2020 Volume 68 Issue 5 Pages 452-465
2020 Volume 68 Issue 5 Pages 452-465
This study reports the synthesis and evaluation of novel indirect AMP-activated protein kinase (AMPK) activators. The series of compounds selectively inhibited cell growth in several human breast cancer cell lines by activating AMPK. We performed back-up medicinal chemistry synthetic research on ASP4132, a previously reported as a compound for clinical development that acts as an indirect AMPK activator. This led to the successful identification of 4-({4-[5-({1-[(5-ethoxypyrazin-2-yl)methyl]-4-fluoropiperidin-4-yl}methoxy)-3-methylpyridine-2-carbonyl]piperazin-1-yl}methyl)benzonitrile succinate (27b), a potent, highly aqueous soluble and metabolically stable compound in human hepatocytes. Compound 27b also showed weaker human Ether-a-go-go Related Gene (hERG) inhibitory activity than that of compound 13 and ASP4132. Therefore, 27b was a promising AMPK activator and a second-generation clinical candidate for treatment for human cancer.
AMP-activated protein kinase (AMPK), a heterotrimeric serine/threonine protein kinase, was first described for its important role in lipid metabolism and regulation of cholesterol and fatty acid levels.1,2) Since then, AMPK has been known as a master player in metabolism because it maintains energy homeostasis during metabolic stress both at the cellular and physiological levels.1,3) AMPK is generally activated in response to a negative energy balance by sensing increases in the ratios of AMP to ATP and adenosine ADP to ATP, and regulates energy balance by inhibiting consumption of ATP while promoting generation of ATP.1,4)
Disruption of cellular energetics is a core feature of cancer.5–7) Therefore, AMPK activation may lead to metabolic tumor suppression as a result of its regulation of energy balance, enforcement of metabolic checkpoints and inhibition of cell growth.8) In fact, a number of studies have demonstrated that AMPK exerts its tumor suppressor function in human cancers via several downstream effectors, such as mammalian target of rapamycin (mTOR),9) p5310,11) and acetyl-CoA carboxylase (ACC).12,13) Thus, AMPK activators may be suitable therapeutic targets for the treatment of cancer.
We recently reported ASP4132, (5-{1-[(6-methoxypyridin-3-yl)methyl]piperidin-4-yl}-1H-benzimidazol-2-yl)(4-{[4-(trifluoromethyl)phenyl]methyl}piperazin-1-yl)methanone ditosylate, as a compound for clinical trial and an indirect AMPK activator that shows potent and selective cell growth inhibitory activity against human breast cancer cell lines, and suitable in vivo anti-tumor efficacy in a xenograft mouse model following oral administration14) (Fig. 1).

While ASP4132 showed potent in vitro and in vivo anti-tumor activity, good in vitro metabolic stability in human hepatocytes and favorable in vivo animal pharmacokinetics (PK) profiles, its aqueous solubility at gastrointestinal pH was moderate. Considering the potential issues that may arise due to its moderate aqueous solubility, which may lead to limited oral absorption at high doses in clinical dose escalation studies, we planned to identify a second generation clinical candidate with good aqueous solubility at gastrointestinal pH while maintaining the pharmacological and absorption, distribution, metabolism, excretion (ADME) profiles of ASP4132.
Previously, a study of GlaxoSmithKline reported that oral drug candidates with more than three aromatic rings typically have poorer compound developability.15) According to this review, aqueous solubility dramatically decreases with increasing aromatic ring count (the number of aromatic and heteroaromatic rings). ASP4132 contains four aromatic rings, suggesting that decreasing the number of aromatic rings is one strategy to improve aqueous solubility. A structure–activity relationship study of ASP4132 and its derivatives indicated that both of the terminal aromatic moieties were important for their AMPK activation activities. Therefore, we considered that the benzimidazole moiety of ASP4132 should be replaced with a monocyclic aromatic moiety.
We previously reported that anilide linkage compound 1, which has three aromatic rings, was a potent AMPK activator14) (Fig. 2). While the benzimidazole scaffold of ASP4132 was effective for producing good animal PK profiles, probably due to elimination of the need for metabolic hydrolysis of an anilide linkage,14) we decided to conduct optimization using a different approach, one that does not increase the aromatic ring count, to acquire a compound with both good aqueous solubility and animal PK profiles.

Herein, we report the discovery of a second generation orally active, potent and selective AMPK activator. Our backup medicinal chemistry strategy using compound 1 as the lead compound led to the identification of 4-({4-[5-({1-[(5-ethoxypyrazin-2-yl)methyl]-4-fluoropiperidin-4-yl}methoxy)-3-methylpyridine-2-carbonyl]piperazin-1-yl}methyl)benzonitrile succinate (27b). Compound 27b showed good aqueous solubility, excellent animal PK and weak human ether-a-go-go related gene (hERG) inhibitory activity.
Chart 1 illustrates the synthesis of compound 6. Benzylation of commercially available ethyl piperidine-4-carboxylate (2) and subsequent ester hydrolysis yielded carboxylic acid 3. Next, commercially available 5-aminopyridine-2-carboxylic acid (4) was condensed with 1-{[4-(trifluoromethyl)phenyl]methyl}piperazine to obtain compound 5. Finally, condensation of 3 with 5 yielded compound 6.

Reagents and conditions: (a) 4-methoxybenzyl chloride, DMF, room temperature (r.t.); (b) 4.0 mol/L NaOHaq., THF, EtOH, r.t., 63% for 2 steps; (c) 1-{[4-(trifluoromethyl)phenyl]methyl}piperazine, WSC·HCl, HOBt, DMF, r.t., 79%; (d) 3, (COCl)2, DMF, CH2Cl2, 0°C to r.t., then 5, pyridine, CH2Cl2, 0°C to r.t., then 4.0 mol/L HCl in 1,4-dioxane, MeOH, r.t., 40%.
The synthetic route for 13–16 is described in Chart 2. Esterification of commercially available 5-hydroxypyridine-2-carboxylic acid (7) with methanol yielded 8. Alkylation of 8 with tert-butyl 4-(hydroxymethyl)piperidine-1-carboxylate by Mitsunobu reaction, and subsequent ester hydrolysis yielded 9. For the synthesis of 13 and 14, condensation of 9 with 1-{[4-(trifluoromethyl)phenyl]methyl}piperazine and a subsequent deprotection reaction produced 10. Alkylation of 10 with the requisite commercially available aryl aldehydes by reductive amination reaction yielded 13 and 14. For the synthesis of 15 and 16a–16c, 9 was condensed with benzyl piperazine-1-carboxylate, the benzyloxycarbonyl group was removed using palladium-catalyzed hydrogenation. The obtained amine was alkylated with the requisite commercially available aryl aldehydes and the Boc protecting group was removed to give 11 and 12. Finally, alkylation of 11 or 12 with the requisite aryl aldehydes by reductive amination reaction yielded 15 and 16a–16c.

Reagents and conditions: (a) c.H2SO4, MeOH, 85°C, 76%; (b) tert-butyl 4-(hydroxymethyl)piperidine-1-carboxylate, 2.2 mol/L DEAD in toluene, PPh3, THF, 0°C to r.t.; (c) 1.0 mol/L NaOHaq., THF, MeOH, 60°C, 99% for 2 steps; (d) 10: 1-{[4-(trifluoromethyl)phenyl]methyl}piperazine, WSC·HCl, HOBt, CH2Cl2, r.t., then 4.0 mol/L HCl in 1,4-dioxane, MeOH, r.t., 88%; 11, 12: benzyl piperazine-1-carboxylate, WSC·HCl, HOBt, CH2Cl2, r.t., then 10% Pd/C (wetted with approx. 50% water), H2 (1.0 kgf/cm2), EtOH, r.t., then ArCHO, NaBH(OAc)3, AcOH, CH2Cl2, r.t., then 4.0 mol/L HCl in 1,4-dioxane, MeOH, r.t., 33%; (e) 14, 15, 16a, 16c: ArCHO, NaBH(OAc)3, AcOH, CH2Cl2, r.t., then p-TsOH·H2O, acetone, r.t., 53–72%; 13, 16b: ArCHO, NaBH(OAc)3, AcOH, CH2Cl2, r.t., then 4.0 mol/L HCl in 1,4-dioxane, MeOH, r.t., 38–51%.
Analogs 21a–21c were synthesized as shown in Chart 3. Dealkylation of the methyl ether of 1716) using AlCl3 gave 18. As with 10, described above, 18 was alkylated with tert-butyl 4-(hydroxymethyl)piperidine-1-carboxylate, hydrolyzed, condensed with 31, and subsequent deprotection of the Boc group yielded 20. Compound 20 was coupled with aldehyde 29 or benzyl chloride 35 by reductive amination reaction or alkylation reaction to give 21a–21c.

Reagents and conditions: (a) AlCl3, CH2Cl2, 55°C, 80%; (b) tert-butyl 4-(hydroxymethyl)piperidine-1-carboxylate, 2.2 mol/L DEAD in toluene, PPh3, THF, 0°C to r.t.; (c) 1.0 mol/L NaOHaq., THF, MeOH, 60°C, 69% for 2 steps; (d) 31, WSC·HCl, HOBt, Et3N, CH2Cl2, r.t.; (e) 4.0 mol/L HCl in 1,4-dioxane, MeOH, r.t., quant. for 2 steps; (f) 21a: 29, NaBH(OAc)3, AcOH, CH2Cl2, r.t., then p-TsOH·H2O, acetone, r.t., 56%; 21b: 35a, K2CO3, MeCN, r.t., then p-TsOH·H2O, MeOH, r.t., 59%; 21c: 35b, DIPEA, MeCN, r.t., then succinic acid, EtOH, r.t., 74%; Structures of compounds 29, 31, 35a and 35b were indicated in Chart 5.
Analogs 26 and 27 were generated using the synthetic route outlined in Chart 4. Alkylation of 18 with the requisite 4-(hydroxymethyl)piperidine derivatives by Mitsunobu reaction using cyanomethylenetributylphosphorane (Tsunoda’s reagent),17) and subsequent ester hydrolysis gave 22 and 23. Compounds 22 and 23 were condensed with 31 and subsequent removal of the Boc protecting group gave 24 and 25. As with 21 in Chart 3, compounds 24 and 25 were coupled with aldehyde 29 or benzyl chloride 35b to yield 26a, 26b, 27a and 27b.

Reagents and conditions: (a) 22: 37, cyanomethylenetributylphosphorane, toluene, 100°C; 23: tert-butyl 4-fluoro-4-(hydroxymethyl)piperidine-1-carboxylate,18) cyanomethylenetributylphosphorane, toluene, 110°C; (b) 1.0 mol/L NaOHaq., MeOH, 60°C, 54–91% for 2 steps; (c) 31, WSC·HCl, HOBt, Et3N, CH2Cl2, r.t.; (d) 4.0 mol/L HCl in 1,4-dioxane, MeOH, r.t., quant. for 2 steps; (e) 26a, 27a: 29, NaBH(OAc)3, AcOH, CH2Cl2, r.t., then succinic acid, 61–68%; 26b, 27b: 35b, DIPEA, MeCN, r.t. to 50°C, then succinic acid, 65–68%; Structures of compounds 29, 31 35b and 37 were indicated in Chart 5.
