2021 Volume 69 Issue 10 Pages 947-952
2021 Volume 69 Issue 10 Pages 947-952
Closed bilayer membranes of amphiphiles in water, termed vesicles, represent one of the promising models of primitive cellular compartments. Herein, we reviewed studies on the design and construction of vesicle-based cell models capable of sequential growth and division and their underlying analysis methods. We discussed the potential contribution of these studies to the universal understanding of the chemical/physical logics behind the steady reproduction of cellular membranes.
All living organisms on earth have cellular compartments that distinguish them from the external environment and enable the exchange of substrates and energy within an organism. Since the earliest emergence of life-like chemical systems, their overall dynamics are oriented in a specific direction, that is, only a living organism can reproduce another living organism and the death of an organism is irreversible. Reconstructing artificial molecular assemblies with such properties in a non-equilibrium environment, where the inflow and outflow of substrates and energy are significant, would provide clues to understand the essence of the living system and the emergence of life. As cells are the fundamental building blocks of living organisms, it can be speculated that the primitive form of life is derived from a cell-like compartment. Thus, the design and construction of a primitive model of a cell-like system is a promising approach to explore the emergence of life on earth.1)
In this review, we focus on closed bilayer membranes composed of amphiphiles, termed vesicles. Liposomes are also a commonly used term. Liposomes are closed bilayer membranes formed primarily by phospholipids in water. Since phospholipids are amphiphiles, liposomes are considered to be included in vesicles in this paper. Although a cell membrane is a molecular assembly of the lipid bilayer membrane (with proteins) with a thickness of only 5 nm, it performs diverse functions: permeation and transport of molecules; protection, compartmentalization, and redistribution of informational molecules; provision of support to the folding and functionalization of membrane proteins; and characterization of the growth, deformation, fission, and fusion. Thus, it is reasonable to choose vesicles as a scaffold to construct a model of primitive cells. In particular, creating vesicles capable of consuming substrate and energy and giving birth to new vesicles is of considerable interest in this regard.
Before moving on to the aforementioned dynamics of vesicles, let us briefly summarize the general analysis methods for physical and static properties of vesicles.2–5) The size of vesicles in the range from nanometers to micrometers is conventionally measured by transmission electron microscopy (TEM) and dynamic light scattering (DLS). Due to the high vacuum inside the sample chamber of TEM, it is difficult to observe vesicles as they are. Therefore, when observing vesicles with TEM, the freeze-fracture replica method is one of powerful tools of TEM. In this method, a replica film of a cross section of frozen vesicle dispersion is taken using carbon deposition and following metal sputtering, and the replica film is observed by TEM. In TEM observation, not only the particle size and shape of the vesicle but also the film thickness can be measured. The freeze-fracture replica method for vesicle sample is now being replaced by the cryogenic transmission electron microscopy. In DLS, the vesicle dispersion is irradiated with laser light, and the scattered light is observed by a photon detector. The scattered light from vesicles interferes with each other, but since the dispersed vesicles exhibit Brownian motion, the intensity distribution due to the interference of the scattered light also changes. The diffusion coefficient and particle size distribution are measured by observing the fluctuation of the scattered light signal, converting it into an autocorrelation function. Phase transition of vesicle membranes is measured by differential scanning calorimetry (DSC). DSC analyze the temperature of the reference substance and the vesicle dispersion at the same time while applying a certain amount of heat. The measured time course of the temperature difference between the reference and the vesicle dispersion can reflect the transition of the thermal properties of the vesicle dispersion because the state of the vesicle membrane affects the endothermic and exothermic process. The zeta potential, which represents the state of charge on the vesicle surface, is measured using the laser Doppler method. In the laser Doppler method, the surface charged vesicles being electrophoresed are irradiated with a laser light. The frequency of scattered light from vesicles shifts due to the Doppler effect, and the amount of shift is proportional to the electrophoresis speed of surface charged vesicles. The zeta potential can be evaluated by determining the electrophoresis speed through measuring the shift. The evaluation of vesicle entrapment yield is performed by fluorescence quenching. In this method, a fluorescent molecule is encapsulated inside the vesicle in preparation, and the sample of vesicle dispersion obtained by dilution is measured with a fluorescence spectrometer (Ftot). As reference, the fluorescence intensity (Fv) of the vesicle dispersion prepared without the fluorescent molecule is also measured. When a solution containing a quenching reagent is added to this vesicle dispersion containing the fluorescent molecule, the fluorescent molecules existing in the bulk are extinguished, but those inside of the vesicles are retained. The fluorescence intensity (Fin) of the vesicle dispersion at this time is measured. Finally destroying the vesicles with surfactant and measuring the fluorescence intensity to measure the fluorescence intensity of vesicles themselves (F′v) afford entrapment yield e (%) as follows: e = (Fin − Fv)/(Ftot − F′v) × 100.
