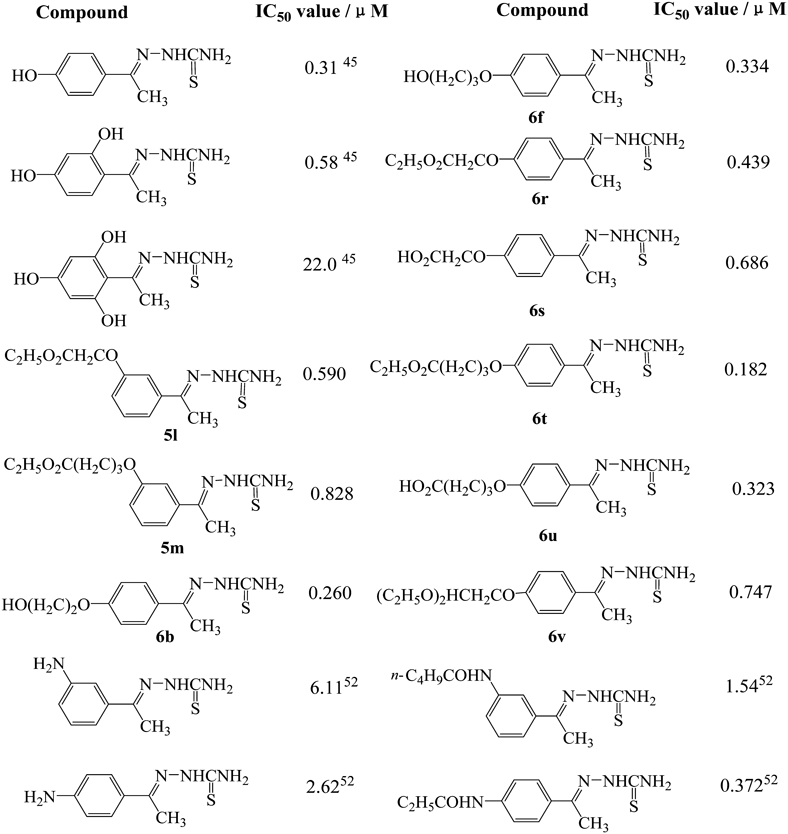
c) Compared to unsubstituted 1-(1-phenylethylidene)thiosemicarbazide, compounds 5l, 5m, 6b, 6f, 6r, 6s, 6t, 6u and 6v, bearing a side chain with hydrophilic group at the top, all showed efficient inhibitory activities and their IC50 values were of the same order of magnitude (IC50 values from 0.182 to 0.828 µM), showing that the type of hydrophilic group at the top of the chain has no obvious influence on inhibitory activity in the present investigation. Subsequently, comparing the IC50 values of compounds 5l, 5m, 6b, 6f, 6r, 6s, 6t, 6u and 6v with that of compounds 5a–e, 6a, 6c–e and 6g–m, it could be concluded that the type of the substituent chain (with or without hydrophilic group at the top) has no obvious influence on inhibitory activity.
d) We find from Fig. 2 that the introduction of the lipophilic group, hydroxyl or amino, to phenyl ring could enhance the inhibitory activity of thiosemicarbazone compounds, the reason for this phenomenon can be suggested as follows.
The active site structure of tyrosinase was shown in Fig. 3.57–59) There is a lipophilic “narrow gorge” in the middle of the center of the enzyme and two copper ions are in middle of the “narrow gorge.” It was found that there is a cap covering “narrow gorge” and the cap is consisted of 378 nucleotides60,61) which are flexible and prevent inhibitor binding to the active site of tyrosinase.57,62)
Thiosemicarbazone molecule needs to enter and pass through the “narrow gorge” before combining with two copper ions. As thiosemicarbazo group is hydrophilic and easy to bind to nucleotides through van der Waals’ force. When the thiosemicarbazone molecule approaches the “nucleotide” cap, thiosemicarbazo group might induce the “nucleotide” cap to change its conformation, which could provide convenience for thiosemicarbazone molecule to enter the gorge. Subsequently, after the gate of the “narrow gorge” opens, the lipophilic characteristic of the other end of thiosemicarbazone molecule would promote thiosemicarbazone molecule to get into the “narrow gorge” and further, to push thiosemicarbazone molecule forward to the activity center of tyrosinase to combine with these two copper ions.
Therefore, the introduction of lipophilic group to the other end of the thiosemicarbazo group is benefit to enhance the inhibitory activity, but the lipophilicity and hydrophilicity of the two ends of thiosemicarbazone molecule should be properly adjusted, if the lipophilicity or hydrophilicity is too large the inhibitory activity of the molecule could be reduced.
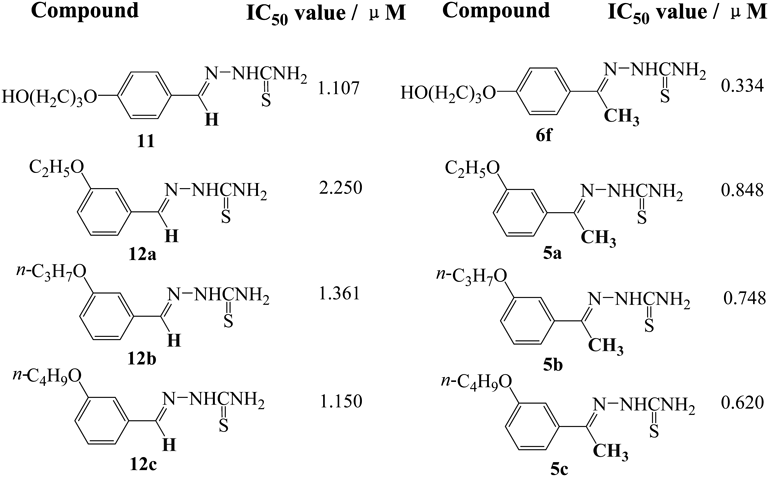
e) In general, the compounds bearing the same alkoxy group at position-4 of the phenyl ring displayed more profound tyrosinase inhibitory activities than the ones bearing it at position-3 of the phenyl ring. For example, the IC50 value of 5a was 0.848 µM, but the IC50 value of 6a was 0.461 µM (Table 1). The IC50 value of 5m was 0.828 µM and the IC50 value of 6t was 0.182 µM. The results implied that the position of the alkoxy substituent influenced the tyrosinase inhibitory activity, and in the investigation, the alkoxy group attached at position-4 of the phenyl ring was more efficacious for the inhibitory potency. It was consistent with previous reports by Kubo,63) Jimenez64) and our recent report,52) and this phenomenon is illustrated as follows through Fig. 4.