The synthetic routes of the intermediates derived in Chart 1–4 are summarized in Chart 5. Compound 29 was synthesized by lithiation of commercially available bromide 28 with n-BuLi, and subsequent trapping with DMF. tert-Butyl piperazine-1-carboxylate (30) was alkylated with 4-formylbenzonitrile, and subsequent deprotection of the Boc group yielded 31. For the synthesis of 35, ipso-substitution of commercially available 32 with methanol or ethanol gave 33a and 33b. Reduction of the ester group of 33a and 33b using NaBH4, and subsequent chlorination of the benzyl alcohol group gave 35a and 35b. Compound 37 was synthesized by p-toluenesulfonic acid-catalyzed epoxide opening of known 3619) with methanol.

Reagents and conditions: (a) 1.7 mol/L n-BuLi in hexane, THF, −68°C then DMF, −68°C to r.t., 59%; (b) 4-formylbenzonitrile, NaBH(OAc)3, AcOH, CH2Cl2, r.t.; (c) 4.0 mol/L HCl in EtOAc, EtOAc, CHCl3, r.t., 77% for 2 steps; (d) 33a: MeONa, MeOH, 0°C to r.t., 81%; 33b: t-BuOK, EtOH, 0°C, 82%; (e) NaBH4, MeOH, 0°C to r.t., 75–77%; (f) SOCl2, CH2Cl2, 0°C to r.t., quant.; (g) p-TsOH·H2O, MeOH, r.t., 52%.
The AMPK activation activity of the synthesized compounds was measured by phospho-ACC whole cell Enzyme-Linked ImmunoSorbent Assay (ELISA) using the MDA-MB-453 human breast cancer cell line. Selected compounds were also evaluated for aqueous solubility in the Japanese Pharmacopoeia 2nd fluid for disintegration test (JP2; pH = 6.8), in vitro metabolic stability in human liver microsomes (HLM) and hERG inhibitory activity.
First, we introduced nitrogen atoms into the central phenyl moiety to improve the aqueous solubility of 1 (Table 1). Introducing hetero atoms into compounds to reduce the hydrophobicity is a widely used strategy for improving aqueous solubility20) because a compound’s aqueous solubility generally depends on its hydrophobicity.21) Compound 6 exhibited good aqueous solubility at gastrointestinal pH (≥100 µM) with 6-fold lower AMPK activating activity than that of 1. Next, we attempted to replace the anilide linkage with an ether linkage, which we expected would eliminate the potential for metabolic hydrolysis. Surprisingly, compound 13 with an ether linkage maintained good aqueous solubility, despite showing similar hydrophobicity to compound 1. Recently, Walker suggested that intermolecular hydrogen-bonding in the solid state may lead to increased crystalline stability and reduced aqueous solubility.22) Compound 13 possesses no hydrogen bond donors, suggesting that it may have lower crystalline stability than 6, which would contribute to the maintenance of good aqueous solubility. Furthermore, compound 13 showed about 10-fold more potent cellular activity than that of 6.
 |
a The EC50 values were examined by whole cell ELISA using the MDA-MB-453 cell line and determined in triplicate in one experiment. b Aqueous solubility in JP2 (pH = 6.8). c c Log P values were calculated using ACD Log P prediction software.23) d Dihydrochloride. e Trihydrochloride.
Given that compound 13 showed both good aqueous solubility and potent AMPK activating activity, we subjected it to further in vitro profiling studies. The results are summarized in Table 2. Compound 13 showed good metabolic stability in human liver microsomes. In contrast, potent inhibitory activity for the hERG channel was confirmed in the auto patch clamp hERG blockade assay (IC50 = 0.74 µM). Inhibition of the hERG channel can cause delayed ventricular cell repolarization, which results in prolongation of the QT interval, leading to serious risk of arrhythmias and sudden death.24,25) In general, the pharmacophore of hERG blockers contains a basic center flanked by a hydrophobic group.26) As shown in Table 2, compound 13 showed high basicity and hydrophobicity. Therefore, we attempted to decrease both the basicity and hydrophobicity of 13 to reduce the hERG inhibitory activity.
 | ||||||
|---|---|---|---|---|---|---|
| Compound | AMPK activation EC50 (µM)a | JP2 (µM)b | HLM CLint, vitro (mL/min/kg) | hERG inhibition IC50 (µM) | c Log Pc | Calculated most basic pKac |
| 13d | 0.0083 | ≥100 | 97 | 0.74 | 4.8 | 9.2 |
a The EC50 value was examined by whole cell ELISA using the MDA-MB-453 cell line and determined in triplicate in one experiment. b Aqueous solubility in JP2 (pH = 6.8). c c Log P and pKa values were calculated using ACD Log P prediction software.23) d Trihydrochloride.
First, we introduced another nitrogen atom into the left aromatic moiety to reduce its hydrophobicity and basicity. Resulting compound 14 showed good aqueous solubility and metabolic stability, but continued to show moderate inhibitory activity against the hERG channel. Next, we replaced the hydrophobic CF3 moiety with a less hydrophobic chloro group (15). Compound 15 exhibited good aqueous solubility and potency, but dramatically elevated in vitro CLint in HLM. Previously, a study of Pfizer reported that the nitrile group was a less hydrophobic isostere of the chloro group, and that such modification led to improved medicinal chemistry properties, such as physicochemical and ADME properties.27) In reference to this report, we attempted to replace the chloro group in 15 with a cyano group (16a). While compound 16a showed 7-fold less potent cellular activity (EC50 = 0.036 µM) than the CF3 congener (14), the metabolic stability of 16a was dramatically improved (HLM CLint, vitro = 58 mL/min/kg) compared to that of 15. Furthermore, 16a showed a 4-fold reduction in hERG channel inhibitory activity compared to 14. The hydrophobicity and basicity of 16a was the lowest among the compounds in Table 3. This suggests that the favorable physical properties led to improved metabolic stability and reduced hERG inhibitory activity as expected.
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compound | X | R | AMPK activation EC50 (µM)a | JP2 (µM)b | HLM CLint, vitro (mL/min/kg) | hERG inhibition IC50 (µM) | c Log Pc | Calculated most basic pKac |
| 13d | CH | CF3 | 0.0083 | ≥100 | 97 | 0.74 | 4.8 | 9.2 |
| 14e | N | CF3 | 0.0051 | ≥100 | 94 | 3.4 | 3.8 | 8.0 |
| 15e | N | Cl | 0.018 | ≥100 | 523 | NTf | 3.4 | 8.0 |
| 16ae | N | CN | 0.036 | ≥100 | 58 | 13 | 2.5 | 8.0 |
a The EC50 values were examined by whole cell ELISA using the MDA-MB-453 cell line and determined in duplicate or triplicate in one experiment. b Aqueous solubility in JP2 (pH = 6.8). c c Log P and pKa values were calculated using ACD LogP prediction software.23) d Trihydrochloride. e Ditosylate. f NT: not tested.
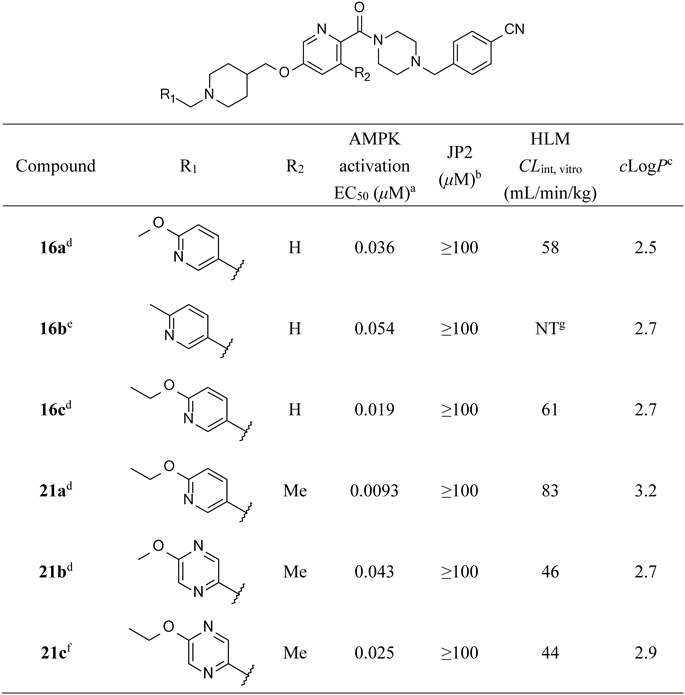
While compound 16a showed moderate AMPK activating activity, its other in vitro properties were promising, including excellent aqueous solubility, good in vitro metabolic stability and weak hERG inhibitory activity. Therefore, we tried to further optimize substituents of the center and left aromatic moiety of 16a to improve its in vitro cellular activity (Table 4). Replacement of the methoxy group on the left moiety with a methyl group (16b) produced a compound with slightly weaker potency (EC50 = 0.054 µM) than that of 16a. However, replacement with an ethoxy group (16c) resulted in a slight improvement in potency (EC50 = 0.019 µM). Further improvement in cellular activity was observed when a methyl group was introduced into the central pyridine moiety (21a), with the resulting compound showing an EC50 of 0.0093 µM. Replacement of the left pyridine moiety with a pyrazine moiety (21b and 21c) led to further improvement in the metabolic stability (HLM CLint, vitro = 46 and 44 mL/min/kg, respectively) probably due to decreased hydrophobicity. However, cellular activity was reduced in methoxy analog 21b (EC50 = 0.043 µM). Similar to the pyridine series, ethoxy analog 21c showed slightly more potent cellular activity (EC50 = 0.025 µM) than 21b in the pyrazine series. Based on these results, we chose compound 21a and 21c as a promising compound and conducted further modifications.
 |
a The EC50 values were examined by whole cell ELISA using the MDA-MB-453 cell line and determined in duplicate or triplicate in one experiment. b Aqueous solubility in JP2 (pH = 6.8). c c Log P values were calculated using ACD Log P prediction software.23) d Ditosylate. e Tetrahydrochloride. f Succinate. g NT: not tested.
To further improve the AMPK activating activity, we focused on introducing substituents into the 4-position of the piperidine linker, into which we had yet to introduce any substituents (Table 5). Introduction of a methoxy group into the 4-position of the piperidine linker (26a and 26b) produced compounds with 3-fold weaker potency (EC50 = 0.035 and 0.082 µM, respectively) than that of non-substituted analogs. Surprisingly, introduction of a fluoro group (27a and 27b) resulted in 2- to 3-fold more potent cellular activity (EC50 = 0.0038 and 0.011 µM, respectively) than that of non-substituted analogs. Unfortunately, ethoxypyridine analog 27a showed high in vitro metabolic clearance (HLM CLint, vitro = 168 mL/min/kg). However, its pyrazine analog 27b showed significant metabolic stability (HLM CLint, vitro = 89 mL/min/kg) that was similar to that of 21a.
 |
a The EC50 values were examined by whole cell ELISA using the MDA-MB-453 cell line and determined in duplicate or triplicate in one experiment. b Aqueous solubility in JP2 (pH = 6.8). c cLogp values were calculated using ACD Log P prediction software.23) d Ditosylate. e Succinate. f NT: not tested.
