2020 年 68 巻 8 号 p. 797-801
2020 年 68 巻 8 号 p. 797-801
The side effects of kwao keur dietary supplements (obtained from the tuberous root of Pueraria mirifica) have recently been reported by the Ministry of Health, Labour and Welfare, Japan. To control the quality of kwao keur products, its ingredients need to be maintained by characteristic marker compounds, such as miroestrol, deoxymiroestrol, and kwakhurin (KWA). In this study, we described the facile synthesis of KWA, a marker compound of P. mirifica. Our revised synthetic method produced KWA with shorter steps and higher yield than the reported method. Furthermore, the absolute purity of KWA was determined by quantitative NMR analysis for standardization as a reagent, and its purity was 92.62 ± 0.12%.
Kwao keur, which is obtained from the tuberous roots of Pueraria mirifica (Pueraria candollei var. mirifica), has been used in Thai traditional medicine as a hormonal dietary supplement for rejuvenation and for the treatment of menopausal symptoms by the action of phytoestrogen components such as miroestrol, deoxymiroestrol, kwakhurin (KWA), genistein, daidzein, and puerarin.1) Especially, miroestrol, deoxymiroestrol, and KWA are the characteristic components of P. mirifica.1,2) Miroestrol isolated from P. mirifica has been shown to be an estrogenically active compound3) with very strong estrogenic activity (i.e., approximately 1000-fold stronger than so-called estrogenic isoflavones such as genistein and daidzein).1,2) Subsequently, Ishikawa and colleagues isolated deoxymiroestrol as an alternative and more active compound (approximately 10-fold stronger) than miroestrol and suggested that miroestrol is an artifact because of the easy chemical conversion of deoxymiroestrol to miroestrol.1) KWA was also isolated as a characteristic isoflavone from P. mirifica with a moderate estrogenic activity comparable to that of daidzein.2)
Recently, the side effects of kwao keur (P. mirifica) dietary supplements have been reported by the Ministry of Health, Labour and Welfare, Japan.4) Therefore, it is important to control the quality of kwao keur dietary supplements. To maintain the quality of the kwao keur products, a control over the amounts of ingredients is required, possibly by the use of the characteristic marker compounds such as miroestrol, deoxymiroestrol, and KWA. Ishikawa and colleagues reported a quantitative analysis focusing on the more stable miroestrol and a characteristic isoflavone KWA as a marker compound for the standardization of P. mirifica.5) Thus, miroestrol and KWA might be used as external standards for the quantitative analysis of P. mirifica. However, they are minor components of P. mirifica (isolation yields < 0.001%).2,5) Therefore, only few commercial reagents of miroestrol and KWA are available as it is difficult to be isolated or chemically synthesized them. Considering that these compounds are synthesized and provided for use as commercially available standards, miroestrol tends to be difficult for the synthetic target molecule because of its complicated structure compared with KWA. Although the total synthesis of miroestrol was previously reported by Corey and Wu, the convergent route involving two components was performed in multiple steps, requiring manipulation for stereoselective reaction and separation of structural isomers.6) As for the chemical synthesis of KWA, one method has been reported by Ishikawa and colleagues,7) and it tended to be reproducible and admissible to synthesize KWA with higher yield. Therefore, we tried to develop a more facile and efficient synthetic route for KWA.
The purity of the commercial reagent used as a reference standard is crucial for the quantification of active components in dietary supplements, including natural products. Although the purity of the commercial reagent is usually determined by HPLC, quantitative NMR (qNMR) has recently emerged as a new absolute quantification method for low-molecular compounds, including pure natural compounds.8,9) We have previously reported the absolute purity of commercial reagents such as glabridin, a component of licorice, for the quantification of functional substances determined by qNMR.10)
In this study, we first described the facile synthesis of KWA with a shorter synthetic route and higher yield than those of the reported method.7) Second, we determined the absolute purity of the synthesized KWA by qNMR analysis to provide a practical approach for its use as a reagent.
Chart 1 shows the synthetic route of KWA reported by Ishikawa and colleagues.7) The isoflavone skeleton of 4 was constructed via the Suzuki–Miyaura coupling reaction using boronic acid 2 prepared from compound 1 and bromide 3. Then, the formyl group of 4 was changed to the hydroxy group by Baeyer–Villiger oxidation using 3-chloroperbenzoic acid (mCPBA) followed by alkaline hydrolysis to afford compound 5.