From Fig. 4 it was found that the value of molecular section height of 5a is greater than that of 6a (h1 > h2), which made 6a more favorable than 5a to enter and pass through the “narrow gorge” of tyrosinase, and then, to combine with these two copper ions in the center of tyrosinase. In addition, the difference of electronic effect of group at 3-positon or 4-positon of the phenyl ring on thiosemicarbazone group may also participate in and affect the inhibitory activity of these two compounds.
f) 1-Alkoxyphenylethylidenethiosemicarbazide compounds exhibited higher inhibitory activity than 2-alkoxybenzylidenehydrazine-1-carbothioamide compounds with about a two-fold of IC50 value, which indicated that on the benzylidene group of the molecule of thiosemicarbazide compounds methyl can enhance the activity more than hydrogen (Fig. 5). Therefore, methyl group on benzylidene unit is another important group to enhance inhibitory activity.
The above SAR results were summarized and illustrated in Fig. 6.

The inhibition mechanism of compounds 5a and 6a against mushroom tyrosinase for the oxidation of L-DOPA was investigated and the relationship between enzyme activity and concentration of compounds 5a and 6a were respectively shown in Fig. 7. The results demonstrated that the plots gave a family of straight lines which all passed through the origin point. The increase of the inhibitor concentration resulted in the decrease of the line slope, indicating that the inhibitory action of compounds 5a and 6a on mushroom tyrosinase were reversible.45,65–69)

The concentrations of compound 5a for curves 1–4 were 0.0, 0.5, 1.0 and 2.0 µM, respectively. The concentrations of compound 6a for curves 1–4 were 0.0, 0.1, 0.5 and 1.0 µM, respectively.
To further insight into the inhibitory mechanism, the inhibitory types of selected compounds 5a and 6a against mushroom tyrosinase for the oxidation of L-DOPA was determined by the Lineweaver–Burk double reciprocal plots with three different concentrations of inhibitors 5a and 6a, respectively (Fig. 8). The results indicated that the plots of 1/V versus 1/[S] gave three straight lines with different slopes, and they intersected one another at the ordinate. The values of Vmax remained the same and the values of Km increased with the increase of inhibitor concentrations. The results demonstrated that compounds 5a and 6a were the competitive tyrosinase inhibitors and also suggested that they could only bind with the free enzyme.3,47,52,64,70)

The concentrations of 5a for curves 1–3 were 2.0, 1.0 and 0.5 µM, respectively. The concentrations of 6a for curves 1–3 were 1.0, 0.5 and 0.1 µM, respectively.
The 293T cell line rarely express endogenous receptors required for extracellular ligands, and is easy to transfect, which is a very common cell line to express endogenous genes. In order to verify the safeties of these thiosemicarbazone compounds, four compounds 5a, 6e, 6g and 6t, considering the diversity of structure and inhibition activity, were selected to test the cytotoxicity to the 293T cell line.71–74) As demonstrated in Fig. 9, the results showed that these compounds had no obvious growth inhibitory effect on the 293T cells at the concentrations of 0, 31.25, 62.5, 125, 250, 500, 1000 µmol/L.

Since all of the selected compounds didn’t show any obvious growth inhibition effect and toxicity to the 293T cell line even at a high concentration (1000 µmol/L), we concluded that the compounds designed and selected in this study have low cytotoxicity. In consideration of the cytotoxicity and promising inhibitory effects against tyrosinase, these compounds should have a broad application potential in food industry, agriculture and cosmetics for the treatment of tyrosinase-related disorders.
In summary, we developed for the first time thirty-nine 3-/4-alkoxy substituted phenylethylidenethiosemicarbazides as new, potent and safe tyrosinase inhibitors. The results showed that thirty-one structure-based compounds had remarkable inhibitory activities with IC50 value of lower than 1 µM, and among them compound 6t exhibited the highest inhibitory activity with an IC50 value of 0.182 µM. The SAR analysis indicated that: i) the type of alkoxy group has no obvious influence on the activity; ii) methyl group on benzylidene group can enhance inhibitory activities; iii) the position of the alkoxy group obviously influenced the activity, and in the investigation, the alkoxy group attached at position-4 was optimal. Moreover, the inhibition mechanism and the inhibitory kinetics of compounds 5a and 6a revealed that these compounds exhibited such tyrosinase inhibitory effects by acting as a reversible and competitive inhibitor, and the process of the interaction was considered deeply between inhibitors and the active center of tyrosinase, based on the polarity characteristics of the two ends of inhibitors and structural characteristics of the active site of tyrosinase. Besides, the selected compounds 5a, 6e, 6g and 6t did not display any toxicity to the 293T cell line even at the concentration of 1000 µmol/L. Taken together, these results suggested that these compounds could serve as the promising candidates for the treatment of tyrosinase-related disorders. Further investigation of these compounds using human tyrosinase and a human melanoma cell line is being conduct, and the results will be reported in due course.
Melting points were determined on a WRS-1B digital instrument without correction. NMR spectra were recorded on a Varian Mercury-Plus 300 spectrometer in dimethyl sulfoxide (DMSO)-d6. All chemical shifts (δ) were quoted in parts per million and coupling constants (J) were given in Hertz. Mass spectra were obtained from VG ZAB-HS, LCMS-2010 A or LCQ DECA XP spectrometer. Elemental analyses were performed on a Vario EL instrument and were within ±0.4% of the theoretical values. All commercially available reagents and solvents were used without further purification. Mushroom tyrosinase (specific activity of the enzyme is 6680 U/mg) and L-DOPA (L-3,4-dhydroxyphenylalanine) were purchased from Sigma Chemical Co.
Procedure for the Synthesis of Targeted Compounds 5a–m, 6a–v, 11 and 12a–c (Chart 1)Into 50 mL of anhydrous acetone were added 10.0 mmol of compound 1 or 2, 13.0 mmol of the corresponding alkoxy bromide or alkyl iodide and 20.0 mmol of K2CO3, the above mixture was stirred at room temperature for 4–8 h. After completion of the reaction as indicated by TLC, the reaction mixture was filtered and the solvent was removed by evaporation at vacuum to get crude products, followed by chromatography to provide the pure intermediates 3a–m, 4a–n, 4p–v. Compounds 9 and 10a–c were prepared as the same method. Then, they respectively reacted with thiosemicarbazide in the presence of acetic acid (0.5 mL) at 50–80°C for 3 h to deliver the desired products 5a–m, 6a–v, 11 and 12a–c.
2-(1-(3-Ethoxyphenyl)ethylidene)hydrazine-1-carbothioamide (5a)Solid product, yield 81%, mp 152–153°C. 1H-NMR (300 MHz, DMSO-d6) δ: 10.15 (s, 1H), 8.25 (s, 1H), 7.91 (s, 1H), 7.46–7.37 (m, 2H), 7.26 (t, J = 7.9 Hz, 1H), 6.95–6.89 (m, 1H), 4.06 (q, J = 6.9 Hz, 2H), 2.27 (s, 3H), 1.32 (t, J = 6.9 Hz, 3H). 13C-NMR (75 MHz, DMSO-d6) δ: 178.8, 158.5, 147.8, 139.1, 129.2, 119.0, 115.3, 112.4, 63.0, 14.6, 14.2. Electrospray ionization (ESI)-MS, m/z = 238.2 [M + H]+. Anal. Calcd for C11H15N3OS: C, 55.67; H, 6.37; N, 17.71. Found C, 55.99; H, 6.39; N, 17.79.