Based on their pharmacological, physicochemical and ADME properties, compounds 21a and 27b were selected for further profiling. The hERG inhibitory activity of these compounds is summarized in Table 6. Compound 21a showed 22-fold lower inhibitory activity (IC50 = 16 µM) than that of 13. Compound 27b showed even lower hERG inhibitory activity, with an IC50 over 30 µM. In addition, the hERG inhibitory activity of 27b was significantly weaker than ASP4132. A clear reverse correlation was observed between the hERG inhibitory activity and physical properties; that is, decreasing hydrophobicity and basicity was effective for weakening a compound’s hERG inhibitory activity.
| Compound | hERG inhibition IC50 (µM) | c Log Pa | Calculated most basic pKaa |
|---|---|---|---|
| 13b | 0.74 | 4.8 | 9.2 |
| 21ac | 16 | 3.2 | 8.0 |
| 27bd | > 30 | 2.7 | 7.1 |
| ASP4132c | 2.0 | 3.5 | 8.3 |
a c Log P and pKa values were calculated using ACD Log P prediction software.23) b Trihydrochloride. c Ditosylate. d Succinate.
Overall, 27b displayed promising AMPK activating activity, aqueous solubility, metabolic stability and hERG inhibitory activity, which reflect the fact that it had the lowest hydrophobicity and basicity among the series of compounds. Next, we evaluated the pharmacological, pharmacokinetic and physicochemical properties of 27b in vitro and in vivo (Table 7). Compound 27b showed comparable AMPK activating activity and cell growth inhibitory activity against MDA-MB-453 to that of ASP4132. Furthermore, 27b showed relatively weak antiproliferative activity against SK-BR-3, suggesting that it maintained the selective cell growth inhibitory activity observed in ASP4132. In addition, the aqueous solubility of 27b at gastrointestinal pH was dramatically improved over that of ASP4132.
| Compound | AMPK activation EC50 (µM) | MDA-MB-453 growth inhibition IC50 (µM) | SK-BR-3 growth inhibition IC50 (µM) | HLM CLint, vitro (mL/min/kg) | Aqueous solubility (µM)a JP1/JP2/JP2 + TCb |
|---|---|---|---|---|---|
| ASP4132 | 0.018d | 0.014d | >3d | 61 | ≥100/5.6/ ≥ 100 |
| 27bc | 0.011e | 0.026f | 21% inh. at 3 µMf | 89 | ≥100/ ≥ 100/ ≥ 100 |
a Aqueous solubility in JP1 (pH = 1.2) and JP2 (pH = 6.8). b TC: taurocholic acid. c Succinate. d The geometric mean of the EC50 and IC50 values from 3 independent experiments are shown. e The EC50 value was examined by whole cell ELISA using the MDA-MB-453 cell line and determined in triplicate in one experiment. f The IC50 value was determined in triplicate in one experiment.
Next, we conducted a pharmacokinetic study of compound 27b in Sprague-Dawley (SD) rats. The PK parameters of 27b are shown in Table 8. Compound 27b showed good oral bioavailability (F = 83%) and relatively low total body clearance (CLtot = 2.5 mL/min/kg) compared to the hepatic blood flow rate in rats (55.2 mL/min/kg).28)
| Compound | i.v.b (1 mg/kg) | p.o.b (1 mg/kg) | ||||||
|---|---|---|---|---|---|---|---|---|
| AUC24hc (ng∙h/mL) | t1/2d (h) | Vsse (L/kg) | CLtotf (mL/min/kg) | AUC24hc (ng·h/mL) | Cmaxg (ng/mL) | tmaxh (h) | Fi (%) | |
| 27ba | 6580 | 2.7 | 0.42 | 2.5 | 5470 | 1090 | 1.3 | 83 |
a Succinate. b i.v.: intravenous, p.o.: oral administration. c Area under the plasma concentration versus time curve from time zero to 24 h after dosing. d Elimination half-life from plasma. e Volume of distribution at steady state. f Total body clearance. g Maximum plasma concentration. h Time to reach maximum plasma concentration. i Absolute oral bioavailability.
To evaluate the in vivo efficacy of 27b, MDA-MB-453 xenografts were established in nude mice. Upon tumor establishment, dosing of compound 27b was initiated with per os (p.o.) doses of 1 to 4 mg/kg, twice daily (BID). The results are summarized in Fig. 3. The tumor growth inhibition (TGI) rate was 83% at 1 mg/kg (BID), and the tumor regression rates were 13% and 64% at 2 and 4 mg/kg (BID), respectively. All doses of 27b were well tolerated over the 14-d dosing window without body weight loss.

a Succinate. b Mice were treated with 27b from Days 1 to 14. Each point represents the mean ± S.E.M. (N = 5). Statistical analysis was performed the values on Day 15. *: p < 0.05, **: p < 0.01, and ***: p < 0.001 compared with the value of vehicle on Day 15 (Dunnett’s multiple comparison test). c Mice were treated with 27b from Days 1 to 14. Each point represents the mean ± S.E.M. (N = 5).
We reported the medicinal chemistry study behind the development of the second-generation clinical candidate, 4-({4-[5-({1-[(5-ethoxypyrazin-2-yl)methyl]-4-fluoropiperidin-4-yl}methoxy)-3-methylpyridine-2-carbonyl]piperazin-1-yl}methyl)benzonitrile succinate (27b), as an indirect AMPK activator. We conducted a structure–activity relationship study with the aim of identifying a compound with improved aqueous solubility at gastrointestinal conditions (JP2; pH = 6.8) compared to ASP4132. As a result, we identified the compound 13, which showed good aqueous solubility at pH = 6.8 but potent inhibition of hERG potassium channels. Further optimization efforts along with strategies to decrease hydrophobicity and basicity resulted in the identification of compound 27b, which had potent cellular activity, good metabolic stability and aqueous solubility, favorable animal PK properties and desirable in vivo anti-tumor efficacy on oral dosing. Our findings suggested that 27b was a promising AMPK activator and a second-generation clinical candidate for treatment for human cancer.
1H-NMR spectra were recorded on a Varian VNS-400, Varian 400-MR, JEOL JNM Lambda-300, JEOL JNM Lambda-400 or Bruker AVANCE III-HD500 spectrometer. Chemical shifts were expressed in δ values (ppm) using tetramethylsilane as the internal standard (s = singlet, d = doublet, t = triplet, q = quartet, dd = double doublet, ddd = double double doublet, m = multiplet and br = broad peak). MS were recorded on a JEOL GC Mate II, Waters SQD, Waters ZQ-2000, Thermo Fisher LCQ Advantage or Thermo Fisher Exactive Plus Orbitrap. All reactions were performed using commercially available reagents and solvents without further purification. The following abbreviations are used: AcOH, acetic acid; Boc, tert-butoxycarbonyl; BuLi, butyllithium; t-BuOK, potassium tert-butoxide; DEAD, diethyl (E)-diazene-1,2-dicarboxylate; DMF, N,N-dimethylformamide; DIPEA, N,N-diisopropylethylamine; DMSO, dimethyl sulfoxide; ESI, electrospray ionization; Et3N, triethylamine; Et2O, diethylether; EtOAc, ethyl acetate; EtOH, ethanol; HOBt, 1H-benzotriazol-1-ol; IPA, isopropyl alcohol; MeCN, acetonitrile; MeOH, methanol; NaBH(OAc)3, sodium triacetoxyborohydride; PPh3, triphenylphosphine; THF, tetrahydrofuran; p-TsOH·H2O, 4-methylbenzenesulfonic acid monohydrate; WSC·HCl, N-[3-(dimethylamino)propyl]-N′-ethylcarbodiimide hydrochloride.
1-[(4-Methoxyphenyl)methyl]piperidine-4-carboxylic Acid (3)To a solution of 4-methoxybenzyl chloride (1.1 mL, 7.7 mmol) in DMF (10 mL) was added ethyl piperidine-4-carboxylate (2; 3.0 mL, 19 mmol). After stirring at room temperature for 16 h, the mixture was diluted with H2O and extracted with EtOAc. The organic layer was washed with H2O, saturated NaHCO3 aqueous solution and brine. The organic layer was dried over Na2SO4 and concentrated in vacuo. The residue was purified by column chromatography on silica gel (CHCl3/MeOH). To a solution of the residue in THF (15 mL) and EtOH (10 mL) was added 4.0 mol/L NaOH aqueous solution (13 mL, 52 mmol). After stirring at room temperature for 3 d, the mixture was acidified with concentrated HClaq. (approximately pH 5) and concentrated in vacuo. To the residue was added CHCl3/MeOH (2 : 1), the mixture was filtered and the filtrate was concentrated in vacuo. To the residue was added EtOAc, and the precipitated solid was collected by filtration to obtain the product (1.2 g, 63%). 1H-NMR (DMSO-d6) δ: 1.46–1.63 (2H, m), 1.69–1.88 (2H, m), 1.90–2.30 (3H, m), 2.64–2.88 (2H, m), 3.08–3.63 (2H, m), 3.73 (3H, s), 6.88 (2H, d, J = 8.4 Hz), 7.21 (2H, d, J = 8.4 Hz); MS m/z: 250 (M + H)+.
(5-Aminopyridin-2-yl)(4-{[4-(trifluoromethyl)phenyl]methyl}piperazin-1-yl)methanone (5)To a solution of 5-aminopyridine-2-carboxylic acid (4; 200 mg, 1.4 mmol) in DMF (3.3 mL) were added 1-{[4-(trifluoromethyl)phenyl]methyl}piperazine (360 mg, 1.5 mmol), WSC·HCl (330 mg, 1.7 mmol) and HOBt (250 mg, 1.9 mmol). After stirring at room temperature overnight, the mixture was diluted with H2O and extracted with EtOAc. The organic layer was washed with H2O, saturated NaHCO3 aqueous solution and brine. The organic layer was dried over Na2SO4 and concentrated in vacuo. The residue was purified by column chromatography on silica gel (CHCl3/MeOH) to obtain the product (420 mg, 79%). 1H-NMR (CDCl3) δ: 2.32–2.69 (4H, m), 3.58 (2H, s), 3.72–3.84 (4H, m), 3.90 (2H, s), 7.01 (1H, dd, J = 2.7, 8.4 Hz), 7.46 (2H, d, J = 8.0 Hz), 7.54–7.60 (3H, m), 7.98 (1H, d, J = 2.7 Hz); MS m/z: 365 (M + H)+.
1-[(4-Methoxyphenyl)methyl]-N-[6-(4-{[4-(trifluoromethyl)phenyl]methyl}piperazine-1-carbonyl)pyridin-3-yl]piperidine-4-carboxamide Trihydrochloride (6)To a solution of 1-[(4-methoxyphenyl)methyl]piperidine-4-carboxylic acid (3; 110 mg, 0.45 mmol) in CH2Cl2 (4.0 mL) were added (COCl)2 (44 µL, 0.51 mmol) and DMF (1 drop) at 0°C. After stirring at room temperature for 1 h, to the mixture were added a solution of (5-aminopyridin-2-yl)(4-{[4-(trifluoromethyl)phenyl]methyl}piperazin-1-yl)methanone (5; 140 mg, 0.37 mmol) in CH2Cl2 (2.0 mL) and pyridine (76 µL, 0.95 mmol) at 0°C. After stirring at room temperature for 4 h, the mixture was diluted with H2O and extracted with CHCl3. The organic layer was washed with brine, dried over Na2SO4 and concentrated in vacuo. The residue was purified by column chromatography on silica gel (CHCl3/MeOH). To a mixture of the residue in MeOH (3.0 mL) was added 4.0 mol/L HCl in 1,4-dioxane (0.50 mL, 2.0 mmol). After stirring at room temperature for 15 min, the mixture was concentrated in vacuo. To the residue was added Et2O, and the precipitated solid was collected by filtration to obtain the product (110 mg, 40%). 1H-NMR (CD3OD) δ: 1.93–2.36 (4H, m), 2.73–2.86 (1H, m), 3.03–3.16 (2H, m), 3.20–3.74 (10H, m), 3.83 (3H, s), 4.28 (2H, s), 4.52 (2H, s), 7.00–7.07 (2H, m), 7.43–7.48 (2H, m), 7.77–7.86 (5H, m), 8.28 (1H, dd, J = 2.4, 8.6 Hz), 8.94 (1H, d, J = 2.4 Hz); MS m/z: 596 (M + H)+; ESI-MS m/z: 596.2836 (M + H)+ (Calcd for C32H37O3N5F3: 596.2843).