The Baeyer–Villiger oxidation of compound 4 was attempted according to the reported method.7) Some over-oxidized products were observed in addition to the target product 4′, even when examining certain conditions (temperature, time, equivalent of mCPBA), especially in the case of a large-scale synthesis. Each over-oxidized product exhibited 16-Da mass increase over the target product 4′, suggesting that the isoflavone moiety of 4′ was further oxidized (Fig. 1).

Therefore, we reconstructed a synthetic route acceptable for large-scale synthesis, as shown in Chart 2. We planned to construct an isoflavone skeleton of 5 with the hydroxy group via the Suzuki–Miyaura coupling reaction using boronic acid 8 and bromide 7. The compound 7 was easily synthesized from compound 1 without any by-product formation, and boronic acid 8 was prepared from compound 3. The desired compound 5 was obtained via Suzuki–Miyaura coupling from boronic acid 8 and bromide 7 in 2 steps at an yield of 64%. Furthermore, Pd-catalyzed alkylation of the phenolic hydroxy group on compound 5 with tert-butyl (2-methylbut-3-en-2-yl) carbonate11) was directly converted to the desired 1,1-dimethylallyl ether 6 in high yield.12,13) Thus, our revised synthetic method enabled intermediate 6 to be obtained with shorter steps and higher yield (3 steps, 57% yield from 3) than the reported method (5 steps, 33% yield from 3). Finally, KWA in approx. 1.0 g was obtained from 6 according to the reported protocols. Based on the NMR and LC–high-resolution mass spectroscopy (HRMS) data, the synthesized KWA was identified by directly comparing the its data with those of the isolated authentic KWA.

First, the quantitation signals of KWA in 1H-qNMR using 1,4-BTMSB-d4 as an internal standard were examined. KWA had no interference signal when the signal of position 2 in isoflavone (around 7.56 ppm) was used as a signal to be integrated (Fig. 2). This signal was used as a quantitation signal to calculate absolute purity. As a result, the purity of KWA was 92.62 ± 0.12%.
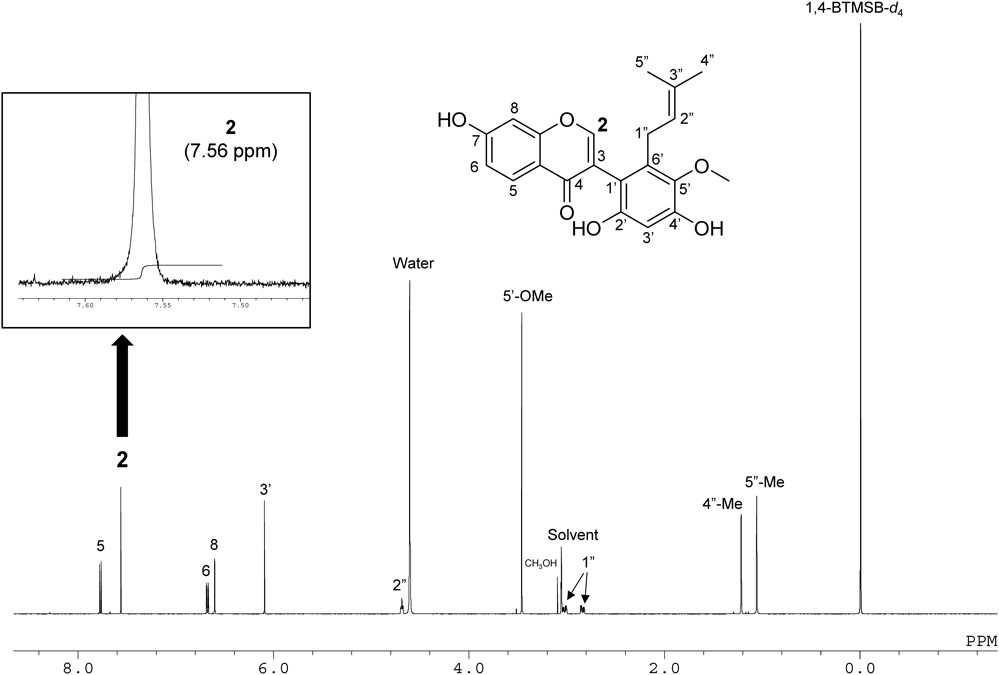
In this report, we described the facile synthesis of KWA as a candidate marker compound in P. mirifica. Our revised synthetic method provides KWA with shorter steps and higher yield (3 steps, 57% yield from 3) than the reported method (5 steps, 33% yield from 3). Furthermore, the absolute purity of KWA determined by qNMR, and its purity was 92.62 ± 0.12%. The quantitative analysis of P. mirifica using the synthesized KWA as an external standard is proceeding.