2-(1-(3-Propoxyphenyl)ethylidene)hydrazine-1-carbothioamide (5b)Solid product, yield 82%, mp 114–115°C. 1H-NMR (300 MHz, DMSO-d6) δ: 10.23 (s, 1H), 8.33 (s, 1H), 7.98 (s, 1H), 7.46 (dd, J = 7.4, 1.1 Hz, 2H), 7.29 (t, J = 8.1 Hz, 1H), 6.98–6.92 (m, 1H), 3.97 (t, J = 6.5 Hz, 2H), 2.31 (s, 3H), 1.82–1.66 (m, 2H), 1.00 (t, J = 7.4 Hz, 3H). 13C-NMR (75 MHz, DMSO-d6) δ: 178.9, 158.7, 147.9, 139.1, 129.2, 119.0, 115.2, 112.4, 68.9, 22.1, 14.2, 10.4. ESI-MS m/z = 252.2 [M + H]+. Anal. Calcd for C12H17N3OS: C, 57.34; H, 6.82; N, 16.72. Found C, 57.45; H, 6.78; N, 16.66.
2-(1-(3-Butoxyphenyl)ethylidene)hydrazine-1-carbothioamide (5c)Solid product, yield 78%, mp 105–106°C. 1H-NMR (300 MHz, DMSO-d6) δ: 10.14 (s, 1H), 8.24 (s, 1H), 7.91 (s, 1H), 7.46–7.36 (m, 2H), 7.25 (t, J = 7.9 Hz, 1H), 6.97–6.89 (m, 1H), 4.00 (t, J = 6.4 Hz, 1H), 1.76–1.61 (m, 1H), 1.50–1.37 (m, 1H), 0.92 (t, J = 7.3 Hz, 1H). 13C-NMR (75 MHz, DMSO-d6) δ: 178.8, 158.7, 148.0, 139.1, 129.2, 119.0, 115.3, 112.40, 7.1, 30.8, 18.7, 14.2, 13.7. ESI-MS m/z = 266.2 [M + H]+. Anal. Calcd for C13H19N3OS: C, 58.84; H, 7.22; N, 15.83. Found C, 59.06; H, 7.18; N, 15.86.
2-(1-(3-(4-Chlorobutoxy)phenyl)ethylidene)hydrazine-1-carbothioamide (5d)Solid product, yield 74%, mp 114–115°C. 1H-NMR (300 MHz, DMSO-d6) δ: 10.15 (s, 1H), 8.26 (s, 1H), 7.91 (s, 1H), 7.43 (d, J = 8.9 Hz, 2H), 7.26 (t, J = 7.9 Hz, 1H), 6.93 (d, J = 8.1 Hz, 1H), 4.04 (t, J = 5.3 Hz, 2H), 3.70 (t, J = 5.7 Hz, 2H), 2.27 (s, 3H), 1.99–1.75 (m, 4H). 13C-NMR (75 MHz, DMSO-d6) δ: 178.8, 158.5, 147.9, 139.1, 129.2, 119.1, 115.4, 112.4, 66.7, 5.2, 28.9, 26.13, 14.2. ESI-MS m/z = 300.3 [M + H]+. Anal. Calcd for C13H18ClN3OS: C, 52.08; H, 6.05; N, 14.02. Found C, 52.31; H, 6.08; N, 13.91.
2-(1-(3-Pentoxyphenyl)ethylidene)hydrazine-1-carbothioamide (5e)Solid product, yield 78%, mp 103–104°C. 1H-NMR (300 MHz, DMSO-d6) δ: 10.23 (s, 1H), 8.33 (s, 1H), 7.97 (s, 1H), 7.46 (d, J = 7.6 Hz, 2H), 7.29 (t, J = 7.9 Hz, 1H), 6.99–6.90 (m, 1H), 4.00 (t, J = 6.4 Hz, 2H), 2.31 (s, 3H), 1.78–1.62 (m, 2H), 1.47–1.25 (m, 4H), 0.90 (t, J = 7.1 Hz, 3H). 13C-NMR (75 MHz, DMSO-d6) δ: 177.20, 157.00, 146.17, 137.40, 127.52, 117.28, 113.53, 110.74, 65.74, 26.76, 26.09, 20.25, 12.55, 12.22. ESI-MS m/z = 280.3 [M + H]+. Anal. Calcd for C14H21N3OS: C, 60.18; H, 7.58; N, 15.04. Found C, 60.33; H, 7.47; N, 15.02.
2-(1-(3-iso-Pentoxyphenyl)ethylidene)hydrazine-1-carbothioamide (5f)Solid product, yield 79%, mp 120–121°C. 1H-NMR (300 MHz, DMSO-d6) δ: 10.19 (s, 1H), 8.28 (s, 1H), 7.94 (s, 1H), 7.49–7.38 (m, 2H), 7.28 (t, J = 7.9 Hz, 1H), 6.96 (dd, J = 8.1, 1.9 Hz, 1H), 4.04 (t, J = 6.6 Hz, 2H), 2.29 (s, 3H), 1.88–1.71 (m, 1H), 1.65–1.57 (m, 2H), 0.93 (d, J = 6.6 Hz, 6H). 13C-NMR (75 MHz, DMSO-d6) δ: 178.8, 158.7, 147.9, 139.1, 129.2, 119.0, 115.3, 112.4, 65.8, 37.5, 24.5, 22.4, 14.2. ESI-MS m/z = 280.3 [M + H]+. Anal. Calcd for C14H21N3OS:C, 60.18; H, 7.58; N, 15.04. Found C, 60.48; H, 7.54; N, 15.10.
2-(1-(3-Hexoxyphenyl)ethylidene)hydrazine-1-carbothioamide (5g)Solid product, yield 82%, mp 102–103°C. 1H-NMR (300 MHz, DMSO-d6) δ: 10.19 (s, 1H), 8.29 (s, 1H), 7.94 (s, 1H), 7.48–7.49 (m, 2H), 7.28 (t, J = 7.9 Hz, 1H), 6.95 (dd, J = 8.1, 1.9 Hz, 1H), 4.01 (t, J = 6.4 Hz, 2H), 2.29 (s, 3H), 1.79–1.62 (m, 2H), 1.51–1.20 (m, 6H), 0.88 (t, J = 6.9 Hz, 3H). 13C-NMR (75 MHz, DMSO-d6) δ: 178.8, 158.7, 147.9, 139.1, 129.2, 119.0, 115.3, 112.4, 67.4, 31.0, 28.7, 25.2, 22.1, 14.2, 13.9. ESI-MS m/z = 294.3 [M + H]+.
