2020 Volume 68 Issue 7 Pages 618-627
2020 Volume 68 Issue 7 Pages 618-627
A novel polymer (PEG2000-carborane), self-assembling into spherical vesicles (boron-containing vesicles, BCVs), could be quickly taken up by tumor cells and had an enhance stability in the bloodstream in previous study. To have more comprehensive understanding of BCVs, endocytic mechanism and cytotoxicity assessment were conducted. The results showed that BCVs were taken up in the intact form with cholesterol-dependent pathway during endocytosis, and BCVs exhibited nearly no cytotoxicity. BCVs could accumulate within tumors for at least 24 h. The data would provide reference information and guidance for BCVs’ multifunctional application serving as a boron delivery agent for boron neutron capture therapy (BNCT), a hydrophilic and/or hydrophobic drug carrier and a diagnostic imaging fluorescent probe.
Boron neutron capture therapy (BNCT) is an effective radiotherapeutic modality, which is considered suitable for therapy of many cancers, such as malignant meningiom, recurrent malignant glioma, recurrent glioblastoma multiforme, head and neck cancer, recurrent lung cancer and so on,1,2) particularly brain cancer.3) Enough boron in tumor cells is required in this radiotherapy. So far clinical practice has used two main boron delivery agents: sodium borocaptate (Na2B12H11SH, BSH) and 4-borono-L-phenylalanine (C9H12BNO4, BPA).4,5) BSH agent was the first BNCT drug approved by Food and Drug Administration (FDA) for human glioblastoma, squamous cell carcinoma, head and neck and so on. BSH has 12 boron atoms per molecular but is low selective to tumors. The highest boron uptake of BSH was detected in the dura and there was an exceptionally low uptake in bone, cerebrospinal fluid and in the brain. BPA could be transported into tumor cells actively via L-amino acid transporter (LAT1)-mediated uptake but needs larger doses due to only one boron atom per molecule. Accordingly, neither BSH nor BPA proved to be an optimal boron compound for BNCT.6)
Many researchers focused on novel boron delivery systems in order to achieve more selective to tumors and longer circulation time in blood. Carborane, with a high content of boron and the highest neutron capture cross section, is a perfect potential candidate for boron delivery.7) Polyethylene glycol (PEG)-modified liposomes encapsulated carborane physically have been developed, but the premature leakage of the encapsulated BSH from liposome was a limitation.8) So many efforts have focused on the self-assembled carborane fabricated by covalently linking, such as boron-polymer/microbubble complex,9) self-assembled nanogel containing carborane clusters,10) PEGylated blocked copolymer-boron cluster conjugate,11) micelles12,13) and so on. In Wu’s group,14) PEG2000-carborane was designed and synthesized with a linear PEG chain (M.W. = 2000 g·mol−1) and this polymer could self-assemble into spherical vesicles with narrow distribution above critical aggregation concentration (CAC approx. 1 × 10−5 g·mL−1), called boron-containing vesicles (BCVs, Fig. 1A). The BCVs would be potentially multifunctional in biomedical applications, namely, serving as a boron delivery agent for BNCT, a hydrophilic and/or hydrophobic drug carrier and a diagnostic imaging fluorescent probe. The results also revealed that BCVs could be quickly taken up by tumor cells, had an enhanced stability in the bloodstream, and had nearly no cytotoxicity by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay on Hep-B cells.
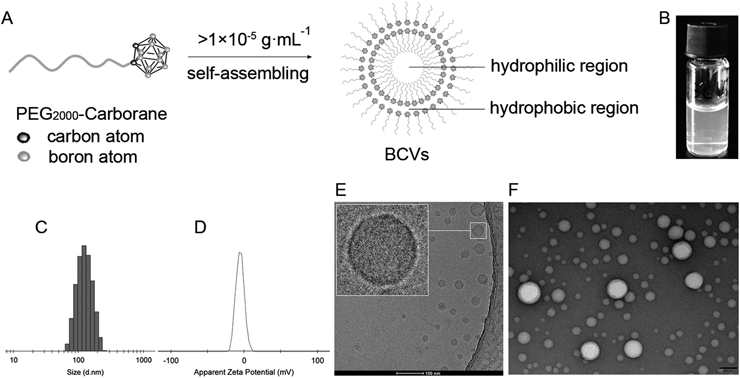
(A) Schematic diagram of preparation of BCVs. (B) Picture of BCVs suspension. (C) Size (intensity) by dynamic light scattering (DLS) and (D) Zeta potential of BCVs in water at 25°C (Malvern Zetasizer, Nano-ZS, Malvern, U.K.). (E) Cryo-transmission electron microscopy (cryo-TEM) image of BCVs at 120 kV (cryo-TEM, Talos L120C, Thermo Fisher Scientific, U.S.A.), Scale bars 100 nm. (F) TEM image of BCVs at 100 kV with negative stained by 1% uranium acetate at atmosphere (TEM, JEM-1400 plus, JEOL, Japan), Scale bars 100 nm.
To have more comprehensive understanding of BCVs, we conducted the studies on the endocytic mechanism and cytotoxicity assessment, including the uptake, influence on cell membrane during endocytosis, endocytosis mechanism, apoptosis, cell cycle and morphological observation so on. These further investigations on BCVs would provide reference information and guidance for BCVs’ multifunctional application.