Methyl 5-Hydroxypyridine-2-carboxylate (8)To a solution of 5-hydroxypyridine-2-carboxylic acid (7; 10 g, 72 mmol) in MeOH (120 mL) was added concentrated H2SO4 (8.0 mL, 150 mmol). The mixture was stirred at 85°C overnight. After cooling to 0°C, the mixture was basified with 1.0 mol/L NaOH aqueous solution (approximately pH 9), and then acidified with 10% citric acid aqueous solution (approximately pH 5). The mixture was extracted with CHCl3/IPA (4 : 1), the organic layer was dried over MgSO4 and concentrated in vacuo to obtain the product (8.4 g, 76%). 1H-NMR (DMSO-d6) δ: 3.82 (3H, s), 7.27 (1H, dd, J = 2.8, 8.6 Hz), 7.94 (1H, dd, J = 0.5, 8.6 Hz), 8.22 (1H, dd, J = 0.5, 2.8 Hz), 10.81 (1H, s); MS m/z: 154 (M + H)+.
5-{[1-(tert-Butoxycarbonyl)piperidin-4-yl]methoxy}pyridine-2-carboxylic Acid (9)To a solution of methyl 5-hydroxypyridine-2-carboxylate (8; 2.0 g, 13 mmol), tert-butyl 4-(hydroxymethyl)piperidine-1-carboxylate (3.0 g, 14 mmol) and PPh3 (5.0 g, 19 mmol) in THF (40 mL) was added dropwise 2.2 mol/L DEAD in toluene (9.0 mL, 20 mmol) at 0°C. After stirring at room temperature overnight, the mixture was concentrated in vacuo. The residue was purified by column chromatography on silica gel (hexane/EtOAc). To a solution of the residue in THF (20 mL) and MeOH (20 mL) was added 1.0 mol/L NaOH aqueous solution (40 mL, 40 mmol). The mixture was stirred at 60°C for 1 h. After cooling to room temperature, the mixture was acidified with 10% citric acid aqueous solution (approximately ~pH 5). The mixture was extracted with CHCl3/IPA (4 : 1), the organic layer was dried over MgSO4 and concentrated in vacuo to obtain the product (4.4 g, 99%). 1H-NMR (DMSO-d6) δ: 1.10–1.25 (2H, m), 1.40 (9H, s), 1.70–1.81 (2H, m), 1.91–2.05 (1H, m), 2.60–2.91 (2H, m), 3.91–4.09 (4H, m), 7.50 (1H, dd, J = 2.8, 8.6 Hz), 8.01 (1H, d, J = 8.6 Hz), 8.36 (1H, d, J = 2.8 Hz), 8.97 (1H, s); MS m/z: 337 (M + H)+.
{5-[(Piperidin-4-yl)methoxy]pyridin-2-yl}(4-{[4-(trifluoromethyl)phenyl]methyl}piperazin-1-yl)methanone (10)To a mixture of 5-{[1-(tert-butoxycarbonyl)piperidin-4-yl]methoxy}pyridine-2-carboxylic acid (9; 1.4 g, 4.2 mmol), 1-{[4-(trifluoromethyl)phenyl]methyl}piperazine (0.85 mL, 4.3 mmol) and CH2Cl2 (25 mL) were added WSC·HCl (1.1 g, 5.7 mmol) and HOBt (750 mg, 5.6 mmol). After stirring at room temperature overnight, the mixture was diluted with saturated NaHCO3 aqueous solution and extracted with CHCl3. The organic layer was dried over MgSO4 and concentrated in vacuo. The residue was purified by column chromatography on silica gel (CHCl3/MeOH). To a solution of the residue in MeOH (10 mL) was added 4.0 mol/L HCl in 1,4-dioxane (10 mL, 40 mmol). After stirring at room temperature for 2.5 h, the mixture was basified with saturated NaHCO3 aqueous solution and extracted with CHCl3/IPA (4 : 1). The organic layer was dried over MgSO4 and concentrated in vacuo to obtain the product (1.7 g, 88%). 1H-NMR (DMSO-d6) δ: 1.10–1.24 (2H, m), 1.64–1.74 (2H, m), 1.77–1.93 (1H, m), 2.24–2.57 (6H, m), 2.91–3.02 (2H, m), 3.32–3.73 (6H, m), 3.92 (2H, d, J = 6.4 Hz), 7.47 (1H, dd, J = 2.9, 8.8 Hz), 7.52–7.58 (3H, m), 7.69 (2H, d, J = 8.2 Hz), 8.24 (1H, dd, J = 0.7, 2.9 Hz); MS m/z: 463 (M + H)+.
{4-[(4-Chlorophenyl)methyl]piperazin-1-yl}{5-[(piperidin-4-yl)methoxy]pyridin-2-yl}methanone (11)To a mixture of 5-{[1-(tert-butoxycarbonyl)piperidin-4-yl]methoxy}pyridine-2-carboxylic acid (9; 3.0 g, 8.8 mmol), benzyl piperazine-1-carboxylate (1.8 mL, 9.3 mmol) and CH2Cl2 (50 mL) were added WSC·HCl (2.3 g, 12 mmol) and HOBt (1.6 g, 12 mmol). After stirring at room temperature overnight, the mixture was diluted with saturated NaHCO3 aqueous solution and extracted with CHCl3. The organic layer was dried over MgSO4 and concentrated in vacuo. The residue was purified by column chromatography on silica gel (CHCl3/MeOH). To a solution of the residue in EtOH (40 mL) was added 10% Pd/C (wetted with approx. 50% water, 1.0 g). After stirring under a hydrogen atmosphere (1.0 kgf/cm2) at room temperature for 3 d, to the mixture was added 10% Pd/C (wetted with ca. 50% water, 1.5 g). After stirring under a hydrogen atmosphere (1.0 kgf/cm2) at room temperature for 2 d, the mixture was filtered through a Celite pad and the filtrate was concentrated in vacuo. The residue was purified by column chromatography on silica gel (CHCl3/MeOH) to obtain the intermediate (1.2 g). To a solution of the obtained intermediate (600 mg, 1.5 mmol) in CH2Cl2 (12 mL) were added 4-chlorobenzaldehyde (240 mg, 1.7 mmol), acetic acid (10 µL, 0.17 mmol) and NaBH(OAc)3 (800 mg, 3.8 mmol). After stirring at room temperature overnight, the mixture was diluted with saturated NaHCO3 aqueous solution and extracted with CHCl3. The organic layer was dried over MgSO4 and concentrated in vacuo. The residue was purified by column chromatography on silica gel (CHCl3/MeOH). To a solution of the residue in MeOH (6.0 mL) was added 4.0 mol/L HCl in 1,4-dioxane (6.0 mL, 24 mmol). After stirring at room temperature for 1 h, the mixture was diluted with saturated NaHCO3 aqueous solution and extracted with CHCl3/IPA (4 : 1). The organic layer was dried over MgSO4 and concentrated in vacuo to obtain the product (640 mg, 33%). 1H-NMR (DMSO-d6) δ: 1.10–1.25 (2H, m), 1.64–1.72 (2H, m), 1.76–1.91 (1H, m), 2.26–2.55 (6H, m), 2.91–3.00 (2H, m), 3.41–3.69 (6H, m), 3.91 (2H, d, J = 6.4 Hz), 7.29–7.42 (4H, m), 7.46 (1H, dd, J = 2.9, 8.8 Hz), 7.55 (1H, dd, J = 0.7, 8.8 Hz), 8.24 (1H, dd, J = 0.7, 2.9 Hz); MS m/z: 429 (M + H)+.
4-[(4-{5-[(Piperidin-4-yl)methoxy]pyridine-2-carbonyl}piperazin-1-yl)methyl]benzonitrile (12)Compound 12 was prepared from 9 and 4-formylbenzonitrile in 33% yield using a similar approach to that described for 11. 1H-NMR (DMSO-d6) δ: 1.10–1.29 (2H, m), 1.64–1.73 (2H, m), 1.75–1.92 (1H, m), 2.30–2.56 (6H, m), 2.90–3.01 (2H, m), 3.48–3.68 (6H, m), 3.92 (2H, d, J = 6.4 Hz), 7.44–7.59 (4H, m), 7.76–7.83 (2H, m), 8.24 (1H, dd, J = 0.4, 2.4 Hz); MS m/z: 420 (M + H)+.
[5-({1-[(4-Methoxyphenyl)methyl]piperidin-4-yl}methoxy)pyridin-2-yl](4-{[4-(trifluoromethyl)phenyl]methyl}piperazin-1-yl)methanone Trihydrochloride (13)To a solution of {5-[(piperidin-4-yl)methoxy]pyridin-2-yl}(4-{[4-(trifluoromethyl)phenyl]methyl}piperazin-1-yl)methanone (10; 300 mg, 0.65 mmol) in CH2Cl2 (6.0 mL) were added 4-methoxybenzaldehyde (90 µL, 0.74 mmol), acetic acid (5.0 µL, 87 µmol) and NaBH(OAc)3 (300 mg, 1.4 mmol). After stirring at room temperature overnight, the mixture was diluted with saturated NaHCO3 aqueous solution and extracted with CHCl3. The organic layer was dried over MgSO4 and concentrated in vacuo. The residue was purified by column chromatography on silica gel (CHCl3/MeOH). To a solution of the residue in MeOH was added excess 4.0 mol/L HCl in 1,4-dioxane. The mixture was concentrated in vacuo. To the residue was added Et2O, and the precipitated solid was collected by filtration to obtain the product (230 mg, 51%). 1H-NMR (DMSO-d6) δ: 1.54–2.15 (5H, m), 2.75–3.73 (10H, m), 3.78 (3H, s), 3.91–4.06 (2H, m), 4.11–4.37 (3H, m), 4.38–4.68 (3H, m), 7.01 (2H, d, J = 8.8 Hz), 7.43–7.61 (3H, m), 7.68 (1H, d, J = 8.8 Hz), 7.78–7.97 (4H, m), 8.27 (1H, d, J = 2.9 Hz), 10.52 (1H, br s), 11.89 (1H, br s); MS m/z: 583 (M + H)+; ESI-MS m/z: 583.2888 (M + H)+ (Calcd for C32H38O3N4F3: 583.2891).
