2020 Volume 68 Issue 5 Pages 443-446
2020 Volume 68 Issue 5 Pages 443-446
Coumarin moiety has garnered momentous attention especially in the design of compounds with significant biological activities. In this work, a series of 3-substituted coumarin derivatives 6a–6l were synthesized and fully characterized. Most of the compounds could obviously inhibit the activity of cyclooxygenase-1 (COX-1) at the concentration of 10 µM. Besides, 6h and 6l exhibited highest inhibitory effects against COX-2 with inhibition rates of 33.48 and 35.71%, respectively. Detailed structure–activity relationships (SARs) were also discussed. In vivo studies, 6b, 6i and 6l could remarkably repress the xylene-induced ear swelling in mice at the dose of 20 mg/kg. Especially, 6l seemed to be the most effective compound at the dose of 10 mg/kg, displaying favorable anti-inflammatory activity comparable to indomethacin. All of these findings suggested that 6l might be utilized as a candidate for the treatment of inflammatory diseases.
Inflammation is a primary defensive response of living tissue to various damage factors, such as biological pathogens, toxic chemicals, irritants and other harmful stimuli.1,2) As a complex biological and physiological process, inflammation is characterized by five main symptoms, including swelling, redness, heat, pain and local dysfunction.3) Inflammation is a protective immune response and is usually beneficial. However, persistent and exaggerated inflammation will promote tissue damage and lead to diseases, for instance, arthritis, sepsis, atherosclerosis, and even cancer.4–6)
Based on the structure and therapeutic mechanism, anti-inflammatory drugs can be divided into two types, of which nonsteroidal anti-inflammatory drugs (NSAIDs) are the most widely administered drugs for the treatment of inflammation.7) NSAIDs, such as indomethacin and ibuprofen, act their antipyretic, analgesic and anti-inflammatory effects by inhibiting cyclooxygenase-1 and cyclooxygenase-2 (COX-1 and COX-2), which are key enzymes involved in the pathway that produces prostaglandins (PGs).8,9) However, NSAIDs may cause some unexpected side effects, such as peptic ulcer, liver damage and anaphylaxis.10) Thus, it is still quite necessary for us to develop and explore anti-inflammatory drugs with better therapeutic effects.
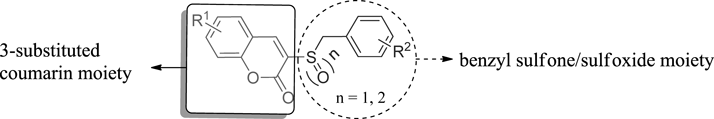
The coumarin skeleton, also known as benzo-α-pyrone, has attracted voluminous attention for its ability to form non-covalent interaction with the active sites of the target protein.11) Given its favorable pharmacological activity, benzo-α-pyrone has been used as an indispensible structural subunit for the discovery of drugs with improved pharmacological profiles.12) In recent years, coumarins and related derivatives have displayed their diverse biological activities, such as anti-cancer,13) antibacterial,14) antioxidant15) and anti-inflammatory.16) Furthermore, some coumarins with different pharmacophores at C-3 position have been evaluated for anti-inflammatory activities.17–20)
Sulfone and sulfoxide derivatives containing heterocyclic moieties belong to an important class of active compounds possessing various biological activities.21,22) It has been reported that the combination of distinct pharmacophores in the same structure is very likely to obtain compounds with significant activity.23) Thus, in order to develop novel anti-inflammatory agents, benzyl sulfone/sulfoxide moieties were introduced to the C-3 position of coumarin skeleton and the target compounds, 3-substituted coumarin derivatives were designed and synthesized (Fig. 1 and Chart 1). Anti-inflammatory activity of compounds 6a–6l was preliminarily evaluated in mouse RAW 264.7 macrophages. Most of the compounds could markedly restrain the release of lipopolysaccharide (LPS)-induced tumor necrosis factor (TNF)-α at non-cytotoxic concentrations. Besides, all compounds were evaluated for cyclooxygenase inhibitory activity in cellular level by the enzyme-linked immuno-sorbent assay (ELISA) in vitro. In addition, 6b, 6c, 6d, 6h, 6i, 6l were selected for further anti-inflammatory study in vivo by the xylene-induced ear swelling method.

Reagents and Conditions: (i) HSCH2COOH, NaOH, CH3OH, rt, 1–2 h, 63–87%; (ii) H2O2, NaOH, H2O, rt, 4 h, 61–88%; (iii) H2O2, CH3COOH, 55°C, 5 h, 63–77%; (iv) EDCI, DMAP, CH3CN, rt, 1 h, 26–58%; (v) CH3COONa, (CH3CO)2O, 110°C, 0.5 h, 29–62%.