To understand the dynamic behaviors of vesicles accompanying their deformation during the propagation of vesicles, considering theoretical models based on soft matter physics is useful. For example, the area difference elasticity model is known to appropriate to explain the experimental behaviors of vesicle deformations.6) Among them, growth and division are of particular interests.7–9) Here, as a terminology in this research area, “growth” denotes the increase of volume and/or surface area of the vesicles, and “division” means physical detachment of newborn vesicles from its mother vesicle following the constricted shape (Fig. 1). Some experimental results demonstrated that vesicles composed of simple amphiphiles can grow and divide only by incorporating constituent molecules or energy.10)

Metabolism, molecular transformation, is an essential life process, and the growth driven only by physical processes without molecular transformation is very simple. Luisi discussed the importance of the energy and molecular transformation of the precursors incorporated into the constituents of the system in the growth and division of the vesicles, invoking the argument of autopoiesis.11) He termed the dynamics “self-reproduction” to distinguish it from the physical growth and division of vesicles (Fig. 1). This review discusses studies on the molecular transformation and underlying analysis methods regarding self-reproducing vesicles to facilitate the development of vesicle-based compartments and verification of their growth and division, respectively.
In the 1990 s, Luisi and colleagues investigated a basic aqueous dispersion of a micelle of a fatty acid mixed with its anhydride and found a sudden increase in the amount of fatty acid and the resultant micelle after a certain interval from mixing.12) The autocatalytic mechanism was as follows: the hydrolysis of the anhydride of fatty acid (Chart 1) was enhanced when the anhydride was incorporated into the micelle. Because the resulting fatty acids were the constituents of the micelle and the micelles could accommodate only a certain number of molecules, the produced fatty acid molecules destabilized the structure, resulting in the formation of another micelle. As the newly formed micelle also acted as a catalyst for hydrolysis, the production of fatty acids was catalyzed by the newly formed fatty acids, which indicated autocatalycity.13) Later, they reported the self-reproduction of vesicles, whose underlying mechanism was similar to the abovementioned mechanism, comprising oleic acid and oleate formed at a particular pH.14) They performed a series of studies on the concept of autopoiesis reported by Varela15) and provided a fundamental discussion on the origin of life with respect to the self-reproduction of the cellular compartment.11)
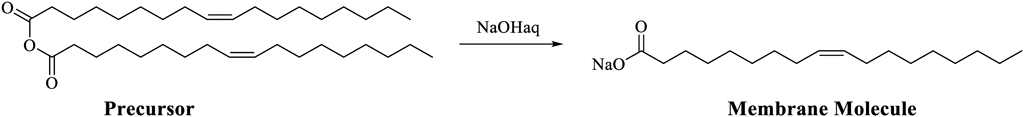
From a technical viewpoint, the measurement and quantification of molecular dynamics at two different hierarchies were required for these studies: the progress of the reaction inside the chemical system and the time course of the number of micelles and vesicles. Fourier transform IR spectroscopy (FTIR) was mainly used for analyzing the progress of the reaction. IR spectroscopy is an analytical technique employed to examine and quantify the structure of a sample by irradiating the sample with IR light and measuring the reflected or transmitted light. In FTIR, a sample is irradiated with full-wavelength light interfered by an optical interferometer. The transmitted or reflected light is measured, and its Fourier transform IR spectrum is obtained. FTIR is superior to conventional dispersive IR in terms of brightness, sensitivity, scanning speed, and effective utilization of light owing to the non-use of diffraction gratings. Luisi and colleagues tracked the changes in absorption at specific wavelengths corresponding to the carbonyl group of each anhydride and fatty acid to confirm the progress of the reaction in the entire system.