[5-({1-[(6-Methoxypyridin-3-yl)methyl]piperidin-4-yl}methoxy)pyridin-2-yl](4-{[4-(trifluoromethyl)phenyl]methyl}piperazin-1-yl)methanone Ditosylate (14)To a solution of {5-[(piperidin-4-yl)methoxy]pyridin-2-yl}(4-{[4-(trifluoromethyl)phenyl]methyl}piperazin-1-yl)methanone (10; 300 mg, 0.65 mmol) in CH2Cl2 (6.0 mL) were added 6-methoxypyridine-3-carbaldehyde (100 mg, 0.73 mmol), acetic acid (5.0 µL, 87 µmol) and NaBH(OAc)3 (300 mg, 1.4 mmol). After stirring at room temperature overnight, the mixture was diluted with saturated NaHCO3 aqueous solution and extracted with CHCl3. The organic layer was dried over MgSO4 and concentrated in vacuo. The residue was purified by column chromatography on silica gel (CHCl3/MeOH). To a solution of the residue (230 mg, 0.40 mmol) in acetone was added p-TsOH·H2O (150 mg, 0.79 mmol). The mixture was concentrated in vacuo. To the residue was added Et2O, and the precipitated solid was collected by filtration to obtain the product (320 mg, 53%). 1H-NMR (DMSO-d6) δ: 1.38–1.65 (2H, m), 1.78–2.17 (3H, m), 2.29 (6H, s), 2.85–3.56 (10H, m), 3.89 (3H, s), 3.99 (2H, d, J = 6.0 Hz), 4.12–4.73 (6H, m), 6.93 (1H, d, J = 8.4 Hz), 7.07–7.16 (4H, m), 7.43–7.60 (5H, m), 7.70 (1H, d, J = 8.6 Hz), 7.75 (2H, d, J = 7.9 Hz), 7.80–7.93 (3H, m), 8.24–8.31 (2H, m), 9.32 (1H, br s), 10.07 (1H, br s); MS m/z: 584 (M + H)+; ESI-MS m/z: 584.2840 (M + H)+ (Calcd for C31H37O3N5F3: 584.2843).
{4-[(4-Chlorophenyl)methyl]piperazin-1-yl}[5-({1-[(6-methoxypyridin-3-yl)methyl]piperidin-4-yl}methoxy)pyridin-2-yl]methanone Ditosylate (15)Compound 15 was prepared from 11 in 72% yield using a similar approach to that described for 14. 1H-NMR (DMSO-d6) δ: 1.39–1.65 (2H, m), 1.81–2.19 (3H, m), 2.29 (6H, s), 2.77–3.60 (10H, m), 3.70–4.74 (11H, m), 6.93 (1H, d, J = 9.0 Hz), 7.12 (4H, dd, J = 0.7, 8.4 Hz), 7.45–7.59 (9H, m), 7.62–7.75 (1H, m), 7.80–7.87 (1H, m), 8.18–8.36 (2H, m), 9.27 (1H, br s), 9.89 (1H, br s); MS m/z: 550 (M + H)+; ESI-MS m/z: 550.2584 (M + H)+ (Calcd for C30H37O3N5Cl: 550.2579).
4-({4-[5-({1-[(6-Methoxypyridin-3-yl)methyl]piperidin-4-yl}methoxy)pyridine-2-carbonyl]piperazin-1-yl}methyl)benzonitrile Ditosylate (16a)Compound 16a was prepared from 12 in 66% yield using a similar approach to that described for 14. 1H-NMR (DMSO-d6) δ: 1.35–1.68 (2H, m), 1.78–2.14 (3H, m), 2.29 (6H, s), 2.77–3.60 (10H, m), 3.89 (3H, s), 3.99 (2H, d, J = 6.0 Hz), 4.09–4.71 (6H, m), 6.93 (1H, d, J = 8.4 Hz), 7.12 (4H, d, J = 7.9 Hz), 7.46–7.60 (1H, m), 7.49 (4H, d, J = 7.9 Hz), 7.65–7.76 (3H, m), 7.83 (1H, dd, J = 2.4, 8.6 Hz), 7.97 (2H, d, J = 8.4 Hz), 8.20–8.35 (2H, m), 9.27 (1H, br s), 10.01 (1H, br s); MS m/z: 541 (M + H)+; ESI-MS m/z: 541.2925 (M + H)+ (Calcd for C31H37O3N6: 541.2922).
4-({4-[5-({1-[(6-Methylpyridin-3-yl)methyl]piperidin-4-yl}methoxy)pyridine-2-carbonyl]piperazin-1-yl}methyl)benzonitrile Tetrahydrochloride (16b)Compound 16b was prepared from 12 and 6-methylpyridine-3-carbaldehyde in 38% yield using a similar approach to that described for 13. 1H-NMR (DMSO-d6) δ: 1.54–2.22 (5H, m), 2.57–4.86 (21H, m), 7.54 (1H, dd, J = 2.9, 8.8 Hz), 7.68 (1H, d, J = 8.8 Hz), 7.79–8.04 (4H, m), 8.27 (1H, d, J = 2.9 Hz), 8.38–8.68 (2H, m), 8.86–9.08 (1H, m), 11.34 (1H, br s), 11.91 (1H, br s); MS m/z: 525 (M + H)+; ESI-MS m/z: 525.2971 (M + H)+ (Calcd for C31H37O2N6: 525.2973).
4-({4-[5-({1-[(6-Ethoxypyridin-3-yl)methyl]piperidin-4-yl}methoxy)pyridine-2-carbonyl]piperazin-1-yl}methyl)benzonitrile Ditosylate (16c)Compound 16c was prepared from 12 and 29 in 59% yield using a similar approach to that described for 14. 1H-NMR (DMSO-d6) δ: 1.33 (3H, t, J = 7.1 Hz), 1.41–1.63 (2H, m), 1.76–2.17 (3H, m), 2.29 (6H, s), 2.84–3.54 (10H, m), 3.99 (2H, d, J = 6.0 Hz), 4.12–4.77 (8H, m), 6.89 (1H, d, J = 9.0 Hz), 7.11 (4H, d, J = 8.2 Hz), 7.41–7.59 (1H, m), 7.48 (4H, d, J = 8.2 Hz), 7.65–7.75 (3H, m), 7.82 (1H, dd, J = 2.4, 8.6 Hz), 7.97 (2H, d, J = 8.4 Hz), 8.22–8.34 (2H, m), 9.25 (1H, br s), 10.01 (1H, br s); MS m/z: 555 (M + H)+; ESI-MS m/z: 555.3079 (M + H)+ (Calcd for C32H39O3N6: 555.3078).
Methyl 5-Hydroxy-3-methylpyridine-2-carboxylate (18)To a solution of methyl 5-methoxy-3-methylpyridine-2-carboxylate16) (17; 2.4 g, 13 mmol) in CH2Cl2 (140 mL) was added AlCl3 (20 g, 150 mmol). The mixture was stirred at 55°C overnight under an argon atmosphere. After cooling to 0°C, the mixture was diluted with 1.0 mol/L HCl aqueous solution and stirred at room temperature. The mixture was basified with 1.0 mol/L NaOH aqueous solution and acidified with 10% citric acid aqueous solution. The mixture was extracted with CHCl3/IPA (4 : 1), and the organic layer was dried over MgSO4 and concentrated in vacuo to obtain the product (1.8 g, 80%). 1H-NMR (DMSO-d6) δ: 2.44 (3H, s), 3.79 (3H, s), 7.08 (1H, dd, J = 0.8, 2.6 Hz), 8.02 (1H, dd, J = 0.4, 2.6 Hz), 10.56 (1H, br s); MS m/z: 168 (M + H)+.
5-{[1-(tert-Butoxycarbonyl)piperidin-4-yl]methoxy}-3-methylpyridine-2-carboxylic Acid (19)Compound 19 was prepared from 18 in 69% yield using a similar approach to that described for 9. 1H-NMR (DMSO-d6) δ: 1.03–1.27 (2H, m), 1.40 (9H, s), 1.66–1.82 (2H, m), 1.86–2.06 (1H, m), 2.46–2.54 (3H, m), 2.61–2.92 (2H, m), 3.92–4.03 (2H, m), 3.99 (2H, d, J = 6.4 Hz), 7.36 (1H, d, J = 2.7 Hz), 8.16 (1H, d, J = 2.6 Hz), 12.57 (1H, br s); MS m/z: 351 (M + H)+.
4-[(4-{3-Methyl-5-[(piperidin-4-yl)methoxy]pyridine-2-carbonyl}piperazin-1-yl)methyl]benzonitrile (20)To a solution of 5-{[1-(tert-butoxycarbonyl)piperidin-4-yl]methoxy}-3-methylpyridine-2-carboxylic acid (19; 600 mg, 1.7 mmol), 4-[(piperazin-1-yl)methyl]benzonitrile dihydrochloride (31; 480 mg, 1.8 mmol), Et3N (0.49 mL, 3.5 mmol) in CH2Cl2 (9.0 mL) were added WSC·HCl (450 mg, 2.3 mmol), HOBt (320 mg, 2.4 mmol). After stirring at room temperature overnight, the mixture was diluted with saturated NaHCO3 aqueous solution and extracted with CHCl3. The organic layer was dried over MgSO4 and concentrated in vacuo. The residue was purified by column chromatography on silica gel (CHCl3/MeOH). To a solution of the residue in MeOH (5.0 mL) was added 4.0 mol/L HCl in 1,4-dioxane (5.0 mL, 20 mmol). After stirring at room temperature for 1.5 h, the mixture was basified with saturated NaHCO3 aqueous solution and extracted with CHCl3/IPA (4 : 1). The organic layer was dried over MgSO4 and concentrated in vacuo to obtain the product (790 mg, quantitative yield). 1H-NMR (DMSO-d6) δ: 1.07–1.33 (2H, m), 1.62–1.73 (2H, m), 1.75–1.91 (1H, m), 2.21 (3H, s), 2.25–2.37 (2H, m), 2.38–2.55 (4H, m), 2.89–3.04 (2H, m), 3.06–3.16 (2H, m), 3.58–3.61 (2H, m), 3.61–3.69 (2H, m), 3.88 (2H, d, J = 6.6 Hz), 7.32 (1H, d, J = 2.7 Hz), 7.52 (2H, d, J = 8.4 Hz), 7.79 (2H, d, J = 8.4 Hz), 8.06 (1H, d, J = 2.6 Hz); MS m/z: 434 (M + H)+.
4-({4-[5-({1-[(6-Ethoxypyridin-3-yl)methyl]piperidin-4-yl}methoxy)-3-methylpyridine-2-carbonyl]piperazin-1-yl}methyl)benzonitrile Ditosylate (21a)Compound 21a was prepared from 20 and 29 in 56% yield using a similar approach to that described for 14. 1H-NMR (DMSO-d6) δ: 1.33 (3H, t, J = 7.1 Hz), 1.41–1.59 (2H, m), 1.78–2.14 (3H, m), 2.26 (3H, s), 2.29 (6H, s), 2.79–3.78 (10H, m), 3.95 (2H, d, J = 6.0 Hz), 4.06–4.82 (8H, m), 6.90 (1H, d, J = 8.6 Hz), 7.07–7.15 (4H, m), 7.37 (1H, d, J = 2.4 Hz), 7.48 (4H, d, J = 7.9 Hz), 7.61–7.76 (2H, m), 7.82 (1H, dd, J = 2.4, 8.6 Hz), 7.91–8.02 (2H, m), 8.08 (1H, d, J = 2.4 Hz), 8.26 (1H, d, J = 2.4 Hz), 9.27 (1H, br s), 10.04 (1H, br s); MS m/z: 569 (M + H)+; ESI-MS m/z: 569.3238 (M + H)+ (Calcd for C33H41O3N6: 569.3235).