The target compounds 6a–6l were synthesized via a three-step synthetic route from substituted benzylchloride/bromide (1a–1j) as outlined in Chart 1. The starting material 1a–1c were treated with mercaptoacetic acid at the presence of sodium hydroxide to give benzylmercaptoacetic acids 2a–2c in 63–87% yields.24) Treatment of 2a–2c with 30% hydrogen peroxide at room temperature gave benzylsulfinylacetic acids 3a–3c24) or at heating condition gave benzylsulfonylacetic acids 4a–4c with satisfactory yields.25) Finally, the target compounds 6a–6l were synthesized via knoevenagel reaction26) between 3a–3c or 4a–4c and substituted salicylaldehydes (5a–5d). The expected compounds 6a–6h were prepared from 3a, 3b, 3c or 4c with 5a–5d at the presence of EDCI in 26–58% yields. And 6i–6l were obtained from 4a, 4b with 5a, 5c or 5d in acetic anhydride with different yields ranging from 29 to 62%. All the target compounds were purified by recrystallization or flash chromatography and their structures were confirmed by 1H-NMR, 13C-NMR and high resolution (HR) MS spectra analysis.
Cell Viability AssayThe cytotoxicity of coumarin derivatives 6a–6l (2.5, 5, 10 and 20 µM) on RAW264.7 macrophages was evaluated by CCK-8 (Cell Counting Kit-8, WST-8) assay27) after 24 h of treatment. As observed from the cell viability data in Fig. 2(A), at the concentration of up to 10 µM, all compounds generated no cytotoxicity to RAW264.7 with cell viability higher than 85%. At the concentration of 20 µM, the viability of 6h-treated cells was just 81.79%. Thus, the concentration of 10 µM was selected to evaluate coumarin derivatives in the following TNF-α detection.

Data were expressed as the mean ± S.D. of three independent experiments. Compared with the LPS group, * p < 0.01, ** p < 0.001. DEX: dexamethasone; IND: indomethacin.
Macrophages are well known to play an important role in the initiation and development of inflammation.28) Activated macrophages induced by LPS produce cytokines such as TNF-α, interleukin, and pre-inflammatory mediators, including nitric oxide (NO) and PGs.29) Studies have proved that over release of cytokines and pro-inflammatory mediators will lead to inflammatory diseases.30) In order to evaluate the anti-inflammatory activity of all the target compounds 6a–6l in vitro, ELISA was used to screen the production of TNF-α induced by LPS in RAW264.7 macrophages.31) As showed in Fig. 2(B), at the concentration of 10 µM, most of the tested compounds could significantly inhibit the secretion of TNF-α compared with the LPS group. Especially, 6c, 6d, 6h and 6i most strongly restrained the secretion of TNF-α.
In Vitro Cyclooxygenase Inhibition and Structure–Activity Relationship (SAR) StudyThe 12 newly synthesized compounds 6a–6l were first evaluated for cyclooxygenase inhibitory activity in cellular level by ELISA assay,7) using indomethacin as a comparison. Based on the results displayed in Table 1, all compounds exhibited favorable inhibitory activity against COX-1 at the concentration of 10 µM, except for 6j. Compounds 6a, 6e and 6g exhibited excellent inhibitory potency with inhibition rates of 46.76, 46.24 and 45.57%, respectively, which were comparable to that of indomethacin (51.11%). Compounds 6b, 6c, 6d, 6h, 6i, 6k and 6l also exhibited moderate inhibitory effects against COX-2 with inhibition rates above 25% at the concentration of 10 µM. Besides, 6h and 6l exhibited the highest potency with inhibition rates of 33.48 and 35.71%, respectively, and could be potent COX-2 inhibitors.
| Compound | Inhibition Rates (%) | |||
|---|---|---|---|---|
| COX-1 | COX-2 | |||
| 5 µM | 10 µM | 5 µM | 10 µM | |
| IND | 35.14 ± 5.88 | 51.11 ± 6.59 | 19.02 ± 2.17 | 35.26 ± 5.34 |
| 6a | 21.66 ± 3.97 | 46.76 ± 4.56 | 13.01 ± 3.54 | 21.58 ± 4.59 |
| 6b | 21.00 ± 2.97 | 34.74 ± 6.44 | 16.80 ± 5.56 | 25.47 ± 4.89 |
| 6c | 29.99 ± 2.81 | 43.59 ± 2.87 | 16.24 ± 3.93 | 21.02 ± 2.37 |
| 6d | 23.25 ± 5.28 | 41.61 ± 4.24 | 13.79 ± 4.79 | 24.36 ± 3.36 |
| 6e | 28.67 ± 3.90 | 46.24 ± 4.64 | 8.90 ± 2.00 | 12.46 ± 3.91 |
| 6f | 23.65 ± 3.60 | 36.20 ± 6.00 | 10.01 ± 2.37 | 13.46 ± 3.21 |
| 6g | 25.63 ± 6.44 | 45.57 ± 1.99 | 10.12 ± 1.90 | 15.24 ± 2.41 |
| 6h | 30.38 ± 2.78 | 31.70 ± 5.96 | 16.13 ± 2.59 | 33.48 ± 5.02 |
| 6i | 19.82 ± 2.39 | 41.22 ± 4.38 | 20.58 ± 5.24 | 32.15 ± 3.72 |
| 6j | 12.15 ± 4.59 | 19.02 ± 4.20 | 11.90 ± 3.18 | 19.35 ± 4.54 |
| 6k | 11.62 ± 4.80 | 40.29 ± 7.05 | 9.45 ± 2.22 | 22.91 ± 2.52 |
| 6l | 31.04 ± 5.24 | 38.84 ± 5.17 | 18.91 ± 2.73 | 35.71 ± 4.01 |
From the data of COX-1 inhibitory activities, some SARs can be observed: (i) the bioactivity of sulfoxides was higher than the corresponding sulfones (6a > 6i, 6c > 6b); (ii) the type, number and position of R1 seemed to play important roles for the activity: 7-OCH3 > 5,7-(OCH3)2 > 6-Br (6g > 6f, 6a > 6h). When it came to COX-2 inhibitory activity, some interesting SARs were illustrated: (i) the bioactivity of sulfones was higher than the corresponding sulfoxides (6i > 6a, 6b > 6c); (ii) the type, number and position of R1 also played important roles for the activity: 6-Br (6h) > 5,7-(OCH3)2 (6a), 7-OCH3 (6g) > 5,7-(OCH3)2 (6f).