BCVs were formed by self-assembly with PEG2000-carborane above critical aggregation concentration (>1 × 10−5 g·mL−1) (Figs. 1A, 1B). The average particles size of BCVs was 118.5 ± 5.1 nm with a narrow polydispersity index (PDI, 0.13 ± 0.06) determined by dynamic light scattering (DLS) (Fig. 1C), and average zeta potential was −3.5 ± 1.2 mV (Fig. 1D). BCVs showed bilayer structure contained inner water phase, called as vesicle,14) and like liposome or core-shell structure (Figs. 1E, 1F), the hydrophilic drugs could be loaded in the inner core, and the hydrophobic in the bilayer. Doxorubicin (Dox) was physically loaded in the inner core of BCVs (BCVs-Dox). Fluorescent probe 3,3′-dioctadecyloxacarbocyanine perchlorate (DiO) [or 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI), or 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide (DiR), 100 µg] was physically encapsulated in the hydrophobic bilayer to form BCVs-DiO (or BCVs-DiI, or BCVs-DiR). Fluorescent probes both DiO and DiI (50 + 50 µg), as a donor–acceptor fluorescence resonance energy transfer (FRET) pair, were physically co-encapsulated within the highly hydrophobic bilayer to form DiO-BCVs-DiI.
The transmission electron microscopy (cryo-TEM and TEM) images of BCVs revealed clearly that BCVs was dispersed as individual particles with a well-defined spherical bilayer/core-shell structure. This similar case was reported that Nie’s group15) prepared DOX and paclitaxel (TAX) co-loaded mPEG-PLGA nanoparticles (NPs-DOX-TAX) with size of approximate 250 nm by DLS. The morphology of NPs-DOX-TAX was examined by TEM. The image showed a well-defined spherical core-shell structure, and the bilayer structure of BCVs was proved by previous studies by Wu’s group in Chinese University of Hong Kong else.14)
We tried to replace tetrahydrofuran (THF) with CHCl3 or CH2Cl2 to prepared BCVs (Table S1 and Fig. S1), respectively. The average size of BCVs prepared by THF was the smallest (120 ± 5 nm), and the biggest (194 ± 4 nm) with most narrow PDI by CHCl3. The size (158 ± 7 nm) was applicable by CH2Cl2. There was no significant difference in the zeta potential of BCVs. Taking lab safety into consideration, we would optimize the procedure of BCVs and would replace THF with CHCl3 or CH2Cl2.
Cellular UptakeBNCT has been applied to treat various cancers and most BNCT-related activities are focused on brain tumors, such as glioblastoma.3) The human glioblastoma cell line U87 MG (U87 cells) is one of the typical glioblastoma cell lines, and the U87 cells had been widely used in cellular uptake and so on.16,17) Accordingly, we selected the U87 cells as model to explore BCVs’ cellular uptake and endocytic mechanism.
In Wu’s group, “BCVs–rhodamine, labeling vesicles made of PEG2000-carborane–rhodamine conjugation (rhodamine bonded to PEG2000-carborane), were quickly taken up by the U87 cells and most of the cell was fully colored with red rhodamine.”14) This result indicated clearly the BCVs–rhodamine or/and PEG2000-carborane–rhodamine conjugation had penetrated the cell membrane into the cells.
For nanovesicles formed by self-assembly, structural integrity is particularly important for maintaining functionality. In order to elucidate whether BCVs were taken up as whole by cells or not dismantling during endocytosis, the uptake of BCVs-Dox was performed (Dox, having fluorescence properties, was physically encapsulated in the inner hydrophilic region of BCVs), and uptake of DiO-BCVs-DiI was also carried out (as a donor–acceptor FRET pair, physically co-encapsulated within the highly hydrophobic bilayer).
Uptake of BCVs-Dox under Confocal Laser Scanning Microscope (CLSM)To observe the uptake of BCVs, the endocytosis of BCVs-Dox was imaged in the U87 cells by CLSM. The results showed fluorescent intensity in the BCVs-Dox group was higher than that in free Dox group, indicating BCVs could remarkably improve the uptake of Dox (Fig. 2A), and BCVs should be taken up in the intact form during endocytosis.

(A) CLSM images of U87 cells incubated with free Dox or BCVs-Dox at 37°C for 1 h, Scale bar 100 µm. (B) The mean fluorescence intensity from intracellular Dox in U87 cells at 37°C for 1 h incubation (without MβCD) analyzed quantitatively by flow cytometer. BCVs-Dox represented Dox encapsulated physically in the inner core of BCVs, “BCVs/Dox mixed” meant simple mixture of BCVs and free Dox. (C) The mean fluorescence intensity from intracellular Dox in U87 cells at 37°C for 1 h incubation with MβCD analyzed quantitatively by flow cytometer. (D) FRET imaging of DiO-BCVs-DiI or BVCs-DiO + BCVs-DiI in U87 cells at 37°C for 4 h or 12 h incubation by CLSM (Ex/Em = 480 nm/550-650 nm). Scale bar 50 µm. (F) Histograms of quantitative analysis of (D). Data presented as mean ± S.D. (n = 6). ** p < 0.01, *** p < 0.001.
To further prove whether BCVs were taken up in the intact form during endocytosis or not, the fluorescence intensity from intracellular Dox in free Dox group, BCVs-Dox group and BCVs/Dox mixed group were determined by flow cytometry on U87 cells, respectively. The results showed there was the strongest fluorescence intensity in BCVs-Dox group (118 ± 6.3), the middle in BCVs/Dox mixed group (56.9 ± 3.9), and the weakest in free Dox group (36.0 ± 1.6), and there was highly significant difference in the fluorescence intensity between BCVs-Dox and free Dox group, and so was BCVs-Dox and BCVs/Dox mixed group. There was significant difference between BCVs/Dox mixed and free Dox group. It was drawn that BCVs-Dox were stable, and non-destroyed before endocytosis from the data of highly significant difference (p < 0.001) between BCVs-Dox and BCVs/Dox mixed groups. The above suggested that a lot of Dox penetrated membrane into cells with the help of BCVs carriers (encapsulating Dox physically), and this could prove BCVs were taken up as vesicle whole by cells (Fig. 2B).