2-(1-(3-Octyloxyphenyl)ethylidene)hydrazine-1-carbothioamide (5h)Solid product, yield 72%, mp 107–108°C. 1H-NMR (300 MHz, DMSO-d6) δ: 10.18 (s, 1H), 8.28 (s, 1H), 7.94 (s, 1H), 7.48–7.49 (m, 2H), 7.28 (t, J = 7.9 Hz, 1H), 6.95 (dd, J = 8.1, 2.0 Hz, 1H), 4.00 (t, J = 6.4 Hz, 2H), 2.28 (s, 3H), 1.79–1.62 (m, 2H), 1.51–1.17 (m, 10H), 0.86 (t, J = 6.9 Hz, 3H). 13C-NMR (75 MHz, DMSO-d6) δ: 178.8, 158.7, 147.9, 139.1, 129.2, 119.0, 115.3, 112.4, 67.4, 31.2, 28.7, 28.7, 28.7, 25.6, 22.1, 14.2, 13.9. ESI-MS m/z = 322.4 [M + H]+. Anal. Calcd for C17H27N3OS: C, 63.51; H, 8.47; N, 13.07. Found C, 63.62; H, 8.40; N, 13.13.
2-(1-(3-Benzyloxyphenyl)ethylidene)hydrazine-1-carbothioamide (5i)Solid product, yield 86%, mp 143–144°C. 1H-NMR (300 MHz, DMSO-d6) δ: 10.21 (s, 1H), 8.32 (s, 1H), 7.93 (s, 1H), 7.57–7.54 (m, 1H), 7.50–7.29 (m, 7H), 7.04 (dd, J = 8.0, 2.1 Hz, 1H), 5.17 (s, 2H), 2.29 (s, 3H). 13C-NMR (75 MHz, DMSO-d6) δ: 178.9, 158.3, 147.7, 139.1, 137.1, 129.3, 128.4, 127.8, 127.7, 119.4, 115.8, 112.6, 69.3, 14.2. ESI-MS m/z = 300.3 [M + H]+.
2-(1-(3-((4-Bromobenzyl)oxy)phenyl)ethylidene)-hydrazine-1-carbothioamide (5j)Solid product, yield 83%, mp 163–164°C. 1H-NMR (300 MHz, DMSO-d6) δ: 10.28 (s, 1H), 8.40 (s, 1H), 8.00 (s, 1H), 7.63–7.55 (m, 3H), 7.51–7.41 (m, 3H), 7.32 (t, J = 8.0 Hz, 1H), 7.04 (dd, J = 8.1, 2.0 Hz, 1H), 5.16 (s, 2H), 2.32 (s, 3H). 13C-NMR (75 MHz, DMSO-d6) δ: 176.4, 155.6, 145.2, 136.6, 134.0, 128.8, 127.3, 126.8, 118.5, 117.0, 113.3, 110.1, 66.0, 11.7. ESI-MS m/z = 378.2 [M + H]+. Anal. Calcd for C16H16BrN3OS: C, 50.80; H, 4.26; N, 11.11. Found C, 51.08; H, 4.35; N, 10.90.
2-(1-(3-(3-Phenylpropoxy)phenyl)ethylidene)hydrazine-1-carbothioamide (5k)Solid product, yield 81%, mp 143–144°C. 1H-NMR (300 MHz, DMSO-d6) δ: 10.21 (s, 1H), 8.31 (s, 1H), 7.96 (s, 1H), 7.49–7.43 (m, 2H), 7.36–7.13 (m, 6H), 7.02–6.91 (m, 1H), 4.02 (t, J = 6.3 Hz, 2H), 2.83–2.69 (m, 2H), 2.30 (s, 3H), 2.11–1.95 (m, 2H). 13C-NMR (75 MHz, DMSO-d6) δ: 178.9, 158.6, 147.9, 141.4, 139.1, 129.3, 128.3, 125.8, 119.1, 115.3, 112.5, 66.7, 31.5, 30.4, 14.2. ESI-MS m/z = 328.3 [M + H]+. Anal. Calcd for C18H21N3OS: C, 66.02; H, 6.46; N, 12.83. Found C, 66.16; H, 6.47; N, 12.73.
Ethyl 2-(3-(1-(2-Carbamothioylhydrazono)ethyl)phenoxy)-acetate (5l)Solid product, yield 84%, mp 154–155°C. 1H-NMR (300 MHz, DMSO-d6) δ: 10.21 (s, 1H), 8.32 (s, 1H), 7.97 (s, 1H), 7.55–7.42 (m, 2H), 7.34–7.27 (m, 1H), 7.02–6.91 (m, 1H), 4.86 (s, 2H), 4.17 (q, J = 7.1 Hz, 2H), 2.29 (s, 3H), 1.21 (t, J = 7.1 Hz, 3H). 13C-NMR (75 MHz, DMSO-d6) δ: 178.9, 168.7, 157.6, 147.5, 139.1, 129.3, 119.8, 115.7, 112.1, 64.7, 60.6, 14.1, 14.0. ESI-MS m/z = 296.2 [M + H]+. Anal. Calcd for C13H17N3O3S: C, 52.86; H, 5.80; N, 14.23. Found C, 53.08; H, 5.80; N, 14.25.
2-(1-(3-(4-(Ethylperoxy)butoxy)phenyl)ethylidene)-hydrazine-1-carbothioamide (5m)Solid product, yield 75%, mp 107–108°C. 1H-NMR (300 MHz, DMSO-d6) δ: 10.20 (s, 1H), 8.30 (s, 1H), 7.94 (s, 1H), 7.45 (t, J = 5.8 Hz, 2H), 7.29 (t, J = 7.9 Hz, 1H), 6.95 (dd, J = 8.1, 1.8 Hz, 1H), 4.15–3.98 (m, 4H), 2.47 (t, J = 7.3 Hz, 2H), 2.29 (s, 3H), 2.04–1.92 (m, 2H), 1.18 (t, J = 7.1 Hz, 3H). 13C-NMR (75 MHz, DMSO-d6) δ: 178.8, 172.6, 158.4, 147.8, 139.1, 129.3, 119.2, 115.3, 112.4, 66.5, 59.9, 30.1, 24.2, 14.2, 14.1. ESI-MS m/z = 324.3 [M + H]+. Anal. Calcd for C15H21N3O3S: C, 55.71; H, 6.54; N, 12.99. Found C, 55.67; H, 6.51; N, 13.02.