4-({4-[5-({1-[(5-Methoxypyrazin-2-yl)methyl]piperidin-4-yl}methoxy)-3-methylpyridine-2-carbonyl]piperazin-1-yl}methyl)benzonitrile Ditosylate (21b)To a mixture of 4-[(4-{3-methyl-5-[(piperidin-4-yl)methoxy]pyridine-2-carbonyl}piperazin-1-yl)methyl]benzonitrile (20; 410 mg, 0.94 mmol) and MeCN (8.0 mL) were added 2-(chloromethyl)-5-methoxypyrazine (35a; 170 mg, 1.1 mmol) and K2CO3 (390 mg, 2.8 mmol). After stirring at room temperature overnight, the mixture was diluted with H2O and extracted with CHCl3. The organic layer was dried over MgSO4 and concentrated in vacuo. The residue was purified by column chromatography on silica gel (CHCl3/MeOH). To a solution of the residue (380 mg, 0.68 mmol) in MeOH was added p-TsOH·H2O (240 mg, 1.3 mmol). The mixture was concentrated in vacuo. To the residue was added Et2O, and the precipitated solid was collected by filtration to obtain the product (500 mg, 59%). 1H-NMR (DMSO-d6) δ: 1.42–1.65 (2H, m), 1.76–2.16 (3H, m), 2.26 (3H, s), 2.29 (6H, s), 2.81–3.82 (10H, m), 3.85–4.05 (5H, m), 4.34–4.75 (4H, m), 4.41 (2H, d, J = 4.9 Hz), 7.07–7.15 (4H, m), 7.37 (1H, d, J = 2.7 Hz), 7.48 (4H, d, J = 7.9 Hz), 7.70 (2H, d, J = 8.2 Hz), 7.97 (2H, d, J = 8.2 Hz), 8.09 (1H, d, J = 2.7 Hz), 8.38 (1H, d, J = 1.3 Hz), 8.43 (1H, d, J = 1.3 Hz), 9.66 (1H, br s), 9.78–10.19 (1H, m); MS m/z: 556 (M + H)+; ESI-MS m/z: 556.3030 (M + H)+ (Calcd for C31H38O3N7: 556.3031).
4-({4-[5-({1-[(5-Ethoxypyrazin-2-yl)methyl]piperidin-4-yl}methoxy)-3-methylpyridine-2-carbonyl]piperazin-1-yl}methyl)benzonitrile Succinate (21c)To a solution of 4-[(4-{3-methyl-5-[(piperidin-4-yl)methoxy]pyridine-2-carbonyl}piperazin-1-yl)methyl]benzonitrile (20; 3.2 g, 7.3 mmol), DIPEA (4.0 mL, 23 mmol) and MeCN (30 mL) was added 2-(chloromethyl)-5-ethoxypyrazine (35b; 1.5 g, 8.4 mmol) in MeCN (20 mL). After stirring at room temperature for 14 h, the mixture was concentrated in vacuo. The residue was purified by column chromatography on amino functionalized silica gel (hexane/EtOAc). The residue was purified by column chromatography on silica gel (CHCl3/MeOH). To a solution of the residue (3.2 g, 5.7 mmol) in EtOH (12 mL) was added succinic acid (670 mg, 5.7 mmol). After stirring at room temperature for 0.5 h, the mixture was concentrated in vacuo. To the residue was added heptane. After stirring at room temperature overnight, the precipitated solid was collected by filtration to obtain the product (3.7 g, 74%). 1H-NMR (DMSO-d6) δ: 1.19–1.43 (2H, m), 1.34 (3H, t, J = 7.0 Hz), 1.64–1.85 (3H, m), 1.98–2.17 (2H, m), 2.20 (3H, s), 2.25–2.36 (2H, m), 2.39–2.45 (2H, m), 2.41 (4H, s), 2.79–2.96 (2H, m), 3.03–3.21 (2H, m), 3.54–3.69 (6H, m), 3.91 (2H, d, J = 6.0 Hz), 4.33 (2H, q, J = 6.9 Hz), 7.32 (1H, d, J = 2.7 Hz), 7.52 (2H, d, J = 8.4 Hz), 7.79 (2H, d, J = 8.4 Hz), 8.06 (1H, d, J = 2.7 Hz), 8.16 (1H, d, J = 1.3 Hz), 8.21 (1H, d, J = 1.3 Hz), 12.10 (2H, br s); MS m/z: 570 (M + H)+; ESI-MS m/z: 570.3185 (M + H)+ (Calcd for C32H40O3N7: 570.3187).
5-{[1-(tert-Butoxycarbonyl)-4-methoxypiperidin-4-yl]methoxy}-3-methylpyridine-2-carboxylic Acid (22)To a mixture of methyl 5-hydroxy-3-methylpyridine-2-carboxylate (18; 960 mg, 5.7 mmol), tert-butyl 4-(hydroxymethyl)-4-methoxypiperidine-1-carboxylate (37; 1.4 g, 5.7 mmol) and toluene (20 mL) was added cyanomethylenetributylphosphorane (2.2 mL, 8.3 mmol). The mixture was stirred at 100°C overnight under an argon atmosphere. After cooling to room temperature, the mixture was concentrated in vacuo. The residue was purified by column chromatography on silica gel (hexane/EtOAc). To a solution of the residue in MeOH (20 mL) was added 1.0 mol/L NaOH aqueous solution (20 mL, 20 mmol). The mixture was stirred at 60°C for 1 h. After cooling to room temperature, the mixture was washed with Et2O and the aqueous layer was acidified with 10% citric acid aqueous solution. The mixture was extracted with CHCl3, and the organic layer was dried over MgSO4 and concentrated in vacuo. To the residue was added Et2O, and the precipitated solid was collected by filtration to obtain the product (2.0 g, 91%). 1H-NMR (DMSO-d6) δ: 1.40 (9H, s), 1.45–1.60 (2H, m), 1.73–1.88 (2H, m), 2.51 (3H, s), 2.85–3.16 (2H, m), 3.19 (3H, s), 3.61–3.82 (2H, m), 4.11 (2H, s), 7.42 (1H, d, J = 2.4 Hz), 8.20 (1H, d, J = 2.4 Hz), 12.60 (1H, br s); MS m/z: 381 (M + H)+.
5-{[1-(tert-Butoxycarbonyl)-4-fluoropiperidin-4-yl]methoxy}-3-methylpyridine-2-carboxylic Acid (23)Compound 23 was prepared from 18 and tert-butyl 4-fluoro-4-(hydroxymethyl)piperidine-1-carboxylate18) in 54% yield using a similar approach to that described for 22. 1H-NMR (DMSO-d6) δ: 1.41 (9H, s), 1.55–1.82 (2H, m), 1.83–2.01 (2H, m), 2.51 (3H, s), 2.89–3.18 (2H, m), 3.73–3.93 (2H, m), 4.27 (2H, d, J = 21.2 Hz), 7.42 (1H, d, J = 2.4 Hz), 8.21 (1H, d, J = 2.4 Hz), 12.64 (1H, br s); MS m/z: 369 (M + H)+.
4-[(4-{5-[(4-Methoxypiperidin-4-yl)methoxy]-3-methylpyridine-2-carbonyl}piperazin-1-yl)methyl]benzonitrile (24)Compound 24 was prepared from 22 in quantitative yield using a similar approach to that described for 20. 1H-NMR (DMSO-d6) δ: 1.41–1.62 (2H, m), 1.64–1.83 (2H, m), 2.22 (3H, s), 2.26–2.36 (2H, m), 2.37–2.48 (2H, m), 2.63–2.81 (4H, m), 3.06–3.18 (2H, m), 3.16 (3H, s), 3.60 (2H, s), 3.60–3.70 (2H, m), 4.00 (2H, s), 7.37 (1H, d, J = 2.4 Hz), 7.52 (2H, d, J = 8.4 Hz), 7.80 (2H, d, J = 8.4 Hz), 8.11 (1H, d, J = 2.4 Hz); MS m/z: 464 (M + H)+.
4-[(4-{5-[(4-Fluoropiperidin-4-yl)methoxy]-3-methylpyridine-2-carbonyl}piperazin-1-yl)methyl]benzonitrile (25)Compound 25 was prepared from 23 in quantitative yield using a similar approach to that described for 20. 1H-NMR (DMSO-d6) δ: 1.52–1.90 (4H, m), 2.21 (3H, s), 2.26–2.35 (2H, m), 2.37–2.48 (2H, m), 2.63–2.87 (4H, m), 3.05–3.19 (2H, m), 3.60 (2H, s), 3.61–3.69 (2H, m), 4.14 (2H, d, J = 21.0 Hz), 7.38 (1H, d, J = 2.4 Hz), 7.52 (2H, d, J = 8.4 Hz), 7.79 (2H, d, J = 8.4 Hz), 8.12 (1H, d, J = 2.4 Hz); MS m/z: 452 (M + H)+.
4-({4-[5-({1-[(6-Ethoxypyridin-3-yl)methyl]-4-methoxypiperidin-4-yl}methoxy)-3-methylpyridine-2-carbonyl]piperazin-1-yl}methyl)benzonitrile Succinate (26a)To a solution of 4-[(4-{5-[(4-methoxypiperidin-4-yl)methoxy]-3-methylpyridine-2-carbonyl}piperazin-1-yl)methyl]benzonitrile (24; 370 mg, 0.79 mmol) in CH2Cl2 (6.0 mL) were added 6-ethoxypyridine-3-carbaldehyde (29; 120 mg, 0.79 mmol), acetic acid (5.0 µL, 87 µmol) and NaBH(OAc)3 (450 mg, 2.1 mmol). After stirring at room temperature for 4 h, the mixture was diluted with saturated NaHCO3 aqueous solution and extracted with CHCl3. The organic layer was dried over MgSO4 and concentrated in vacuo. The residue was purified by column chromatography on silica gel (CHCl3/MeOH). To a solution of the residue (370 mg, 0.62 mmol) in MeOH was added succinic acid (72 mg, 0.61 mmol). The mixture was concentrated in vacuo. To the residue was added heptane, and the precipitated solid was collected by filtration to obtain the product (340 mg, 61%). 1H-NMR (DMSO-d6) δ: 1.30 (3H, t, J = 7.1 Hz), 1.50–1.70 (2H, m), 1.73–1.87 (2H, m), 2.14–2.63 (8H, m), 2.21 (3H, s), 2.41 (4H, s), 3.05–3.16 (2H, m), 3.15 (3H, s), 3.44 (2H, s), 3.54–3.72 (2H, m), 3.59 (2H, s), 4.00 (2H, s), 4.28 (2H, q, J = 7.1 Hz), 6.75 (1H, d, J = 8.6 Hz), 7.37 (1H, d, J = 2.2 Hz), 7.52 (2H, d, J = 8.2 Hz), 7.61 (1H, dd, J = 2.4, 8.6 Hz), 7.79 (2H, d, J = 8.2 Hz), 8.03 (1H, d, J = 2.2 Hz), 8.10 (1H, d, J = 2.4 Hz), 12.22 (2H, br s); MS m/z: 599 (M + H)+; ESI-MS m/z: 599.3348 (M + H)+ (Calcd for C34H43O4N6: 599.3340).