However, IR is not suitable for tracking the molecular assembly dynamics, such as the increase in the number of micelles, because the FTIR spectra reflect the average properties of the whole system. Therefore, turbidity (optical density) was measured to evaluate the number of micelles and vesicles. Turbidimetry is an analytical method employed to measure the transmitted light crossing the sample to quantify the optical density. The transmitted light is affected by absorption, scattering, and refraction. As micelles or vesicles in the measured sample scatter the introduced light, the sample containing micelles or vesicles shows a larger optical density. Variation in the number of micelles or vesicles in the system was estimated by measuring the change in optical density after the addition of the anhydride of the constituent fatty acid (precursor) via appropriate control experiments. Interestingly, the optical density of the system demonstrated a steep increase after a particular induction time, indicating a synergistic effect between the production of micelles or vesicles and the progress of the reaction.
The model proposed by Luisi and colleagues was promising because it contained the fundamentals required for a primitive cell model. However, some researchers struggled to accomplish a higher-order chemical system, aiming to fill the gap between the simple chemical systems and the present living cells. In this regard, elaborate examples were provided by Szostak and colleagues. They found that multilamellar vesicles comprising fatty acids deformed to a thread-like shape upon the addition of the fatty acid, and the deformed vesicles easily divided into small vesicles upon gentle agitation, for example, by compressed air, of the dispersion.10,16) They further elucidated that the membrane disturbance caused by the addition of fatty acids (membrane molecules) enhanced the permeation of nucleotides; however, this enhancement was not significant for oligonucleotides.17) They demonstrated non-enzymatic replication of short-chain DNA inside the vesicles. Another notable study reported by Szostak and colleagues was based on the oligopeptides autocatalytically produced inside the vesicles. Importantly, the oligopeptides were localized in the vesicle membrane and increased the affinity of the external fatty acids to the membrane. They revealed that the growth behaviors within vesicles could be competitive due to the difference in the amount of entrapped oligopeptides.18) Although the dynamics of the compartment were driven by a physical process, the molecular transformation responsible for these dynamics were considerable. They discussed a series of studies to realize the primitive form of a cell through chemical evolution starting from a simple cell model.
The key to these studies was to verify the amplification of DNA and vesicle growth. At first, DNA amplification was confirmed by electrophoresis using polyacrylamide. Electrophoresis is a phenomenon in which charged substances in a solution directly move along the applied electric field. The samples loaded on the supports (membranes or gels with a mesh-like structure) migrate to the oppositely charged electrodes and are separated because of the difference in the speed of electrophoresis, which is affected by various factors including their electric states. The support works as a physical molecular sieve and separates substances depending on their molecular weight as smaller substances travel faster, whereas larger substances move slower on it. In a series of studies reported by Szostak and colleagues, electrophoresis was employed to isolate and purify DNA from a mixture of lipids and short-chain DNA molecules. For example, they demonstrated that 32 bp template DNA was amplified from a 15 bp primer in the vesicles.17)
Moreover, the growth of the vesicles was evaluated by fluorescence resonance energy transfer (Förster resonance energy transfer, FRET). If the following three conditions are realized between two fluorescent molecules, the probability that the excited molecule (donor) will cause excitation of the other molecule (acceptor) instead of relaxation of energy as emission increases: the fluorescence spectrum of the donor and the excitation spectrum of the acceptor overlap; the two fluorescent molecules are close enough (typically within 10 nm); and the dipole moments of the two molecules are appropriately oriented. FRET occurs only when the distance between the donor and acceptor is a few nanometers; thus, the microscopic distance between the two molecules can be visualized. In the abovementioned studies, Adamala and Szostak used the fact that the fluorescence intensity of the acceptor decreases with an increase in the surface area of the vesicle owing to the dilution of the donor and acceptor in the membrane.18) They conceptualized the competition between vesicles for the membrane molecules by tracing the growth behavior of vesicles and discussed the relationship between evolution and diversification of the catalytic activity of the oligopeptides generated in the vesicles.