2-(1-(4-Ethoxyphenyl)ethylidene)hydrazine-1-carbothioamide (6a)Solid product, yield 85%, mp 153–154°C. 1H-NMR (300 MHz, DMSO-d6) δ: 10.15 (s, 1H), 8.24 (s, 1H), 7.88 (d, J = 8.9 Hz, 2H), 6.91 (d J = 8.9 Hz, 2H), 4.12–3.97 (m, 2H), 2.28 (s, 3H), 1.34 (t, J = 6.8 Hz, 3H). 13C-NMR (75 MHz, DMSO-d6) δ: 178.6, 159.5, 147.8, 129.8, 128.1, 113.9, 63.1, 14.6, 13.8. ESI-MS m/z = 238.2 [M + H]+. Anal. Calcd for C11H15N3OS: C, 55.67; H, 6.37; N, 17.71. Found C, 55.94; H, 6.39; N, 17.79.
2-(1-(4-(2-Hydroxyethoxy)phenyl)ethylidene)hydrazine-1-carbothioamide (6b)Solid product, yield 83%, mp 217–218°C. 1H-NMR (300 MHz, DMSO-d6) δ: 10.09 (s, 1H), 8.18 (s, 1H), 7.86 (s, 1H), 7.84 (d, J = 8.6 Hz, 2H), 6.90 (d, J = 8.7 Hz, 2H), 4.86 (t, J = 5.4 Hz, 1H), 4.00 (t, J = 4.7 Hz, 2H), 3.71 (dd, J = 9.7, 4.9 Hz, 2H), 2.25 (s, 3H). 13C-NMR (75 MHz, DMSO-d6) δ: 179.3, 160.3, 148.5, 130.6, 128.8, 114.8, 70.4, 60.4, 14.7. ESI-MS m/z = 254.3 [M + H]+. Anal. Calcd for C11H15N3OS: C, 52.15; H, 5.97; N, 16.59. Found C, 52.43; H, 5.88; N, 16.46.
2-(1-(4-Propoxyphenyl)ethylidene)hydrazine-1-carbothioamide (6c)Solid product, yield 84%, mp 185–186°C. 1H-NMR (300 MHz, DMSO-d6) δ: 10.17 (s, 1H), 8.26 (s, 1H), 7.96–7.83 (m, 3H), 6.92 (d, J = 9.0 Hz, 2H), 3.94 (t, J = 6.5 Hz, 2H), 2.28 (s, 3H), 1.82–1.65 (m, 2H), 0.98 (t, J = 7.4 Hz, 3H). 13C-NMR (75 MHz, DMSO-d6) δ: 178.6, 159.6, 147.8, 129.8, 128.1, 114.0, 68.9, 22.0, 13.8, 10.3. ESI-MS m/z = 252.2 [M + H]+, Anal. Calcd for C12H17N3OS: C, 57.34; H, 6.82; N, 16.72. Found C, 57.46; H, 6.77; N, 16.64.
2-(1-(4-(Allyloxy)phenyl)ethylidene)hydrazine-1-carbothioamide (6d)Solid product, yield 88%, mp 157–158°C. 1H-NMR (300 MHz, DMSO-d6) δ: 10.16 (s, 1H), 8.25 (s, 1H), 7.89 (d, J = 8.9 Hz, 3H), 6.95 (d, J = 8.8 Hz, 2H), 6.11–6.00 (m, 1H), 5.41 (d, J = 15.9 Hz, 1H), 5.27 (d, J = 10.5 Hz, 1H), 4.60 (d, J = 5.1 Hz, 2H), 2.28 (s, 3H). 13C-NMR (75 MHz, DMSO-d6) δ: 178.6, 159.1, 147.8, 133.5, 130.1, 128.1, 117.5, 114.2, 68.2, 13.8. ESI-MS m/z = 250.2 [M + H]+.
2-(1-(4-(Prop-2-yn-1-yloxy)phenyl)ethylidene)hydrazine-1-carbothioamide (6e)Solid product, yield 78%, mp 175–176°C. 1H-NMR (300 MHz, DMSO-d6) δ: 10.10 (s, 1H), 8.19 (s, 1H), 7.87 (d, J = 8.0 Hz, 3H), 6.95 (d, J = 8.5 Hz, 2H), 4.83 (s, 2H), 3.56 (s, 1H), 2.26 (s, 3H). 13C-NMR (75 MHz, DMSO-d6) δ: 179.2, 158.7, 148.3, 131.4, 128.7, 115.2, 79.8, 79.1, 56.3, 14.7. ESI-MS m/z = 248.2 [M + H]+. Anal. Calcd for C12H13N3OS: C, 58.28; H, 5.30; N, 16.99. Found C, 58.41; H, 5.35; N, 16.81.
2-(1-(4-(3-Hydroxypropoxy)phenyl)ethylidene)hydrazine-1-carbothioamide (6f)Solid product, yield 78%, mp 176–177°C. 1H-NMR (300 MHz, DMSO-d6) δ: 10.07 (s, 1H), 8.17 (s, 1H), 7.84 (d, J = 8.0 Hz, 3H), 6.88 (d, J = 8.4 Hz, 2H), 4.54 (t, J = 5.0 Hz, 1H), 4.04 (t, J = 6.2 Hz, 2H), 3.60–3.49 (m, 2H), 2.24 (s, 3H), 1.91–1.79 (m, 2H). 13C-NMR (75 MHz, DMSO-d6) δ: 179.1, 160.2, 148.3, 130.4, 128.6, 114.5, 65.1, 57.7, 32.5, 14.3. ESI-MS m/z = 268.4 [M + H]+.
2-(1-(4-Butoxyphenyl)ethylidene)hydrazine-1-carbothioamide (6g)Solid product, yield 83%, mp 166–167°C. 1H-NMR (300 MHz, DMSO-d6) δ: 10.09 (s, 1H), 8.18 (s, 1H), 7.86 (s, 1H), 7.84 (d, J = 8.8 Hz, 2H), 6.88 (d, J = 8.9 Hz, 2H), 3.98 (t, J = 6.4 Hz, 2H), 2.25 (s, 3H), 1.76–1.61 (m, 2H), 1.47–1.36 (m, 2H), 0.92 (t, J = 7.3 Hz, 3H). 13C-NMR (75 MHz, DMSO-d6) δ: 178.6, 160.0, 147.8, 129.8, 128.1, 114.0, 67.2, 30.7, 18.7, 13.8, 13.7. ESI-MS m/z = 266.2 [M + H]+. Anal. Calcd for C13H19N3OS: C, 58.84; H, 7.22; N, 15.83. Found C, 59.06; H, 7.18; N, 15.86.