We recorded 1H- and 13C-NMR spectra using a JEOL ECZ600 (600 MHz) spectrometer. Chemical shifts were reported in delta (δ) units, part per million (ppm) relative to the center of solvent peaks as an internal reference (CDCl3: 7.26 for 1H-NMR, 77.0 for 13C-NMR; CD3OD: 3.30 for 1H-NMR, 49.0 for 13C-NMR; Acetone-d6: 2.04 for 1H-NMR, 29.8 for 13C-NMR). HR-MS were obtained using a Thermo Scientific UltiMate 3000 UHPLC-Q Exactive MS using the electrospray ionization (ESI) method (conditions: column, ACQUITY UPLC HSS T3 (2.1 × 100 mm, 1.7 µm; Waters); mobile phase, A = 0.1% formic acid in H2O, B = 0.1% formic acid in CH3CN; gradient elution, 5–95%B over 5 min; flow rate, 0.4 mL/min). Column chromatography was performed using medium pressure chromatography (Smart Flash, YAMAZEN) equipped with a Hi-Flash column and an Inject column (40 µm). All experiments were performed under anhydrous conditions in an argon atmosphere, unless otherwise mentioned.
5-Bromo-2,4-bis[(2-methoxyethoxy)methoxy]phenol (7)To a solution of 1 (3.2 g, 8.14 mmol) in CH2Cl2 (40 mL), mCPBA was added (approximately 35% water, 2.6 g, 9.77 mmol), and the reaction mixture was stirred at room temperature for 16 h in the presence of Ar gas. The reaction mixture was quenched with saturated aqueous NaHCO3, and then extracted using CH2Cl2 (20 mL × 2). The combined organic layers were dried over Na2SO4, filtered, and concentrated under reduced pressure. The obtained crude product was dissolved in CH2Cl2 (40 mL), and 10% aqueous KOH/MeOH (10 mL) was added. After stirring at room temperature for 2 h, the reaction mixture was diluted with EtOAc (100 mL), quenched with 10% HCl, and then extracted with EtOAc (30 mL × 2). The combined organic extracts were washed with brine, dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (Hexane/EtOAc = 30 : 70) to give compound 7 as a colorless oil (2.9 g, 93%).
1H-NMR (600 MHz, CDCl3) δ: 7.13 (s, 1H), 6.97 (s, 1H), 6.47 (s, 1H), 5.22 (s, 2H), 5.21 (s, 2H), 3.90–3.87 (m, 4H), 3.60 (td, J = 4.8, 1.8 Hz, 2H), 3.58 (td, J = 4.8, 1.8 Hz, 2H), 3.41 (s, 3H), 3.38 (s, 3H).
13C-NMR (151 MHz, CDCl3) δ: 147.0, 144.4, 143.3, 119.6, 108.1, 106.7, 96.7, 95.3, 71.5, 71.5, 69.1, 67.9, 59.0 (1C overlapped).
HRMS (ESI) m/z Calcd for C14H25BrNO7+ 398.0809 [M + NH4]+ 398.0806.
{7-[(2-Methoxyethoxy)methoxy]-4-oxo-4H-chromen-3-yl}boronic Acid (8)To a solution of 3 (1.6 g, 4.9 mmol) with anhydrous 1,4-dioxane (25 mL), bis(pinacolato)diboron (3.1 g, 12.0 mmol) was added and degassed by argon bubbling for 10 min. To the above mixture, [1,1′-bis(diphenylphosphino)ferrocene]palladium(II) dichloride dichloromethane adduct (198 mg, 0.24 mmol) and AcOK (1.43 g, 14.5 mmol) was added, and the resulting mixture was stirred at 90°C for 6 h. After cooling to room temperature, the reaction mixture was concentrated under reduced pressure, and the residue was dissolved in EtOAc, washed with 1 M HCl and brine. The organic layer was dried over Na2SO4, filtered, and concentrated under reduced pressure to give the crude product 8, which was used in the next reaction without further purification. To determine the structure, the crude product was partially purified by silica gel column chromatography (Hexane/EtOAc = 8 : 2 to 2 : 8) to give 8 as a colorless crystalline solid.