The size of vesicles observed in the models introduced by Luisi’s group and Szostak’s group was in the range of several tens to hundreds of nanometers. This size range would be justified for the origin of life or emergence of a primitive cell model through chemical evolution. It is also important to determine whether vesicles larger than 1 µm (termed giant vesicles) can be a candidate for the primitive cell model. This insight was provided by Sugawara and colleagues. They proposed the growth and division of giant vesicles using a positively charged artificial amphiphile as a precursor. The imine bond in this precursor was hydrolyzed in the vesicle membrane containing the catalyst, forming the constituent molecule of the membrane (Chart 2). They reported destabilization of the spherical giant vesicles followed by several growth and division events within 1 h.19)

They further developed a system by encapsulating DNA and a solution inside the vesicles prepared with a positively charged amphiphile and additional phospholipids for PCR.20) The PCR was conducted with 24-mer primers for 1229-bp DNA template in 20 thermal cycles (94 °C for 15 s and 68 °C for 90 s). The integrated vesicles demonstrated amplification of the inner DNA and then self-reproduction of the compartment with the addition of precursors. Interestingly, the more the DNA inside the vesicles after amplification and the larger the size of the vesicles, the faster the vesicles divided several times. Because DNA is negatively charged, the encapsulated DNA could function as a scaffold to localize the catalyst and precursor molecules in the vicinity of the membrane, resulting in the efficient production of membrane molecules. The reason for size dependency was discussed to be derived from the size dependency of the incorporation of the molecules required for amplification of DNA. Recent studies have reported that the self-reproducing dynamics change depending on the length of the encapsulated DNA21) and self-reproducing vesicles comprising negatively charged amphiphiles without ad hoc catalyst22) (Chart 3).

Flow cytometry and optical microscopy played an indispensable role in the studies reported by Sugawara and colleagues. A flow cytometer contains a flow unit, an optical unit, and an electrical unit, enabling the measurement of multiple features of a single cell at high throughput. Although the system was originally designed for blood cells, it is applicable to other particles such as vesicles. Initially, the vesicles in the suspension were aligned in a row during their passage through the flow unit. Then, a laser was irradiated on the aligned vesicles in the flow unit and was scattered by the vesicles. If the vesicles were stained with fluorescent probes, light emission was detected. The interaction between the laser light and the vesicles was evaluated by light scattering and fluorescence intensity measurements using an electrical unit. The scattered light can indicate the size and shape of the vesicles, and the fluorescence intensity of the emission implies the amount of the probed molecule. The main advantage of flow cytometry is throughput: it is easy to measure approximately 10000 vesicles in a short time. Although the detailed time course of each vesicle is not traceable, the transition over time is detected by the change in the distribution whose individual data points reflect information about individual vesicles. In a series of studies reported by Sugawara and colleagues, microscopic observation and flow cytometry were combined to acquire information about the comprehensive features of giant vesicle dynamics.
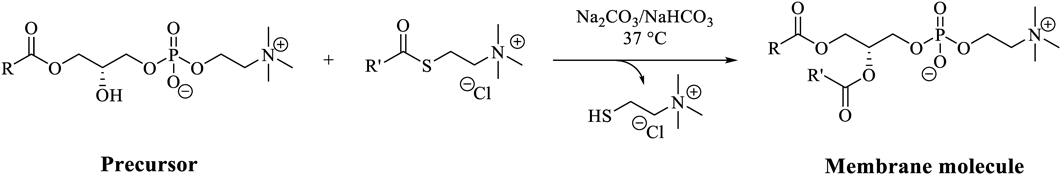
Studies reported by Sugawara’s group were different from those reported by Luisi’s group and Szostak’s group as Sugawara and colleagues designed and synthesized artificial molecules to achieve the goal. However, the catalyst, an imidazol derivative, used in the studies was prepared before the experiment and was not produced in the vesicle. Devaraj and colleagues proposed giant vesicles enabling the production of not only membrane molecules, but also the catalyst.23) Huisgen cycloaddition is catalyzed by Cu(I) ions, which form a complex with the triazole rings generated by this cycloaddition (Chart 4). When alkyl azide was used as a reactant for Huisgen cycloaddition, the resulting oligotriazole tended to localize in the vesicle membrane and thus more efficiently catalyzed the transformation of the precursor to the membrane molecule. The growth and division of the giant vesicles linked to the production of membrane molecules by Huisugen cycloaddition have also been reported by Toyota and colleagues.24) They reported the redistribution of the encapsulated lambda phage DNA between the original and new born vesicles during the division process.