2-(1-(4-iso-Butoxyphenyl)ethylidene)hydrazine-1-carbothioamide (6h)Solid product, yield 86%, mp 148–149°C. 1H-NMR (300 MHz, DMSO-d6) δ: 10.07 (s, 1H), 8.17 (s, 1H), 8.86 (s, 1H), 7.84 (d, J = 8.7 Hz, 2H), 6.89 (d, J = 8.8 Hz, 2H), 3.76 (d, J = 6.5 Hz, 2H), 2.25 (s, 3H), 2.07–1.94 (m, 1H), 0.98 (d, J = 6.7 Hz, 6H). 13C-NMR (75 MHz, DMSO-d6) δ: 179.3, 160.4, 148.5, 130.6, 128.7, 114.8, 74.6, 28.5, 19.9, 14.7. ESI-MS m/z = 266.2 [M + H]+. Anal. Calcd for C13H19N3OS: C, 58.84; H, 7.22; N, 15.83. Found C, 59.14; H, 7.15; N, 15.84.
2-(1-(4-(4-Chlorobutoxy)phenyl)ethylidene)hydrazine-1-carbothioamide (6i)Solid product, yield 83%, mp 159–160°C. 1H-NMR (300 MHz, DMSO-d6) δ: 10.15 (s, 1H), 8.26 (s, 1H), 7.91 (s, 1H), 7.43 (d, J = 8.9 Hz, 2H), 7.26 (t, J = 7.9 Hz, 1H), 6.93 (d, J = 8.1 Hz, 1H), 4.04 (t, J = 5.3 Hz, 2H), 3.70 (t, J = 5.7 Hz, 2H), 2.27 (s, 3H), 1.99–1.75 (m, 4H). 13C-NMR (75 MHz, DMSO-d6) δ: 178.8, 158.5, 147.9, 139.1, 129.2, 119.1, 115.4, 112.4, 66.7, 45.2, 28.9, 26.1, 14.2. ESI-MS m/z = 300.2 [M + H]+. Anal. Calcd for C13H18ClN3OS: C, 52.08; H, 6.05; N, 14.02. Found C, 52.47; H, 6.09; N, 13.69.
2-(1-(4-Pentoxyphenyl)ethylidene)hydrazine-1-carbothioamide (6j)Solid product, yield 82%, mp 122–123°C. 1H-NMR (300 MHz, DMSO-d6) δ: 10.18 (s, 1H), 8.27 (s, 1H), 7.89 (d, J = 8.9 Hz, 3H), 6.91 (d, J = 9.0 Hz, 2H), 3.96 (t, J = 6.5 Hz, 2H), 2.29 (s, 3H), 1.77–1.63 (m, 2H), 1.45–1.25 (m, 4H), 0.89 (t, J = 7.0 Hz, 3H). 13C-NMR (75 MHz, DMSO-d6) δ: 176.2, 157.3, 145.4, 127.5, 125.7, 111.6, 65.1, 26.0, 25.3, 19.6, 11.5, 11.5. ESI-MS m/z = 280.3 [M + H]+. Anal. Calcd for C14H21N3OS: C, 60.18; H, 7.58; N, 15.04. Found C, 60.46; H, 7.60; N, 14.92.
2-(1-(4-Hexoxyphenyl)ethylidene)hydrazine-1-carbothioamide (6k)Solid product, yield 82%, mp 140–141°C. 1H-NMR (300 MHz, DMSO-d6) δ: 10.15 (s, 1H), 8.24 (s, 1H), 7.88 (d, J = 8.8 Hz, 3H), 6.91 (d, J = 8.9 Hz, 2H), 3.97 (t, J = 6.4 Hz, 2H), 2.27 (s, 3H), 1.78–1.63 (m, 2H), 1.49–1.23 (m, 7H), 0.87 (t, J = 6.7 Hz, 3H). 13C-NMR (75 MHz, DMSO-d6) δ: 178.6, 159.6, 147.7, 129.8, 128.1, 113.9, 67.5, 31.0, 28.6, 25.2, 22.1, 13.9, 13.8. ESI-MS m/z = 294.3 [M + H]+.
2-(1-(4-Cyclohexyloxyphenyl)ethylidene)hydrazine-1-carbothioamide (6l)Solid product, yield 88%, mp 160–161°C. 1H-NMR (300 MHz, DMSO-d6) δ: 10.09 (s, 1H), 8.18 (s, 1H), 7.84 (d, J = 8.3 Hz, 3H), 6.91 (d, J = 8.6 Hz, 2H), 4.40 (s, 1H), 2.28 (s, 3H), 2.00–1.85 (m, 2H), 1.80–1.65 (m, 2H), 1.60–1.22 (m, 6H). 13C-NMR (75 MHz, DMSO-d6) δ: 179.3, 159.0, 148.6, 130.5, 128.8, 116.0, 75.1, 32.1, 26.0, 23.9, 14.7. ESI-MS m/z = 292.3 [M + H]+.
2-(1-(4-Heptyloxyphenyl)ethylidene)hydrazine-1-carbothioamide (6m)Solid product, yield 79%, mp 126–127°C. 1H-NMR (300 MHz, DMSO-d6) δ: 10.16 (s, 1H), 8.25 (s, 1H), 7.88 (d, J = 8.9 Hz, 3H), 6.90 (d, J = 9.0 Hz, 2H), 3.97 (t, J = 6.4 Hz, 2H), 2.27 (s, 3H), 1.77–1.63 (m, 2H), 1.46–1.18 (m, 8H), 0.87 (t, J = 9.1 Hz, 3H). 13C-NMR (75 MHz, DMSO-d6) δ: 178.58, 159.63, 147.71, 129.82, 128.04, 113.93, 67.45, 31.23, 28.64, 28.47, 25.47, 22.05, 13.89, 13.79. ESI-MS m/z = 308.3 [M + H]+. Anal. Calcd for C16H25N3OS: C, 62.50; H, 8.20; N, 13.67. FoundC, 62.43; H, 8.10; N, 13.48.
2-(1-(4-Octyloxyphenyl)ethylidene)hydrazine-1-carbothioamide (6n)Solid product, yield 77%, mp 127–128°C. 1H-NMR (300 MHz, DMSO-d6) δ: 10.08 (s, 1H), 8.18 (s, 1H), 7.83 (d, J = 8.9 Hz, 3H), 6.87 (d, J = 8.9 Hz, 2H), 3.95 (t, J = 6.4 Hz, 2H), 2.25 (s, 3H), 1.76–1.64 (m, 2H), 1.39–1.25 (m, 10H), 0.83 (d, J = 6.8 Hz, 3H). 13C-NMR (75 MHz, DMSO-d6) δ: 179.2, 160.3, 148.4, 130.5, 128.7, 114.7, 68.3, 32.1, 29.6, 29.5, 26.4, 22.9, 14.8, 14.7. ESI-MS m/z = 322.4 [M + H]+. Anal. Calcd for C17H27N3OS: C, 63.51; H, 8.47; N, 13.07. Found C, 63.44; H, 8.38; N, 12.95.