Uptake of DiO-BCVs-DiI with FRETFRET was a forceful method to confirm the structural integrity of vesicles during endocytic process.18,19) If the donor (DiO, Ex/Em: 488/510 nm) and acceptor (DiI, Ex/Em: 540/575 nm) fluorophores are both incorporated into a vesicle, the higher FRET efficiency could prove the vesicle is taken up in the intact form during endocytosis. If the loss or lower of FRET efficiency, this shows disruption of the vesicle and spatial separation of the fluorophores.20)
Indicated as Fig. 2D, FRET efficiencies in DiO-BCVs-DiI group (co-encapsulated physically both DiO and DiI) were 0.66 ± 0.18 and 0.58 ± 0.19 in 4 h and 12 h incubation, respectively, and those in BCVs-DiO + BCVs-DiI group (mixed both BCVs-DiO and BCVs-DiI physically) were 0.25 ± 0.21 and 0.24 ± 0.18. There was highly significant difference between DiO-BCVs-DiI group and BCVs-DiO + BCVs-DiI group in 4 h incubation, and so was in 12 h incubation. The results indicated that BCVs were taken up in the intact form by cells during endocytosis, presumably because vesicular structures are strongly held together by strong hydrophobic interaction among carborane and each PEG chain is covalently linked to a carborane.14)
In general, surface modification of vesicles or nanoparticles with PEG could decrease the uptake. But it was reported also that PEGylated nanoparticles, formed only by amphiphilic mPEG-PLGA15) or only by PEG-polypeptide,21) did not reduce the uptake of Dox into cells. What was more, micelles of Dox in PEG-PE increased the internalization by A549 cells into lysosomes and enhanced cytotoxicity compared to free Dox, and drug-encapsulated doxorubicin was more effective in inhibiting tumor growth than free Dox,22) and the surfaces of the above three nanoparticles were all with high density of PEG modification.
Our data supported the uptake of BCVs-Dox was significantly higher than that of free Dox, this case should be similar to the micelles of Dox in PEG-PE by Liang’s group.22) The more absorption of BCVs-Dox was also strongly associated with rapid penetration through the cells and release drug rapidly in the cells.14) It was proved that in the presence of esterase (inside cells) in cells, BCVs-Dox were dismantled, released Dox much more (35% at 1 h incubation, 60% at 2 h incubation14)). The uptake of PEG-modified nanoparticles (Liposomal Dox with 4% PEG-coating) was less and slower than that of free-Dox,23) which might be due to the slow Dox release from liposomes.24,25)
Endocytic PathwayTo elucidate the endocytic pathway of BCVs, 6 kinds of pharmacological inhibitors were selected out,26,27) and the U87 cells were incubated in medium with 6 inhibitors, respectively. The endocytosis pathway was estimated according to the boron concentration in U87 cells.
The intracellular boron concentration was 736 ± 164 µg ·g−1 in BCVs group without inhibitors, which was 36 times higher than that in control group (20 ± 3 µg·g−1). The boron concentrations in endocytic inhibitor groups were 565 ± 327 µg·g−1 (cytochalasin D), 568 ± 40 µg·g−1 (5-(N-ethyl-N-isopropyl) amiloride (EIPA)), 630 ± 20 µg·g−1 (chlorpromazine), 965 ± 64 µg·g−1 (Filipin), 878 ± 133 µg·g−1 (Nystatin), and 130 ± 37 µg·g−1 (methyl-β-cyclodextrin (MβCD)), respectively (Table 1, Table S2 and Fig. 3). The results showed that MβCD could obviously inhibit the uptake of BCVs (p = 0.0033). There were no significant differences in boron concentration from cytochalasin D (actin polymerization inhibitor) group [or EIPA (macropinocytosis inhibitor), chlorpromazine (clathrin-dependent pathway inhibitor), Filipin (lipid raft/caveolae-dependent pathway inhibitor), Nystatin (lipid raft/caveolae-dependent pathway inhibitor) group] and BCVs group, but there was significant difference in boron concentration from MβCD (lipid raft/caveolae-dependent pathway inhibitor) and BCVs group.
| Inhibitors | Abbreviation of inhibitors | Concentration of inhibitions in the medium | Function |
|---|---|---|---|
| Cytochalasin D | CytD | 0.5 µM | Actin polymerization inhibitor |
| 5-(N-Ethyl-N-isopropyl)-amiloride | EIPA | 40 µM | Micropinocytosis inhibitor |
| Chlorpromazine | CPZ | 30 µM | Clathrin inhibitor |
| Filipin | Filipin | 0.5 µg·mL−1 | Lipid raft/caveolae inhibitor |
| Nystatin | Nystatin | 30 µM | Lipid raft/caveolae inhibitor |
| Methyl-β-cyclodextrin | MβCD | 10 mM | Lipid raft/caveolae inhibitor |
The final concentration of BCVs in the medium was 100 µg·mL−1, and the final boron concentration in the medium was 5 µg·mL−1 (molecule weight of PEG2000-carborane calculated by 2200, PEG2000-carborane has 10 boron atoms per molecular).

Intracellular boron concentration in U87 cells was determined by inductive coupled plasma emission spectrometer (ICP) after 4 h incubation at 37°C in the presence of 6 endocytic inhibitors, respectively. Data presented as mean ± S.D. (n = 3). ** p < 0.01.
The above data suggested that the endocytosis had nothing to do with micropinocytosis (actin polymerization or/and Na+/H+ exchange), or clathrin-dependent pathway. The endocytosis of BCVs was inhibited by 82% [(736−130)/736] in the presence of MβCD, it showed BCVs were internalized by tumor cells with cholesterol-dependent pathway.