4-({4-[5-({1-[(6-Ethoxypyridin-3-yl)methyl]-4-fluoropiperidin-4-yl}methoxy)-3-methylpyridine-2-carbonyl]piperazin-1-yl}methyl)benzonitrile Succinate (27a)Compound 27a was prepared from 25 in 68% yield using a similar approach to that described for 26a. 1H-NMR (DMSO-d6) δ: 1.30 (3H, t, J = 7.1 Hz), 1.55–2.06 (4H, m), 2.14–2.48 (6H, m), 2.21 (3H, s), 2.42 (4H, s), 2.58–2.74 (2H, m), 3.03–3.18 (2H, m), 3.46 (2H, s), 3.59 (2H, s), 3.60–3.70 (2H, m), 4.15 (2H, d, J = 20.5 Hz), 4.28 (2H, q, J = 7.1 Hz), 6.75 (1H, d, J = 8.5 Hz), 7.37 (1H, d, J = 2.2 Hz), 7.52 (2H, d, J = 8.4 Hz), 7.62 (1H, dd, J = 2.4, 8.5 Hz), 7.79 (2H, d, J = 8.4 Hz), 8.03 (1H, d, J = 2.2 Hz), 8.11 (1H, d, J = 2.4 Hz), 12.17 (2H, br s); MS m/z: 587 (M + H)+; ESI-MS m/z: 587.3137 (M + H)+ (Calcd for C33H40O3N6F: 587.3140).
4-({4-[5-({1-[(5-Ethoxypyrazin-2-yl)methyl]-4-methoxypiperidin-4-yl}methoxy)-3-methylpyridine-2-carbonyl]piperazin-1-yl}methyl)benzonitrile Succinate (26b)Compound 26b was prepared from 24 and 35b in 65% yield using a similar approach to that described for 21c. 1H-NMR (DMSO-d6) δ: 1.34 (3H, t, J = 7.1 Hz), 1.53–1.72 (2H, m), 1.72–1.88 (2H, m), 2.21 (3H, s), 2.25–2.47 (6H, m), 2.41 (4H, s), 2.53–2.65 (2H, m), 3.05–3.18 (2H, m), 3.15 (3H, s), 3.59 (4H, s), 3.61–3.69 (2H, m), 4.01 (2H, s), 4.33 (2H, q, J = 7.1 Hz), 7.37 (1H, d, J = 2.4 Hz), 7.52 (2H, d, J = 8.4 Hz), 7.79 (2H, d, J = 8.4 Hz), 8.10 (1H, d, J = 2.4 Hz), 8.17 (1H, d, J = 1.3 Hz), 8.21 (1H, d, J = 1.3 Hz), 12.22 (2H, br s); MS m/z: 600 (M + H)+; ESI-MS m/z: 600.3292 (M + H)+ (Calcd for C33H42O4N7: 600.3293).
4-({4-[5-({1-[(5-Ethoxypyrazin-2-yl)methyl]-4-fluoropiperidin-4-yl}methoxy)-3-methylpyridine-2-carbonyl]piperazin-1-yl}methyl)benzonitrile Succinate (27b)To a solution of 4-[(4-{5-[(4-fluoropiperidin-4-yl)methoxy]-3-methylpyridine-2-carbonyl}piperazin-1-yl)methyl]benzonitrile (25; 2.0 g, 4.4 mmol) in MeCN (20 mL) were added DIPEA (2.7 mL, 16 mmol) and 2-(chloromethyl)-5-ethoxypyrazine (35b; 960 mg, 5.5 mmol) in MeCN (5.0 mL). After stirring at 50°C for 18 h, the mixture was concentrated in vacuo. The residue was purified by column chromatography on silica gel (CHCl3/MeOH). The residue was purified by column chromatography on amino functionalized silica gel (hexane/EtOAc). To the residue was added Et2O. After stirring at room temperature overnight, the precipitated solid was collected by filtration. The obtained solid was purified by column chromatography on silica gel (CHCl3/MeOH/EtOAc). To the residue was added Et2O, and the precipitated solid was collected by filtration. To a mixture of the obtained solid (2.0 g, 3.3 mmol), acetone (15 mL) and EtOH (15 mL) was added succinic acid (390 mg, 3.3 mmol). After stirring at room temperature 15 min, to the mixture was added EtOH (15 mL). After stirring at room temperature 0.5 h, the mixture was stirred at 50°C for additional 15 min. The mixture was filtered and the filtrate was concentrated in vacuo. To the residue was added heptane. After stirring at room temperature for 0.5 h, the precipitated solid was collected by filtration to obtain the product (2.1 g, 68%). 1H-NMR (DMSO-d6) δ: 1.34 (3H, t, J = 7.1 Hz), 1.61–2.01 (4H, m), 2.21 (3H, s), 2.26–2.47 (6H, m), 2.42 (4H, s), 2.62–2.81 (2H, m), 3.05–3.19 (2H, m), 3.53–3.72 (2H, m), 3.59 (2H, s), 3.62 (2H, s), 4.16 (2H, d, J = 20.7 Hz), 4.34 (2H, q, J = 7.1 Hz), 7.37 (1H, d, J = 2.4 Hz), 7.52 (2H, d, J = 8.4 Hz), 7.79 (2H, d, J = 8.4 Hz), 8.12 (1H, d, J = 2.4 Hz), 8.18 (1H, d, J = 1.3 Hz), 8.22 (1H, d, J = 1.3 Hz), 12.16 (2H, br s); MS m/z: 588 (M + H)+; ESI-MS m/z: 588.3092 (M + H)+ (Calcd for C32H39O3N7F: 588.3093).
6-Ethoxypyridine-3-carbaldehyde (29)To a solution of 5-bromo-2-ethoxypyridine (28; 5.0 g, 25 mmol) in THF (70 mL) was added dropwise n-BuLi (16 mL, 26 mmol, 1.7 mol/L in hexane) at −68°C under a nitrogen atmosphere. After stirring at the same temperature for 0.5 h, to the mixture was added dropwise DMF (10 mL, 130 mmol). The mixture was allowed to warm gradually to room temperature and stirred for 3 h. The mixture was diluted with 10% citric acid aqueous solution and extracted with EtOAc. The organic layer was washed with brine, dried over MgSO4 and concentrated in vacuo. The residue was purified by column chromatography on silica gel (hexane/EtOAc) to obtain the product (2.2 g, 59%). 1H-NMR (DMSO-d6) δ: 1.35 (3H, t, J = 7.1 Hz), 4.43 (2H, q, J = 7.1 Hz), 6.96 (1H, ddd, J = 0.7, 0.7, 8.7 Hz), 8.11 (1H, dd, J = 2.4, 8.7 Hz), 8.75 (1H, dd, J = 0.7, 2.4 Hz), 9.96 (1H, d, J = 0.7 Hz); MS m/z: 152 (M + H)+.
4-[(Piperazin-1-yl)methyl]benzonitrile Dihydrochloride (31)To a solution of tert-butyl piperazine-1-carboxylate (30; 7.0 g, 38 mmol) in CH2Cl2 (47 mL) were added 4-formylbenzonitrile (5.9 g, 45 mmol) and acetic acid (4.3 mL, 75 mmol). After stirring at room temperature for 0.5 h, to the mixture was added NaBH(OAc)3 (16 g, 75 mmol). After stirring at room temperature for 3 h, the mixture was diluted with saturated NaHCO3 aqueous solution and extracted with CHCl3. The organic layer was dried over Na2SO4 and concentrated in vacuo. The residue was purified by column chromatography on silica gel (CHCl3/MeOH). To a solution of the residue in EtOAc (60 mL) and CHCl3 (60 mL) was added 4.0 mol/L HCl in EtOAc (60 mL, 240 mmol). After stirring at room temperature for 3 d, the mixture was diluted with hexane, and then the precipitated solid was collected by filtration to obtain the product (8.0 g, 77%). 1H-NMR (D2O) δ: 3.59 (8H, s), 4.52 (2H, s), 7.70 (2H, d, J = 8.2 Hz), 7.90 (2H, d, J = 8.2 Hz); MS m/z: 202 (M + H)+.
Methyl 5-Methoxypyrazine-2-carboxylate (33a)To a solution of methyl 5-chloropyrazine-2-carboxylate (32; 10 g, 58 mmol) in MeOH (100 mL) was added portion-wise sodium methoxide (5.0 g, 93 mmol) at 0°C under a nitrogen atmosphere. After stirring at room temperature for 3 h, the mixture was diluted with 10% citric acid aqueous solution and extracted with EtOAc. The organic layer was dried over MgSO4 and concentrated in vacuo. The residue was purified by column chromatography on silica gel (hexane/EtOAc) to give the product (7.9 g, 81%). 1H-NMR (DMSO-d6) δ: 3.88 (3H, s), 4.00 (3H, s), 8.41 (1H, d, J = 1.3 Hz), 8.84 (1H, d, J = 1.3 Hz); MS m/z: 169 (M + H)+.
Ethyl 5-Ethoxypyrazine-2-carboxylate (33b)To a solution of t-BuOK (2.3 g, 21 mmol) in EtOH (30 mL) was added portion-wise methyl 5-chloropyrazine-2-carboxylate (32; 3.0 g, 17 mmol) at 0°C. After stirring at the same temperature for 1 h, the mixture was neutralized with 1.0 mol/L HCl aqueous solution and extracted with CHCl3. The organic layer was dried over Na2SO4 and concentrated in vacuo. The residue was purified by column chromatography on silica gel (CHCl3) to give the product (2.8 g, 82%). 1H-NMR (DMSO-d6) δ: 1.33 (3H, t, J = 7.1 Hz), 1.37 (3H, t, J = 7.1 Hz), 4.34 (2H, q, J = 7.1 Hz), 4.44 (2H, q, J = 7.1 Hz), 8.37 (1H, d, J = 1.3 Hz), 8.81 (1H, d, J = 1.3 Hz); MS m/z: 197 (M + H)+.
(5-Methoxypyrazin-2-yl)methanol (34a)To a mixture of methyl 5-methoxypyrazine-2-carboxylate (33a; 2.3 g, 14 mmol) and MeOH (45 mL) was added NaBH4 (1.6 g, 42 mmol) at 0°C. After stirring at the same temperature for 15 min, the mixture was stirred at room temperature for an additional 2 h. The mixture was acidified with 1.0 mol/L HCl aqueous solution and basified with 1.0 mol/L NaOH aqueous solution. The mixture was extracted with CHCl3/IPA (4 : 1), and the organic layer was dried over MgSO4 and concentrated in vacuo. The residue was purified by column chromatography on silica gel (hexane/EtOAc) to obtain the product (1.5 g, 77%). 1H-NMR (DMSO-d6) δ: 3.90 (3H, s), 4.55 (2H, d, J = 5.7 Hz), 5.40 (1H, t, J = 5.7 Hz), 8.20–8.22 (1H, m), 8.22–8.24 (1H, m); MS m/z: 141 (M + H)+.
(5-Ethoxypyrazin-2-yl)methanol (34b)Compound 34b was prepared from 33b in 75% yield using a similar approach to that described for 34a. 1H-NMR (DMSO-d6) δ: 1.34 (3H, t, J = 7.1 Hz), 4.34 (2H, q, J = 7.1 Hz), 4.54 (2H, d, J = 5.7 Hz), 5.39 (1H, t, J = 5.7 Hz), 8.07–8.32 (2H, m); MS m/z: 155 (M + H)+.