1H-NMR (600 MHz, CDCl3) δ: 8.31 (s, 1H), 8.13 (d, J = 9.0 Hz, 1H), 7.14 (d, J = 1.8 Hz, 1H), 7.11 (dd, J = 9.0, 1.8 Hz, 1H), 6.96 (br s, 2H), 3.85 (t, J = 4.5 Hz, 2H), 3.57 (t, J = 4.5 Hz, 2H), 3.38 (s, 3H).
13C-NMR (151 MHz, CDCl3) δ: 182.8, 163.04, 162.0, 158.3, 127.2, 118.3, 116.1, 111.1, 103.5, 93.4, 71.5, 68.3, 59.1.
HRMS (ESI) m/z Calcd for C13H16BO7+ 295.0984 [M + H]+, 295.0981.
5′-Hydroxy-7,2′,4′-tris(2-methoxyethoxymethoxy)isoflavone (5)7)The obtained crude product 8 (4.9 mmol) and 7 (2.2 g, 5.8 mmol) were dissolved in 1,4-dioxane (20 mL) and degassed by argon bubbling under sonication for 10 min. To the above mixture was added [1,1′-bis(diphenylphosphino)ferrocene]palladium(II) dichloride dichloromethane adduct (397 mg, 0.49 mmol) and 2 M aqueous K2CO3 (7.3 mL). The reaction mixture was stirred at 100°C for 2 h. After cooling to room temperature, the reaction mixture was diluted with CH2Cl2 and washed with water and brine. The organic layer was dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (Hexane/Acetone = 65 : 35) to give 5 as a red oil (1.65 g, 62%).
1H-NMR (600 MHz, CD3OD) δ: 8.10 (s, 1H), 8.09 (d, J = 9.0 Hz, 1H), 7.21 (d, J = 1.8 Hz, 1H), 7.15 (dd, J = 9.0, 1.8 Hz, 1H), 7.12 (s, 1H), 6.79 (s, 1H), 5.48 (s, 1H), 5.41 (s, 2H), 5.28 (s, 2H), 5.08 (s, 2H), 3.86 (t, J = 4.8 Hz, 2H), 3.84 (t, J = 4.8 Hz, 2H), 3.69 (t, J = 4.8 Hz, 2H), 3.59 (t, J = 4.8 Hz, 2H), 3.56 (t, J = 4.8 Hz, 2H), 3.48 (t, J = 4.8 Hz, 2H), 3.36 (s, 3H), 3.32 (s, 3H), 3.28 (s, 3H).
7,2′,4′-Tris(2-methoxyethoxymethoxy)-5′-(1,1-dimethylallyloxy)isoflavone (6)A solution of 5 (1.05 g, 1.9 mmol), in anhydrous tetrahydrofuran (25 mL), was degassed by argon bubbling under sonication for 15 min at 0°C. To the above mixture, tetrakis(triphenylphosphine)palladium(0) (110 mg, 0.095 mmol) was added, followed by tert-butyl (2-methylbut-3-en-2-yl) carbonate (1.07 g, 5.7 mmol), and then degassed by argon bubbling under sonication for 5 min at 0°C. The resulting reaction mixture was stirred at room temperature for 1 h. The reaction mixture was diluted with CH2Cl2 and washed with water and brine. The organic layer was dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (Hexane/Acetone = 70 : 30) to give 5 as a yellow oil (1.05 g, 89%).
1H-NMR (600 MHz, CD3OD) δ: 8.11 (s, 1H), 8.09 (d, J = 9.0 Hz, 1H), 7.22 (d, J = 2.4 Hz, 1H), 7.15 (dd, J = 9.0, 2.4 Hz, 1H), 7.13 (s, 1H), 7.00 (s, 1H), 6.16 (dd, J = 18.0, 10.8 Hz, 1H), 5.41 (s, 2H), 5.27 (s, 2H), 5.16 (dd, J = 10.8, 1.2 Hz, 1H), 5.15 (s, 2H), 5.06 (dd, J = 10.8, 1.2 Hz, 1H), 3.86 (td, J = 4.8, 1.8 Hz, 2H), 3.84 (td, J = 4.8, 1.8 Hz, 2H), 3.71 (td, J = 4.8, 1.8 Hz, 2H), 3.59 (td, J = 4.8, 1.8 Hz, 2H), 3.56 (td, J = 4.8, 1.8 Hz, 2H), 3.49 (td, J = 4.8, 1.8 Hz, 2H), 3.36 (s, 3H), 3.32 (s, 3H), 3.28 (s, 3H), 1.42 (6H, s).