2-(1-(4-Phenoxyphenyl)ethylidene)hydrazine-1-carbothioamide (6o)Solid product, yield 82%, mp 171–172°C. 1H-NMR (300 MHz, DMSO-d6) δ: 10.15 (s, 1H), 8.22 (s, 1H), 7.92 (d, J = 8.9 Hz, 2H), 7.88 (s, 1H), 7.43–7.30 (m, 2H), 7.13 (t, J = 7.4 Hz, 1H), 7.03–6.98 (m, 2H), 6.93 (d, J = 8.9 Hz, 2H), 2.25 (s, 3H). 13C-NMR (75 MHz, DMSO-d6) δ: 178.8, 157.7, 156.1, 147.2, 132.7, 130.1, 128.5, 123.8, 119.0, 117.8, 13.9. ESI-MS m/z = 286.4 [M + H]+.
2-(1-(4-((4-Bromobenzyl)oxy)phenyl)ethylidene)-hydrazine-1-carbothioamide (6p)Solid product, yield 81%, mp 197–198°C. 1H-NMR (300 MHz, DMSO-d6) δ: 10.08 (s, 1H), 8.17 (s, 1H), 7.86 (d, J = 8.8 Hz, 3H), 7.57 (d, J = 8.3 Hz, 2H), 7.39 (d, J = 8.4 Hz, 2H), 6.96 (d, J = 8.9 Hz, 2H), 5.12 (s, 2H), 2.24 (s, 3H). 13C-NMR (75 MHz, DMSO-d6) δ: 178.6, 159.0, 147.7, 136.4, 131.3, 130.4, 129.8, 128.13, 120.9, 114.4, 68.4, 13.8. ESI-MS m/z = 378.2 [M + H]+. Anal. Calcd for C16H16BrN3OS: C, 50.80; H, 4.26; N, 11.11. Found C, 50.61; H, 4.33; N, 10.83.
2-(1-(4-(3-Phenylpropoxy)phenyl)ethylidene)hydrazine-1-carbothioamide (6q)Solid product, yield 85%, mp 148–149°C. 1H-NMR (300 MHz, DMSO-d6) δ: 10.10 (s, 1H), 8.19 (s, 1H), 7.85 (d, J = 8.6 Hz, 3H), 7.31–7.11 (m, 5H), 6.89 (d, J = 8.8 Hz, 2H), 3.97 (t, J = 6.2 Hz, 2H), 2.73 (t, J = 7.6 Hz, 2H), 2.25 (s, 3H), 2.06–1.95 (m, 2H). 13C-NMR (75 MHz, DMSO-d6) δ: 179.3, 160.2, 148.5, 142.0, 130.7, 129.0, 128.8, 126.5, 114.8, 67.6, 32.3, 31.2, 14.7. ESI-MS m/z = 328.3 [M + H]+. Anal. Calcd for C18H21N3OS: C, 66.02; H, 6.46; N, 12.83. Found C, 66.13; H, 6.47; N, 12.78.
Ethyl 2-(4-(1-(2-Carbamothioylhydrazono)ethyl)phenoxy)-acetate (6r)Solid product, yield 81%, mp 156–157°C. 1H-NMR (300 MHz, DMSO-d6) δ: 10.09 (s, 1H), 8.18 (s, 1H), 7.85 (d, J = 8.7 Hz, 3H), 6.89 (d, J = 8.8 Hz, 2H), 4.81 (s, 2H), 4.15 (q, J = 7.1 Hz, 2H), 2.25 (s, 3H), 1.21 (t, J = 7.1 Hz, 3H). 13C-NMR (75 MHz, DMSO-d6) δ: 179.3, 169.1, 159.1, 148.3, 131.4, 128.7, 114.8, 65.5, 61.5, 14.9, 14.7. ESI-MS m/z = 296.2 [M + H]+. Anal. Calcd for C13H17N3O3S:C, 52.86; H, 5.80; N, 14.23. Found C, 52.74; H, 5.67; N, 14.19.
2-(4-(1-(2-Carbamothioylhydrazono)ethyl)phenoxy)acetic Acid (6s)Solid product, yield 91%, mp 197–198°C. 1H-NMR (300 MHz, DMSO-d6) δ: 12.97 (s, 1H), 10.10 (s, 1H), 8.18 (s, 1H), 7.85 (d, J = 8.6 Hz, 2H), 6.87 (d, J = 8.7 Hz, 2H), 4.70 (s, 2H), 2.25 (s, 3H). 13C-NMR (75 MHz, DMSO-d6) δ: 179.3, 170.6, 159.3, 148.4, 131.2, 128.7, 114.8, 65.3, 14.7. ESI-MS m/z = 266.1 [M-H]−.
Ethyl 4-(4-(1-(2-Carbamothioylhydrazono)ethyl)phenoxy)-butanoate (6t)Solid product, yield 71%, mp 129–130°C. 1H-NMR (300 MHz, DMSO-d6) δ: 10.09 (s, 1H), 8.18 (s, 1H), 7.84 (d, J = 8.5 Hz, 3H), 6.88 (d, J = 8.6 Hz, 2H), 4.11–3.94 (m, 4H), 2.44 (t, J = 7.2 Hz, 2H), 2.25 (s, 3H), 2.04–1.89 (m, 2H), 1.16 (t, J = 7.1 Hz, 3H). 13C-NMR (75 MHz, DMSO-d6) δ: 179.2, 173.1, 160.0, 148.4, 130.7, 128.8, 114.7, 67.3, 60.6, 31.0, 25.0, 15.0, 14.7. ESI-MS m/z = 324.3 [M + H]+.
4-(4-(1-(2-Carbamothioylhydrazono)ethyl)phenoxy)butanoic Acid (6u)Solid product, yield 89%, mp 197–198°C. 1H-NMR (300 MHz, DMSO-d6) δ: 12.12 (s, 1H), 10.08 (s, 1H), 8.17 (s, 1H), 7.84 (d, J = 8.3 Hz, 2H), 6.89 (d, J = 8.5 Hz, 2H), 4.00 (t, J = 6.2 Hz, 2H), 2.38 (t, J = 7.2 Hz, 2H), 2.25 (s, 3H), 2.02–1.85 (m, 2H). 13C-NMR (75 MHz, DMSO-d6) δ: 179.2, 174.6, 160.1, 148.5, 130.7, 128.8, 114.7, 67.4, 30.9, 25.0, 14.7. ESI-MS m/z = 294.1 [M-H]−.
2-(1-(4-(2,2-Diethoxyethoxy)phenyl)ethylidene)hydrazine-1-carbothioamide (6v)Solid product, yield 75%, mp 136–137°C. 1H-NMR (300 MHz, DMSO-d6) δ: 10.09 (s, 1H), 8.18 (s, 1H), 7.85 (d, J = 8.9 Hz, 3H), 6.92 (d, J = 9.0 Hz, 2H), 4.79 (t, J = 5.1 Hz, 1H), 3.98 (d, J = 5.1 Hz, 2H), 3.75–3.47 (m, 4H), 2.25 (s, 3H), 1.14 (t, J = 7.0 Hz, 6H). 13C-NMR (75 MHz, DMSO-d6) δ: 179.2, 159.8, 148.3, 131.0, 128.8, 114.85, 100.6, 68.9, 62.7, 16.2, 14.7. ESI-MS m/z = 326.3 [M + H]+. Anal. Calcd for C15H23N3O3S: C, 55.36; H, 7.12; N, 12.91. Found C, 55.66; H, 7.14; N, 13.24.