Cholesterol is essential for lipid raft/caveolae-dependent endocytosis and Lipid raft/caveolae-dependent endocytosis is sensitive to cholesterol depletion.28–31) Our following information and data could illustrate clearly that cholesterol is involved in uptake of BCVs. MβCD, a lipid raft/caveolae inhibitor, extracts cholesterol from cell membranes by binding the cholesterol within its nonpolar cyclodextrin rings and forming soluble complexes, which could inhibit the cholesterol-dependent endocytosis,32–34) and filipin and nystatin, the other two lipid rafts/caveolae inhibitors, disrupt rafts by directly inserting into the membrane to bind cholesterol and sequester cholesterol into complexes in cell membranes.35–37) Our data indicated that the uptake of BCVs was inhibited by 82% in the presence of MβCD, and filipin and nystatin both could not inhibit the uptake of BCVs. It showed uptake of BCVs was strongly related to cholesterol in membrane. Similar to this situation, nanoparticle was also reported it entered the cells by lipid raft mediated, such as PEG3000–PLA40000 on caco-2 cells.38) In other words, it meant the presence of cholesterol was indispensable for the endocytosis of BCVs. More importantly, as many studies had clarified the significant effects of cholesterol on membrane fluidity and flexibility, our result directly revealed that the cellular uptake of BCVs depended on the local deformation of cholesterol-rich membrane domains. Nevertheless, filipin and nystatin, both of which disturbed the interactions of cholesterol with other membrane proteins or lipids, showed the unavailability during the endocytosis of BCVs. It was suggested that the signal regulatory role of cholesterol might be dispensable here.
The cholesterol-dependent pathway was confirmed again by the uptake of BCVs-Dox in the presence of MβCD. The amount of intracellular Dox in BCVs-Dox group was 3-fold and over 2-fold higher than that in free Dox group and BCVs/Dox mixed group, respectively (Fig. 2B), while the amount of intracellular Dox was at the same level blocked by MβCD (Fig. 2C), it suggested that the endocytic pathway of BCVs in U87 cells would be cholesterol-dependent pathway.
BCVs’ Influence on Cell Membrane during EndocytosisOn Fluidity and Recovery of Cell Membrane by Fluorescence Recovery after Photobleaching (FRAP)FRAP was applied to explore whether BCVs’ influence on fluidity and recovery of cell membrane or not during endocytosis. Herein, cell membranes were exposed to medium with BCVs, and labelled by DiI, then bleached by laser. Finally, a series of fluorescence intensity pictures in the same bleached area were observed and recorded at different time points (0, 10, 20, 30, 40, 50, 60, 70 and 80 s) to evaluate the fluidity and recovery of cell membrane. The results indicated that there was no significant difference in recovery rate and extent of fluorescence intensity from BCVs group to control group (Figs. 4A and 4B, marked by white ellipses), suggesting BCVs did not influence on the fluidity and recovery of cell membrane.

(A) U87 cells were stained by 4 µg·mL−1 DiI and an intensive laser pulse was locally applied to the micron region of the labeled membranes, and its fluorescence recovery pattern was observed at consecutive time points (0, 10, 20, 30, 40, 50, 60, 70 and 80 s) by CLSM. White ellipses represented the laser bleaching periods. (B) Quantitative analysis of fluorescence recovery percentage normalized to fluorescent signal before photobleaching. (C) Micro-viscosity of cell membrane evaluated by DPH probe on U87 cells and J774A.1 cells. U87 cells and J774A.1 cells were incubated with 100 µg·mL−1 BCVs for 4 h at 37°C, dyed by 0.1 µM·L−1 DPH solution and detected by multifunctional microplate spectrophotometer (Ex/Em = 360/432 nm). (D) The integrity of cell membrane evaluated by LDH on U87 cells and J774A.1 cells. U87 cells and J774A.1 cells were incubated with 100 µg·mL−1 BCVs for 24 h at 37°C, stained by Lactate dehydrogenase (LDH) assay kit and detected at 490 nm by multifunctional microplate spectrophotometer. Data shown as mean ± S.D. (n = 6).
To explore furtherly BCVs’ influence on cellular membrane fluidity, micro-viscosities of the cell membranes were calculated by measuring the fluorescence polarizer of fluorescent probe DPH in U87 cells and J774A.1 cells, respectively.
The cell membrane micro-viscosities were 3.34 ± 1.03 in BCVs group and 3.24 ± 1.01 in control on U87 cells, and were 3.54 ± 0.86 in BCVs group and 3.12 ± 0.66 in control on J774A.1 cells. The results indicated that there was no significant difference in micro-viscosity of cell membrane from BCVs group to control group in U87 cells, and the same for J774A.1 cells (Fig. 4C), suggesting BCVs did not influence on micro-viscosity of the cell membranes.
On Integrity of Cell Membrane by Lactate Dehydrogenase (LDH)LDH presented in the cytoplasm of cells and leaked out in culture medium when cell membrane integrity was disrupted.39) The integrity of cell membranes was also investigated by detecting the leakage of LDH on U87 cells and J774A.1 cells, respectively.
The integrity percentages of cell membrane were 1.02 ± 0.08% in BCVs group and 1.04 ± 0.04% in control on U87 cells, and were 0.97 ± 0.02% in BCVs group and 1.03 ± 0.04% in control on J774A.1 cells. The results indicated that there was no significant difference in integrity percentages of cell membrane from BCVs group to control group on U87 cells, and the same for J774A.1 cells (Fig. 4D), suggesting BCVs did not influenced on the integrity of cell membranes.