NSAIDs exert their anti-inflammatory effects mainly by inhibiting COX-2, while the inhibition of COX-1 may contribute to their unwanted side effects, such as gastric and renal damage.32) Compounds with higher inhibitory potency on COX-2 but lower inhibitory activity against COX-1 were thought to be idea anti-inflammatory agents. Then 6b, 6c, 6d, 6h, 6i, 6l were selected for further study in vivo.
Anti-inflammatory Activity Evaluation in VivoThe process of inflammation is related to the increase of blood flow, capillary permeability and migration of macrophages and neutrophils from capillaries to interstitial spaces. As more fluid continues to accumulate in the interstitial space, the damaged tissue begins to swell.33) Thus, swelling becomes one of the main symptoms of inflammation.34) The anti-inflammatory activities in vivo were screened in mice model of xylene-induced ear swelling, with dexamethasone and indomethacin as reference drugs. According to the results in Table 2, the target compounds (6b, 6c, 6d, 6h, 6i and 6l) exhibited different degrees of anti-inflammatory activities under the experimental conditions. At the dose of 20 mg/kg, these compounds could obviously repress ear swelling with inhibition rates from 27.76 to 41.43%. At the dose of 10 mg/kg, 6b, 6i and 6l suppressed the swelling with inhibition rates above 30%. Remarkably, compound 6l exhibited the best anti-inflammatory activity, with inhibition rate of 34.56%, which was comparable to indomethacin.
| Group | Swelling degree (mg) | Inhibition rate (%) | ||
|---|---|---|---|---|
| 20 mg/kg | 10 mg/kg | 20 mg/kg | 10 mg/kg | |
| Control | 16.01 ± 3.81 | — | ||
| DEX | N.T. | 7.02 ± 1.94** | N.T. | 56.14 |
| IND | N.T. | 9.92 ± 2.14** | N.T. | 38.03 |
| 6b | 9.43 ± 2.19** | 10.96 ± 2.03** | 41.08 | 31.58 |
| 6c | 11.57 ± 2.99* | 12.84 ± 2.84 | 27.76 | 19.78 |
| 6d | 10.83 ± 2.40** | 11.93 ± 2.99* | 32.34 | 25.47 |
| 6h | 10.40 ± 3.07** | 11.40 ± 3.05* | 35.04 | 28.80 |
| 6i | 9.72 ± 1.68** | 11.57 ± 2.67** | 39.28 | 30.46 |
| 6l | 9.38 ± 2.83** | 10.48 ± 3.12** | 41.43 | 34.56 |
Data were expressed as the mean ± S.D., n = 9. Compared with the LPS group, * p < 0.05, ** p < 0.01. “NT”: not test.
In summary, to obtain effective lead compounds that can serve as anti-inflammatory agents, we have designed and synthesized a total of twelve coumarin derivatives linked substituted benzyl sulfone/sulfoxide moieties at C-3 position. The anti-inflammatory effects of these compounds were evaluated in vitro and in vivo, including the inhibition of TNF-α production induced by LPS in RAW264.7 macrophages, cyclooxygenase inhibition study and xylene-induced ear swelling in mice. Results of the in vitro study provided evidence that most of the compounds could repress the release of TNF-α and exhibited favorable inhibitory activity against COX-1 at the concentration of 10 µM. Moreover, 6h and 6l exhibited the highest inhibitory potency on COX-2. In addition, at the dose of 20 mg/kg, the active compounds 6b, 6c, 6d, 6h, 6i and 6l could obviously repress ear swelling in vivo. Especially, 6l displayed satisfactory inhibitory activity similar to indomethacin at the dose of 10 mg/kg. All of these results reveal that 6l may be a lead compound working on cyclooxygenase in inflammation therapy and is worthy of further study and optimization.
General chemistry methods, synthesis procedures, spectral data, and bioassay methods are given in Supplementary materials.
This research was supported by National Natural Science Foundation of China (Grant No. 81273431).
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.