2-(4-(3-Hydroxypropoxy)benzylidene)hydrazine-1-carbothioamide (11)Yellowish solid product, yield 85%, mp 172–173°C. 1H-NMR (300 MHz, DMSO-d6) δ: 11.34 (s, 1H), 8.14 (s, 1H), 8.02 (s, 1H), 7.94 (s, 1H), 7.74 (d, J = 8.8 Hz, 1H), 6.96 (d, J = 8.8 Hz, 1H), 4.60 (t, J = 5.1 Hz, 1H), 4.08 (t, J = 6.4 Hz, 2H), 3.58 (t, J = 6.1 Hz, 2H), 1.88-1.91 (m, 2H). 13C-NMR (75 MHz, DMSO-d6) δ: 177.5, 160.1, 142.3, 128.9, 126.52, 114.5, 64.7, 57.2, 32.0. ESI-MS m/z = 254.3 [M + 1]+. Anal. Calcd for C11H15N3O2S: C, 52.16; H, 5.97. Found C, 52.30; H, 6.01.
2-(3-Ethyoxybenzylidene)hydrazine-1-carbothioamide (12a)Solid product, yield 92%, mp 165–166°C. 1H-NMR (300 MHz, DMSO-d6) δ: 11.44 (s, 1H), 8.27 (d, J = 19.3 Hz, 1H), 8.09 (s, 1H), 8.03 (s, 1H), 7.45 (s, 1H), 7.38–7.17 (m, 2H), 7.02–6.90 (m, 1H), 4.07 (q, J = 7.0 Hz, 2H), 1.41–1.26 (m, 3H). 13C-NMR (75 MHz, DMSO-d6) δ: 177.9, 158.8, 142.2, 135.5, 129.7, 120.5, 116.6, 111.4, 63.1, 14.6. ESI-MS m/z = 224.2 [M + 1]+.
2-(3-Propoxybenzylidene)hydrazine-1-carbothioamide (12b)Solid product, yield 89%. mp 161–162°C. 1H-NMR (300 MHz, DMSO-d6) δ: 11.42 (s, 1H), 8.21 (s, 1H), 8.08 (s, 1H), 8.01 (s, 1H), 7.44 (s, 1H), 7.34–7.20 (m, 2H), 6.99–6.92 (m, 1H), 3.97 (t, J = 6.5 Hz, 2H), 1.81–1.65 (m, 2H), 0.99 (t, J = 7.4 Hz, 3H). 13C-NMR (75 MHz, DMSO-d6) δ: 177.9, 159.0, 142.2, 135.5, 129.7, 120.5, 116.6, 111.5, 69.0, 22.0, 10.4. ESI-MS m/z = 238.2 [M + 1]+.
2-(3-Butoxybenzylidene)hydrazine-1-carbothioamide (12c)Solid product, yield 86%, mp 151–152°C. 1H-NMR (300 MHz, DMSO-d6) δ: 11.35 (s, 1H), 8.18 (s, 1H), 8.04 (s, 1H), 7.97 (s, 1H), 7.41 (s, 1H), 7.3–7.17 (m, 2H), 6.92 (d, J = 9.1 Hz, 1H), 3.99 (t, J = 6.4 Hz, 2H), 1.76–1.61 (m, 2H), 1.51–1.36 (m, 2H), 0.93 (t, J = 7.3 Hz, 3H). 13C-NMR (75 MHz, DMSO-d6) δ: 177.9, 159.0, 142.2, 135.5, 129.6, 120.5, 116.6, 111.5, 67.2, 30.8, 18.7, 13.7. Anal. Calcd for C12H17N3OS: C, 57.34; H, 6.82. Found C, 57.30; H, 6.86.
Assay of Inhibitory Activities of Target CompoundsThe inhibition of target compounds on the diphenolase activity of mushroom tyrosinase was investigated by the reported procedure45–53) with some slight modifications. Briefly, all the synthesized compounds were screened for the diphenolase inhibitory activity of tyrosinase using L-DOPA as substrate. Thiosemicarbazide, kojic acid (used as a control) and all the target compounds were dissolved in DMSO and their final concentration in DMSO was 2.0%. Phosphate buffer, pH 6.8, was used to dilute the DMSO stock solution of test compounds. Thirty units of tyrosinase (0.5 mg/mL) was first pre-incubated with the samples, in 50 mM phosphate buffer, for 10 min at 25°C, L-DOPA solution (0.5 mM) was added to the mixture and the enzyme reaction was monitored by measuring the change in absorbance at 475 nm of formation of DOPAchrome for 1 min. The measurement was completed in triplicate for each concentration and averaged before further calculation. IC50 value was determined by interpolation of the dose-response curves. The assay of inhibition mechanism and inhibition types of selected compounds 5a and 6a on mushroom tyrosinase was finished as the reported protocol.45–53)
Assay of Cytotoxicity of Selected Compounds 5a, 6e, 6g and 6t to 293T Cell LineThe assay of cytotoxicity of selected compounds to the 293T cell line was finished as the generally reported protocol.65–70) Briefly, the 293T cell suspension was prepared and 100 µL of the suspension was injected in each tube placed in 96-hole culture plate, and the culture plate was pre-cultured in the incubator for 24 h (37°C, 5% CO2); 10 µL of different-concentration solution of 5a was added to each cell line tube and the final concentration of 5a was respectively kept in 31.25, 62.5, 125, 250, 500, 1000 µmol/L; The culture plate was incubated for 24 h in the incubator, then 10 µL of CCK-8 solution was added into each tube in 96-hole plate; After the culture plate was incubated for 4 h in the incubator, the absorbance of the mixture in each tube at 450 nm was determined using micro-plate reader.
The cell line tube to which compound 5a was not injected was used as reference and the cell-proliferation number of this cell line was assumed “1.” At this time the cell proliferation number for each cell line tube was determined as 1.012, 1.086, 1.086, 1.027, 1.119, 1.014. With the same method, we also determined the cytotoxicities of compounds 6e, 6g and 6t to 293T cell line.
We thank the GDAS’ Project of Science and Technology Development (2018GDASCX-1014 and 2018GDASCX-0807), the Project of Tianhe District, Guangzhou for Innovation and Entrepreneurship Leading Talents, and the Project of Foshan Science and Technology Innovation for financial support of this study.
The authors declare no conflict of interest.