Cytotoxicity EvaluationThe cytotoxicity experiments, including apoptosis, cell cycle and morphology, were performed using the human umbilical vein endothelial cells (HUVECs), Human glioblastoma cell line U87 MG (U87 cells) and murine macrophage cell line J774A.1 (J774A.1 cells). The HUVECs were especially used for nanotoxicological and/or nanomedicine studies. Nanodrug will inevitably come into blood and contact with blood endothelial cells after intravenous injection. So, the HUVECs are one of the most popular cell models in cytotoxicity test.40–43) Moreover, the U87 cells is one of the typical and common glioblastoma cell lines, and it was used as model cell in section of “Live Imaging” in vivo, and here, the U87 cells were served as model cell in the cytotoxicity evaluation. It is well known that macrophages are the most important type of sentinel cells, as the first defense line against invading pathogens and nanoparticles. Macrophages, with high endocytic ability, are very sensitive cells in the blood and could respond rapidly to nanoparticle toxicity. The J774A.1 cells was reported as cell model to evaluate the cytotoxicity of nanoparticle.44–46)
Accordingly, we chose the HUVECs, U87 cells, and J774A.1 cells as models to evaluate BCVs’ cytotoxicity by the data of apoptosis, cell cycle arrest and morphology.
ApoptosisThe results showed that the percentages of HUVECs was 2.76 ± 0.32 and 6.54 ± 0.74% in early and late apoptosis in BCVs group, 2.76 ± 0.49 and 7.44 ± 1.55% in control, there was no significant difference in the percentage of early or late apoptotic cells between the BCVs and control groups in HUVECs model, respectively (Figs. 5A, 5B, 5C). Similarly, that of U87 cells was 4.17 ± 0.30 and 5.77 ± 1.53% in early and late apoptosis in BCVs group, 4.88 ± 0.40 and 7.39 ± 0.59% in control, there was no significant difference, respectively (Figs. 5D, 5E, 5F). The percentage of J774A.1 cells was below 0.5% in early apoptosis in BCVs group and as the same in control group. The percentages were almost equal both in late apoptosis and in BCVs group (13.54 ± 2.3%) and control group (13.38 ± 3.1%) (Figs. 5G, 5H, 5I). And there was no significant difference in early or late apoptosis between the BCVs and control groups, respectively. It suggested that BCVs did not promote apoptosis in the three cell models.

The HUVECs (A), U87 cells (D) and J774A.1 cells (G) were treated at 37°C for 24 h using Annexin V-FITC/PI staining by flow cytometric analysis. X and Y axes are related to Annexin V and PI, consecutively. (B/C), (E/F) and (H/I) histograms indicating quantitative analysis of early/late apoptotic HUVECs, U87 cells and J774A.1 cells, respectively. Cell cycle distributions were detected by flow cytometry in HUVECs (J), U87 cells (K) and J774A.1 cells (L), respectively. Data presented as mean ± S.D. (n = 6). The BCVs concentrations in all groups were kept at 100 µg·mL−1.
The percentages of HUVEs at G0-G1, S and G2-M phase were 59.2 ± 1.2, 30.6 ± 1.7, 10.3 ± 0.9% in BCVs group and 58.6 ± 1.3, 31.0 ± 1.5, 10.4 ± 1.1% in control, respectively, there was no significant difference at the three phase cells between the BCVs group and the control in HUVECs model (Fig. 5J). In the same way, that of U87 cells were 48.6 ± 1.2, 37.9 ± 1.3, 13.5 ± 0.2% in BCVs group and 46.3 ± 0.1, 39.3 ± 0.8, 14.4 ± 0.9% in control, respectively, there was no significant difference (Fig. 5K). That of J774A.1 cells were 75.1 ± 1.7, 17.3 ± 2.0 and 8.1 ± 1.5% in BCVs group, and 73.1 ± 2.5, 17.2 ± 1.7 and 8.9 ± 2.4% in control, respectively. Here was no significant difference (Fig. 5L).
The results suggested BCVs did not induce cell cycle arrest in the three models of HUVECs, U87 cells and J774A.1 cells.
MorphologyThe diameters of J774A.1 cells were 14.5 ± 0.8, 14.2 ± 0.3, 13.7 ± 0.5 and 14.1 ± 0.3 µm in control, 1 µg·mL−1 BCVs, 10 µg·mL−1 BCVs and 100 µg·mL−1 BCVs groups, respectively. No difference was observed in integrity of cell membrane (Figs. 6a and 6c), nuclear membrane (Figs. 6a and 6c) and mitochondria (Figs. 6b and 6d) from BCVs (100 µg·mL−1) group and control group under TEM.

Scale bar 5 µm (a, c) and 2 µm (b, d).
Totally, the data indicated BCVs had no influence on HUVECs, U87 cells or J774A.1 cells in apoptosis, cell cycle (G0-1, S and G2-M) and morphology (diameter, cell membrane, nuclear membrane and mitochondria). It suggested that BCVs had nearly no cytotoxicity.
Live ImagingBCVs’ tumor accumulating ability had been investigated on the orthotopic glioma model with in vivo imaging system. The fluorescence signals of BCVs-DiR in tumors were detected at 1, 2, 4, 8, 12 and 24 h after injection. The signals in tumors gradually increased with time expansion from 1 h to 8 h, and then gradually decreased from 8 h to 24 h (Fig. 7). The results suggested BCVs could accumulate there at least 24 h, which was feasible for BNCT, and the optimal time window for treatment might be from 2 to 12 h after single injection.