Kwakhurin (KWA)KWA was synthesized from compound 6 according to reported protocols.7)
1H-NMR (600 MHz, Acetone-d6) δ: 7.99 (d, J = 9.0 Hz, 1H), 7.82 (s, 1H), 6.97 (dd, J = 9.0, 2.1 Hz, 1H), 6.90 (d, J = 2.1 Hz, 1H), 6.38 (s, 1H), 4.98 (t, J = 6.9 Hz, 1H), 3.69 (s, 3H), 3.29 (dd, J = 14.5, 6.2 Hz, 1H), 3.07 (dd, J = 14.5, 6.9 Hz, 1H), 1.48 (s, 3H), 1.37 (s, 3H).
13C-NMR (151 MHz, Acetone-d6) δ: 176.4, 163.0, 159.0, 155.7, 153.3, 151.3, 140.1, 136.4, 130.8, 128.3, 124.8, 121.5, 118.8, 115.3, 111.5, 103.1, 102.4, 61.1, 27.5, 25.6, 17.6.
qNMR Measurement1. Reference Standard for qNMR and Solvent
In this study, 1,4-BTMSB-d4 (1,4-bis(trimethylsilyl)benzene-d4, MW = 226.50, Code No. 024-17031, Lot. TWN2900, purity 99.9%), a certified reference material (NMIJ CRM), was purchased from FUJIFILM Wako Pure Chemical Corporation, Ltd. and used as the reference standard for qNMR. Methanol-d4 with the following deuteration rate was used as a solvent for qNMR determination: Lot. A0373436 (100.0 atom%D) by Acros Organics.
2. Instruments and Equipment
The Ultra-Microbalance XPR2UV (Mettler Toledo) with a minimum reading of 0.0001 mg was used. JNM-ECA600 (600 MHz) was used for the NMR. The Wilmad 535-PP-7 was used as an NMR sample tube.
3. Preparation of the Sample Solution
Approximately 3.3 mg of KWA and approximately 1 mg of 1,4-BTMSB-d4 (reference standard for qNMR), which were precisely weighed and placed in the same vial together for each tare, were dissolved in NMR solvent (1 mL). This solution (0.6 mL) was sealed in an NMR sample tube.
4. Conditions for qNMR Determination
The observed spectrum width was 20 ppm. A digital filter was used. The center of the spectrum was set at 5 ppm. The pulse width was set to the time at which a 90-degree pulse was obtained. Acquisition time, 4 s; digital resolution, 0.25 Hz; and delay time, 60 s (The T1 values of the signals of KWA were 0.5 to 4.8 s). An auto FG shim was used for shim adjustment. The determination temperature was set at room temperature (20–30°C). We performed 13C decoupling with MPF8. The dummy scan was performed twice, and the scan was performed 32 times. In principle, the determination was performed 3 times for each sample in accordance with the internal standard method (AQARI: Accurate quantitative NMR with internal reference substance) to ensure that the S/N of the quantitative signal was 200 or higher. Purity Pro, manufactured by JEOL Ltd., was used for NMR data processing. The trimethylsilyl peak of the reference standard for qNMR (1,4-BTMSB-d4) was set at 0 ppm. Phase correction and baseline correction were performed manually. The integration range for each peak was determined using a manual method. All integrated values in this study were expressed in terms of purity (%). The purity of the reagents was calculated using the following formula based on a previous study8–10):
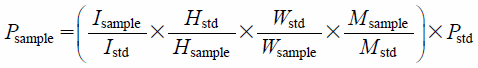 |
The following numbers were used for the calculation: number of protons of methyl groups in 1,4-BTMSB-d4 (reference standard for qNMR), 18; molecular weight of 1,4-BTMSB-d4, 226.50; molecular weight of KWA, 368.38.
We thank Dr. Tsutomu Ishikawa for providing authentic KWA.
This study was supported by a Grant from the Ministry of Health, Labour, and Welfare of Japan.
The authors declare no conflict of interest.