Devaraj and colleagues recently reported non-enzymatic synthesis of natural diacyl phospholipids from a monoacyl phospholipid in water as another notable reaction for the production of phospholipids25) (Chart 5). When the monoacyl phospholipid was transformed to a diacyl phospholipid during the reaction, giant vesicles were formed due to the change in the packing parameter of the phospholipids, causing the formation of a bilayer membrane from the micelle. They discussed that the mechanism of vesicle production involves the fusion of sub-micrometer vesicles as well as the incorporation of newly formed phospholipids. HPLC equipped with evaporative light scattering detection (HPLC/ELSD) was mainly employed by them for reaction analysis. HPLC separates the crude product by passing it through a column with a mobile phase pressurized by a pump owing to the different molecular interactions in the stationary and mobile phases. ELSD is a type of detection method in which the eluent from the HPLC column is evaporated and sprayed on the detector. The detector measures the scattered light. ELSD is particularly sensitive to molecules that do not absorb UV light. The sensitivity is approximately 10 times that of differential refractive index (RI) detection. Therefore, ELSD is suitable for gradient analysis, which is difficult to realize via RI.
To explore a prebiotically plausible reaction system applicable to self-reproducing vesicles, a recent study reported by Sutherland and colleagues should also be considered.26) Amphiphiles, including simple fatty acids, aldehydes, and alcohols, are expected to exist on the early Earth, and methyl isocyanide (MeNC) is believed to have acted as an activating agent. They found that giant vesicles can be formed even under acidic conditions, which are required for the activation reaction using MeNC. Furthermore, using 31P-NMR spectroscopy, they reported that 3′-adenosine monophosphate was transformed into cyclic phosphate (Chart 6), increasing the surface area of the vesicle membrane. They discussed the possibility of co-evolution of the cellular membrane, nucleic acids, and proteins.

Finally, we discuss the relationship between the primitive cell model and the origin of life. Lancet and colleagues proposed the lipid world hypothesis based on the insight that molecular assemblies, including micelles and vesicles, whose constituent production is catalyzed by other constituent molecules (mutual catalysis), can evolve using compositional variety as an information carrier through a ceaseless cycle of growth and division27) (Fig. 2). As discussed so far, the current synthetic approaches have established primitive cell models comprising encapsulated DNA or peptides. As these primitive cell models have the potential to evolve using a combination of macromolecules and composition as the information carrier, a prebiotic scenario describing the transition of the information carrier from composition to macromolecules has also been proposed.28). Implementing chemical systems whose information flow does not necessarily correspond to the current cells, as suggested by the abovementioned theoretical frameworks, can clarify the constraints of the external environment required for the emergence of a life-like chemical system. Furthermore, exploring the behavior of these unique systems is expected to provide complementary insights into the current understanding of biology. For example, if we can investigate the diversification of the relationship between the encapsulated macromolecules and the composition of the primitive cell model during self-reproduction, the logic behind evolution can be elucidated.

To experimentally verify the lipid world hypothesis, these chemical systems should be implemented in practice. Therefore, to scientifically establish the research field of self-reproducing vesicles, we must remember that the synergistic development of analytical techniques to properly quantify and understand the dynamic behavior of this system is necessary. We hope that this review, which summarizes the analytical techniques used in the attractive research field of self-reproducing vesicles and discusses the strengths and limitations of these analytical techniques, will stimulate the interest of researchers in this direction and contribute to the development of this emerging research field.
The study was, in part, supported by Grant-in-Aid for JSPS Research Fellow (to H. S.; Grant number JP18J22004), The ANRI fellowship (to H. S.), and Platform for Dynamic Approaches to Living System (to T. T.) from The Ministry of Education, Culture, Sports, Science and Technology, Japan.
The authors declare no conflict of interest.