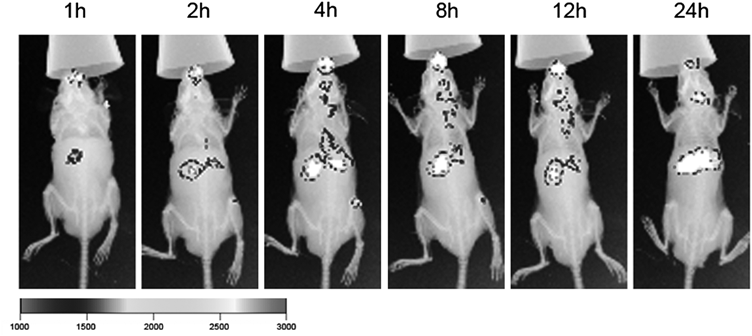
BCVs-DiR (8 mg·kg−1) were intravenously injected through the tail vein into mice bearing orthotopic tumors and their distribution were imaged with in vivo imaging system at predetermined time intervals (1, 2, 4, 8, 12 and 24 h).
Though the fluorescent signals were visible in liver from 1 h to 24 h, in view of no cytotoxicity of BCVs, there was hardly any additional risk on the body for BNCT irradiated locally in brain tumor treatments.
BCVs-DiR exhibited accumulating in tumor tissues in mice head, which was observed on the orthotopic glioma model with fluorescence signals by in vivo imaging system. The signals of BCVs-DiR in tumors were detected after injection, gradually increasing from 1 to 8 h, and then gradually decreasing from 8 to 24 h, and remain at least 24 h, like targeting tumor tissues passively. It was highly possible that apolipoproteins (ApoE and/or ApoA) played an important role in the process of BCVs through the brain–blood barrier (BBB).47) ApoE and/or ApoA could absorb onto the surface of PEGylated nanoparticles (BCVs-DiR) after administration.48–50) The BCVs-DiR apolipoproteins-modified could interact with these apolipoprotein receptors on the BBB, and then cross the BBB, accumulate in the brain tumor site. So, the fluorescence signals of BCVs-DiR around the head in mice was visible stronger after injection. It showed BCVs-DiR could enrich tumor tissues through BBB.
To have more comprehensive understanding of boron-containing vesicles (BCVs), we conducted the studies on the endocytic mechanism and cytotoxicity assessment, including the uptake, influence on cell membrane during endocytosis, Endocytosis mechanism investigations, apoptosis, cell cycle and morphological observation. The results showed that BCVs were taken up in the intact form with cholesterol-dependent pathway during endocytosis, and BCVs showed nearly no cytotoxicity. BCVs could accumulate within tumors for at least 24 h. These further investigations on BCVs would provide reference information and guidance for BCVs’ multifunctional application serving as a boron delivery agent for BNCT, a hydrophilic and/or hydrophobic drug carrier and a diagnostic imaging fluorescent probe.
PEG2000-carborane (10 mg, 10 boron atoms per molecular) was dissolved in tetrahydrofuran (THF) in a round-bottom flask, the solvent was removed by rotary evaporation to obtain PEG2000-carborane-containing film. The film hydrated with 10 mL deionized water to prepare BCVs, and afford the BCVs suspension, the concentration of PEG2000-carborane in the BCVs suspension was 1 mg·mL−1, and boron concentration in this suspension was 50 µg·mL−1 (molecule weight of PEG2000-carborane calculated by 2200).
The prepared PEG2000-carborane-containing film was hydrated by Dox·HCl solution to obtain Dox-loading BCVs (BCVs-Dox) solution, and it was dialyzed to remove unloaded Dox. The final Dox concentration in BCVs-Dox solution was 50 µg·mL−1.
PEG2000-carborane (10 mg) and lipophilic fluorescent dye DiO [or DiI, or DiR, or DiO + DiI (50 µg + 50 µg), 100 µg] were dissolved in THF (10 mL), and the solvent was removed by rotary evaporation to obtain PEG2000-carborane-containing film with DiO [or DiI, DiR, DiO + DiI]. The film was hydrated with deionized water (10 mL) to prepare fluorescent labeling BVCs-DiO (or BCVs-DiI, BCVs-DiR, DiO-BCVs-DiI). The fluorescent dye DiO concentration in the BVCs-DiO suspension was 10 µg·mL−1, DiI concentration in the BVCs-DiI suspension was 10 µg·mL−1, DiR concentration in the BVCs-DiR suspension was 10 µg·mL−1, DiO and DiI in the DiO-BCVs-DiI suspension was 5 µg·mL−1, respectively.
Measurements of Size and Zeta PotentialThe BCVs were characterized in size (diameter, nm) with polydispersity index by DLS and surface charge (zeta potential, mV) by Malvern Zetasizer (Nano-ZS, Malvern, U.K.). The all of determinations were operated at 25°C under appropriate concentration of sample diluted in distilled water.
Morphological CharacterizationThe morphology of BCVs was confirmed using transmission electron microscopy (cryo-TEM and TEM).
Preparation of cryo-TEM sample: 3 µL BCVs suspension was placed on the surface of a glow-discharged holey carbon Cu grids (Quantifoil, R1.2/1.3, 300 mesh) with an automatic plunge device (Leica EM GP). they were incubated for 30 s at 75% humidity and 10°C, the grid was blotted with a filter paper, after 8 s again, flash plunged in cooled liquid ethane (−175°C). The cryo-TEM sample was imaged under Talos L120C electron microscopes with a Ceta camera (Thermo Fisher Scientific, U.S.A.) at 120 kV. This test was completed in the Institute of Biophysics, Chinese Academy of Sciences. TEM sample preparation: 5 µL BCVs suspension was added onto a carbon-coated copper grid (200 mesh), it was blotted with filter paper after being kept for one minute in order to bind the BCVs to the carbon membrane, stained for 1 min by 5 µL 1% uranium acetate, washed twice with 1% uranium acetate, dried naturally in air at room temperature. TEM imaging was obtained under JEM-1400 plus microscope (Tokyo, Japan) at 100 kV. This experiment was finished in the Peking University Health Science Center.