2-(Chloromethyl)-5-methoxypyrazine (35a)To a solution of (5-methoxypyrazin-2-yl)methanol (34a; 700 mg, 5.0 mmol) in CH2Cl2 (10 mL) was added SOCl2 (1.0 mL, 14 mmol) at 0°C. After stirring at room temperature for 0.5 h, the mixture was concentrated in vacuo to obtain the product (800 mg, quantitative yield). 1H-NMR (CDCl3) δ: 3.98 (3H, s), 4.65 (2H, s), 8.19–8.20 (1H, m), 8.20–8.21 (1H, m); MS m/z: 159 (M + H)+.
2-(Chloromethyl)-5-ethoxypyrazine (35b)Compound 35b was prepared from 34b in 100% yield using a similar approach to that described for 35a. 1H-NMR (DMSO-d6) δ: 1.35 (3H, t, J = 7.1 Hz), 4.36 (2H, q, J = 7.1 Hz), 4.80 (2H, s), 8.29 (1H, d, J = 1.3 Hz), 8.33 (1H, d, J = 1.3 Hz); MS m/z: 173 (M + H)+.
tert-Butyl 4-(Hydroxymethyl)-4-methoxypiperidine-1-carboxylate (37)To a solution of tert-butyl 1-oxa-6-azaspiro[2.5]octane-6-carboxylate19) (36; 10 g, 47 mmol) in MeOH (150 mL) was added p-TsOH·H2O (300 mg, 1.6 mmol). After stirring at room temperature overnight, the mixture was diluted with saturated NaHCO3 aqueous solution and H2O. The mixture was extracted with CHCl3. The organic layer was dried over MgSO4 and concentrated in vacuo. The residue was purified by column chromatography on silica gel (hexane/EtOAc) to give the product (6.0 g, 52%). 1H-NMR (DMSO-d6) δ: 1.23–1.43 (2H, m), 1.39 (9H, s), 1.53–1.67 (2H, m), 2.77–3.06 (2H, m), 3.13 (3H, s), 3.30–3.34 (2H, m), 3.53–3.73 (2H, m), 4.54 (1H, t, J = 5.5 Hz); MS m/z: 246 (M + H)+.
The breast cancer cell lines MDA-MB-453 and SK-BR-3 were purchased from American Type Culture Collection (Manassas, VA, U.S.A.). MDA-MB-453 was cultured in Leibovitz’s L-15 Medium (Life Technologies, Carlsbad, CA, U.S.A.) under CO2-free conditions at 37°C. SK-BR-3 was cultured in RPMI 1640 medium (Sigma-Aldrich Co. LLC., St. Louis, MO, U.S.A.) at 37°C in 5% CO2. All media were supplemented with 10% fetal bovine serum (Sigma-Aldrich Co. LLC.) and 1% penicillin/streptomycin (Life Technologies [Cat. No. 15070-063]).
Whole Cell ELISAMDA-MB-453 cells were seeded onto 384-well clear flat plates at 1.5 × 104 cells/well. The following day, the cells were treated with the test compound at final concentrations of 0 (DMSO only), 0.1, 0.3, 1, 3, 10, 30, 100, 300, 1000, 3000 and 10000 nmol/L. The final concentration of DMSO in each well was 0.1% (v/v). Two hours after addition of the test compound, the cells were fixed in 40% glyoxal solution (Nacalai Chemical Ltd., Kyoto, Japan) for 0.5 h at room temperature. For probing, the supernatant was discarded and the cells were permeabilized with 0.1% Triton X-100 in phosphate-buffered saline (PBS) for 10 min at room temperature. Subsequently, the supernatant was discarded and the cells were blocked with ODYSSEY Blocking solution (Li-Cor Biosciences, Lincoln, NE, U.S.A.) for 1 h at room temperature. After discarding the supernatant, a primary phospho- Acetyl-CoA Carboxylase (Ser79) antibody (1 : 500 in ODYSSEY Blocking solution; Cat. No. 3661, Cell Signaling Technologies, Danvers, MA, U.S.A.) was added. After incubating overnight at 4°C, the plates were washed 3 times with Tris-buffered saline containing 0.05% Tween 20 (TBS/Tween). Goat anti-rabbit IRDye 800CW (Li-Cor Biosciences; 1 : 1000 in ODYSSEY Blocking solution) was added, and the plates were incubated for 1 h at room temperature. After the incubation, the plates were washed 3 times with TBS/Tween and dried for at least 3 h at room temperature. Fluorescence signals were quantified using the Aerius automated infrared imaging system (Li-Cor Biosciences). The assays were performed in duplicate or triplicate, and the data were analyzed using Prism5 software (GraphPad Software Inc., San Diego, CA, U.S.A.). The EC50 value of each test compound was calculated using Sigmoid-Emax model non-linear regression analysis. The average signal was normalized by regarding the signal in the DMSO-treated group as 0% in each experiment.
Cell Growth Inhibition AssayEach breast cancer cell line above was seeded onto non-adherent 384-well white plates at 500 cells/well or 96-well white plates at 1000 cells/well. The following day, the test compound was added to each well at final concentrations of 0 (DMSO only), 0.3, 1, 3, 10, 30, 100, 300, 1000, and 3000 nmol/L. The final concentration of DMSO in each well was 0.1% (v/v). Four days after addition of the test compound, cell viability was determined using a CellTiter-Glo® Luminescent Cell Viability Assay (Promega, Madison, WI, U.S.A.). CellTiter-Glo® Reagent was added to each well, and luminescence was measured using ARVO SX® (PerkinElmer, Inc., Waltham, MA, U.S.A.). The assay was performed in triplicate. Cell viability according to luminescence intensity was normalized by regarding the average luminescence intensity following treatment with DMSO only as 100% and no luminescence intensity as 0%. The IC50 value of each test compound was calculated by Sigmoid-Emax non-linear regression analysis using Prism5 software (GraphPad Software Inc., San Diego, CA, U.S.A.).
AnimalsFive-week-old male nude mice (CAnN.Cg-Foxn1nu/CrlCrlj[nu/nu]) were purchased from Charles River Laboratories Japan, Inc. (Kanagawa, Japan). They were maintained on a standard diet and water throughout the experiments under specific-pathogen-free conditions.
In Vivo Antitumor EvaluationMDA-MB-453 cells were suspended in a 1 : 1 BD Matrigel Matrix (BD Biosciences, Franklin Lakes, NJ, U.S.A.) to PBS solution at 3 × 107 cells/mL. The cell suspension was subcutaneously implanted at 3 × 106 cells/animal/100 µL around the lumbar region and allowed to grow. Mice were divided into 4 groups (N = 5) approximately 3 weeks after inoculation such that the mean tumor volume was comparable among the groups. The first day of administration was designated Day 1, and observation was continued until Day 15. 27b was dissolved in 6% (2-hydroxypropyl)-β-cyclo-dextrin (Sigma-Aldrich Co. LLC.) solution as the vehicle, to obtain concentrations of 1, 2 and 4 mg/10 mL. Vehicle or 1, 2 or 4 mg/kg of 27b was orally administered (10 mL/kg) to nude mice twice-daily for 14 d. All dose levels of 27b are expressed as the free base. Tumor diameter and body weight were measured on Days 0, 5, 8, 12 and 15. Tumor volume was calculated as follows:
 |
Dunnett’s multiple comparisons test was used to compare the tumor volume and body weight the day after the last treatment (Day 15) between the vehicle- and 27b-treated groups. p < 0.05 was used to indicate statistical significance.
The tumor growth inhibition rate was calculated as follows:
 |
The tumor regression rate was calculated in groups whose tumor growth inhibition exceeded 100% as follows:
 |
CHO cells that stably expressed hERG channels were cultured in Ham’s F12 containing 10% FBS, 5% ganeticin and 5% hygromycin B. Prior to experiment, the culture medium was removed and the cells were washed with PBS. Cell was suspended with Serum Free Media containing CHO-S-SFM II, 25 mM N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (HEPES), 100 units/mL P/S at concentration of 2–5 × 106 cells/mL. Cell suspension, test compounds solution on plate, and extracellular and intracellular solutions were set at Qpatch system. Used solutions are followings:
Extracellular (mM): 145 NaCl, 4 KCl, 10 HEPES, 2 CaCl2, 1 MgCl2, 10 D(+)-Glucose, adjust pH to 7.4 with NaOH
Intracellular (mM): 120 KCl, 4 Na2-ATP, 10 ethylene glycol bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA)·tetraacetic acid, 10 HEPES, 1.75 MgCl2, 5.374 CaCl2, adjust pH to 7.2 with KOH
The application of pressure for forming gigaseals and the whole-cell patch clamp configuration were established using QPatch Assay Software (Sophion Bioscience).
Addition of test compounds was also set up using the QPatch Assay Software. Compounds were added to the cells with the eight pipettes via the QPlate integrated glass microfluidic pathways. hERG inhibition was evaluated using a stimulus voltage protocol consisting of a 4.8 s activating pulse to 20 mV from a holding potential of −80 mV and a 5 s test pulse to −50 mV (tail current). The pulse pattern was repeated continuously at 15 s intervals. All QPatch data were analyzed and IC50s were calculated using Sophion’s QPatch Assay software.
In Vitro Intrinsic Clearance with Human Liver MicrosomesThe test compounds (0.1–0.2 µmol/L) were incubated with pooled human liver microsomes (0.2 mg protein/mL), reduced nicotinamide adenine dinucleotide phosphate (NADPH) (1 mmol/L) and ethylenediaminetetraacetic acid (EDTA) (0.1 mmol/L) in pH 7.4 phosphate buffer (100 mmol/L) at 37°C for 30 min. The peak area of the test compound was measured using liquid chromatography-tandem mass spectrometry (LC-MS/MS) to calculate the in vitro intrinsic clearance (CLint, vitro).
Pharmacokinetic StudyMale SD rats were administered orally and intravenously with compound 27b. Compound 27b was dissolved in 30–40% PEG400 for oral and intravenous administration. Blood samples were collected using syringes containing heparin sodium at multiple time points up to 24 h after oral or intravenous administrations. Compound concentrations were determined using LC-MS/MS. Pharmacokinetic parameters were calculated by non-compartment analysis.
Aqueous SolubilityThe test compounds in 10 mmol/L DMSO solution (13 µL) were diluted to 130 µmol/L by adding the fluid for disintegration test (JP1: pH = 1.2, JP2: pH = 6.8, JP2 + TC). After incubation at 25°C for 20 h, precipitates were separated by filtration. The filtrate and a standard solution comprising a 100 µmol/L DMSO solution of the test compound were examined using liquid chromatography. The ratio of the peak area of the sample solution to the peak area of the standard solution was calculated to determine the aqueous solubility.
All animal experiments were performed in accordance with the regulation of the Animal Ethics Committee of Astellas Pharma Inc.
The authors thank Dr. Yukinori Shimoshige and Dr. Takamitsu Ikeda for their contribution to the biological evaluations, Dr. Susumu Watanuki for his helpful support in preparing this manuscript, and Dr. Koji Yamazaki and Dr. Satoshi Kitamura for their support. The authors also thank co-workers for conducting the aqueous solubility assay and spectral measurements.
All authors were employees of Astellas Pharma Inc. when the study was conducted and have no further conflicts of interest to declare.