UptakeObservation under CLSMThe U87 cells were seeded onto glass-bottomed dishes and incubated overnight at 37°C for attachment. The medium was replaced by 2 mL fresh one containing free 200 µL Dox (50 µg·mL−1) or 200 µL BCVs-Dox (Dox concentration 50 µg·mL−1), the cells were incubated for 1 h at 37°C, and then washed with phosphate buffered saline (PBS), harvested by trypsinization, observed the uptake under confocal laser microscopy (TCS SP8, Leica, Germany).
Analysis with Flow CytometerThe U87 cells were seeded onto 6-well plates at a density of 4 × 105 cells per well and incubated overnight at 37°C for attachment. The medium was replaced by 2 mL fresh one containing free Dox [or BCVs-Dox, or BCVs/Dox mixed (BCVs mixing with free Dox), or free Dox + MβCD, or BCVs-Dox + MβCD, or BCVs/Dox mixed + MβCD, 200 µL]. The Dox concentrations in the fresh medium in the 6 groups were 5 µg·mL−1, respectively (the Dox concentration was 50 µg·mL−1 in the original solution of free Dox, BCVs-Dox, BCVs/Dox mixed, free Dox + MβCD, BCVs-Dox + MβCD, and BCVs/Dox mixed + MβCD, respectively). And then the cells were incubated for 1 h at 37°C, washed with PBS and harvested by trypsinization. The mean fluorescence intensity of intercellular Dox (Ex/Em: 488/590 nm) was measured with flow cytometer (Becton Dickinson FACS Calibur, U.S.A.).
Uptake of DiO-BCVs-DiI with FRETThe U87 cells were seeded onto a glass bottom culture dish at a density of 4 × 105 cells per dish and incubated overnight at 37°C for attachment. The medium was replaced by 2 mL fresh one containing 200 µL fluorescent labeling BVCs-DiO (or BCVs-DiI, or DiO-BCVs-DiI, or BVCs-DiO + BCVs-DiI). After 4 h or 12 h incubation at 37°C, this medium was replaced by fresh one free from any fluorescent probes. FRET images were observed under a confocal laser scanning microscope (CLSM, TCS SP8, Leica, Germany).
Endocytosis MechanismThe endocytosis pathway was investigated via a pharmacological inhibition strategy. The U87 cells were seeded onto 6-well plates at a density of 4 × 105 cells per well and incubated overnight at 37°C for attachment. The medium (2 mL per well) with endocytic inhibitors (listed in Table 1) was pre-incubated with cells at 37°C for 40 min, then the medium was replaced by fresh one containing 200 µL BCVs solution (1 mg·mL−1) and inhibiters (listed in Table 1), the cells were cultured at 37°C for 4 h and then washed with cold PBS 3 times, trypsinized, collected, divided into 2 parts equally [one part were lysed with RIPA buffer for determining total intracellular protein by BCA method, the other part were used for determining intracellular boron concentration with inductive coupled plasma emission spectrometer (ICP)]. Cells treated only with medium were as control.
Intercellular boron concentration was calculated according to the following formula: Intracellular boron concentration (µg·g−1) = Intracellular boron concentration (µg·g−1) = weight of boron in each well (µg)/ weight of total protein in each well (g).
BCVs’ Influence on Cell Membrane during EndocytosisFluorescence Recovery after Photobleaching (FRAP)The U87 cells were seeded onto glass-bottomed dishes at a density of 4 × 105 cells/well and overnight at 37°C for attachment, cultured again for 4 h at 37°C after the medium was replaced by 2 mL fresh one containing BCVs (200 µL, 1 mg·mL−1), and then DiI was added into the medium to dye the cell membrane (final DiI concentration 4 µg·mL−1 in medium) for 20 min at 37°C. The cells were washed with PBS before FRAP observation (Ex/Em: 540/565–650 nm). Briefly, an intensive laser pulse was locally applied to the micron region of the labeled membranes, and its fluorescence recovery pattern (till reached a plateau) reflected the fluidity of cell membrane during endocytosis. Cells incubated only with medium were set as the control.
Fluorescence Anisotropy of Diphenylhexatriene (DPH)DPH is the most commonly applied rotational probe for estimating micro-viscosity and fluidity of lipid membranes.51,52) The U87 cells and J774A.1 cells were seeded onto 96-well plates at a density of 1 × 104 cells per well and incubated overnight at 37°C for attachment, respectively. The medium was replaced by 200 µL fresh one containing BCVs (20 µL, 1 mg·mL−1), and cells were cultured at 37°C for 4 h, then 1 µL DPH stock solution in DMF was added into the medium (0.1 µM·L−1) for another 1 h incubation in the dark. Samples were excited at 360 nm and the fluorescence intensity was monitored at 432 nm by multifunctional microplate spectrophotometer (Synergy4, Biotek). Cells incubated only with medium were set as the control. Then micro-viscosity of cell membrane (η) was calculated by the following formula according to the fluorescence polarizer (P):
 |
The integrity of cell membrane was accurately quantified by detecting LDH which was rapidly released into the cell culture medium when cell membrane was damaged.53) The U87 cells and J774A.1 cells were seeded onto 96-well plates at a density of 1 × 104 cells per well and incubated overnight at 37°C for attachment, respectively. The medium was replaced by 200 µL fresh one containing BCVs (1 mg·mL−1, 20 µL), and continue to incubate for 24 h at 37°C. Supernatant was aspirated into another 96-well plate, added dye solutions, incubated for 30 min at dark, terminated, and detected at 490 nm by multifunctional microplate spectrophotometer (Synergy4, Biotek). Cells incubated only with medium were set as the negative control.
CytotoxicityThe HUVECs (or U87 cells, or J774A.1 cells) were seeded onto a 6-well plate at a density of 4 × 105 cells per well and cultured overnight at 37°C for attachment. Then, the medium was replaced with 2 mL fresh medium containing BCVs (200 µL, 1 mg·mL−1). After 24 h incubation, cells were washed with PBS, detached by trypsinization, collected, centrifuged (discard the supernatant, 1000 × g for 5 min at 4°C) to obtain centrifuged cells. Cells incubated only with medium were set as the control.
ApoptosisThe above centrifuged cells were resuspended with the binding buffer, and then stained with Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) for 15 min in the dark in order, respectively. The cells were analyzed immediately by FACScan flow cytometer (Becton Dickinson FACS Calibur, U.S.A.).
Cell CycleThe above centrifuged cells were added 0.5 mL 70% cold ethanol overnight, added 1 mL PBS, centrifuged again at 2000 rpm at 4°C for 5 min (discarded supernatant), re-suspended, added 100 µL RNAase, maintaining at 37°C for 30 min, passed through 300 screen, then added 400 µL PI (staining), placed at 4°C for 30 min, analyzed by flow cytometry immediately.
Morphological ObservationThe centrifuged J774A.1 cells were resuspended with PBS, the diameter was measured with forward scattered light and lateral scattered light by flow cytometer (Beckman Coulter Reagents, U.S.A.). The above centrifuged cells were fixed with 2.5% glutaraldehyde overnight at 4°C, dehydrated, sliced, observed by transmission electron microscopy (JEM-1200EX, JEOL).
Orthotopic Tumor ModelThe orthotopic tumor model had been established using Nu/Nu mice.54) In detail, mice were anesthetized using 4% chloral hydrate (10 µL·g−1) and the heads of mice were positioned in a stereotactic instrument. A linear skin incision was made starting in the midline between the eyes and ending in the midline dorsal to the bregma. For intracerebral tumor cell injection, a small hole was drilled with a 0.5 mm rose-head burr 1 mm anterior to the bregma and 3 mm lateral to the midline. Three microliters of the U87 cells suspension (105·cells·µL−1) was injected. Finally, the burr hole was sealed with bone wax and the scalp was closed with a tissue adhesive.
BCVs-DiR were intravenously injected through the tail vein into mice bearing orthotopic tumors at a dose of 8 mg·kg−1 (1 mg·mL−1, 160 µL). Mice were anaesthetized with isoflurane and the distribution of BCVs-DiR were imaged with in vivo imaging system (Carestream Molecular Imaging, New Haven, U.S.A.) at predetermined time intervals (1, 2, 4, 8, 12 and 24 h).
Statistical AnalysisData were presented as mean ± standard deviation (S.D.). The t-test analysis was performed in statistical evaluation. A p-value below 0.05 was supposed to be statistically significant and a p-value below 0.01 was supposed to be highly significant.
MaterialsPEG2000-carborane was gifted by Wu’s group in Chinese University of Hong Kong. Doxorubicin hydrochloride (Dox·HCl) was obtained from Hisun Pharmaceutical (Zhejiang, China). LDH assay kit was bought from Beyotime Biotechnology (Shanghai, China). Bicinchoninic acid (BCA) protein assay kit was purchased from Applygen Company (Beijing, China). Cell cycle detecting kit and Annexin V-FITC/PI apoptosis kit were bought from Macgene Biotech Co., Ltd. (Beijing, China). 6-Diphenyl-1,3,5-hexatriene (DPH), Cytochalasin D (CytD), Chlorpromazine (CPZ), filipin, nystatin, 5-(N-ethyl-N-isopropyl) amirolide (EIPA) and methyl-β-cyclodextrin (MβCD) were all purchased from Sigma-Aldrich (Shanghai, China). Lipophilic fluorescent dyes 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI), 3,3′-dioctadecyloxacarbocyanine perchlorate (DiO), 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine Iodide (DiR) were bought from Biotium, Inc. (Hayward, U.S.A.). Isoflurane was bought from Keyuan Pharma Co., Ltd. (Shandong, China).
Cells and AnimalsHuman umbilical vein endothelial cells (HUVECs), human glioblastoma cell line U87 MG (U87 cells), and murine macrophage cell line J774A.1 (J774A.1 cells) were bought from Basic Medical Science, Chinese Academy of Medical Sciences (Beijing, China). The HUVECs were cultured in Ham’s F 12 nutrient medium with fetal calf serum (10%, v/v) and endothelial cell growth supplement plus heparin (ECGS 35 µg·mL−1, heparin 78 µg·mL−1). The U87 cells were cultured in minimum Eagle’s medium (MEM) with fetal calf serum (10%, v/v), nonessential amino acid (1%, v/v) and penicillin (100 units·mL−1)-streptomycin (100 µg·mL−1) solution. The J774A.1 cells were cultured in high-glucose Dulbecco’s modified Eagles medium (DMEM) with fetal calf serum (10%, v/v) and penicillin (100 units·mL−1)-streptomycin (100 µg·mL−1) solution. The 3 kinds of cells were cultured at 37°C in humidified atmosphere with 5% CO2.
The Nu/Nu mice (female, 6 weeks old, body weight 18 to 22 g) were purchased from Laboratory Animal Center of Peking University (Beijing, China). All care and handling of animals were performed with the approval of Institutional Authority for Laboratory Animal Care.
This work was financially supported by the National Key Research and Development Program of China (2016YFA0201504), NSFC (81673383, 81603063, 81803479) and CIFMS (2016-I2-M-3-013). We felt grateful to Chi Wu’s group in the Chinese University of Hong Kong for giving PEG2000-carborane as a gift.
All authors developed aspects of the protocols. All authors contributed, read and approved the final manuscript.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.