2021 Volume 69 Issue 5 Pages 456-463
2021 Volume 69 Issue 5 Pages 456-463
The purpose of this research was firstly to prepare solifenacin succinate functional particles embedded in a gelling–swelling layer (PEGS) so as to achieve both taste-masking of the unpleasant taste of the drug and rapid drug elution, and secondly to incorporate these PEGS into orally disintegrating tablets (ODTs). In in vitro dissolution tests, initial drug release from the prepared PEGS could be suppressed to less than 1% after 2 min and increased to more than 85% after 30 min by adjusting the composition of the PEGS, in particular the thickness of the outer water-penetration control layer which contains a water-insoluble polymer. For the preparation of ODTs containing PEGS, a semi-direct compression method was adopted in order to prevent damage to the PEGS by processes such as granulation or compaction. The use of a fibre-shaped microcrystalline cellulose with poor fluidity improved the content uniformity of the ODTs, as the crystal fibres became entangled with the PEGS and other additives. The use of spherical mannitol with a hollow structure produced by spray drying imparted relatively high hardness and rapid disintegration properties to the final ODTs containing PEGS, which were tableted using a low compression force. There was no significant difference in the drug-release profiles of the optimally formulated ODTs containing PEGS tableted at different compression forces. The ODTs containing PEGS maintained a drug-release lag time sufficient for taste-masking of solifenacin succinate.
In an aging society, urological diseases such as dysuria which adversely affect the QOL of many patients have become a more common problem. Overactive bladder (OAB), a symptomatic syndrome characterized by urinary urgency, is the main cause of dysuria. OAB patients tend to be reluctant to take drugs with water due to the risk of subsequent urinary urgency.1) Therefore, orally disintegrating tablets (ODTs) that can be taken without water are particularly useful in administering medicines for OAB. ODTs, known as user-friendly dosage form, have been developed and marketed by many companies with various technologies,2,3) and their clinical usage is still increasing. This is mainly because of their advantages for patients with poor swallowability and because they do not need to be taken with water.4,5) Recently, ODTs containing small functional particles such as taste-masking, sustained-release or enteric-release have been developed.6–11)
Solifenacin succinate, an anti-muscarinic agent with high selectivity for the urinary bladder, is used to treat OAB; therapeutic benefits include reduced adverse effects, such as dry mouth.12–14) Solifenacin succinate, however, is both bitter and astringent which presents an obstacle to its inclusion in ODTs. Further, solifenacin succinate is also highly soluble in water and quickly dissolves in even a small amount of saliva in the oral cavity to form a highly concentrated, unpleasant-tasting, solution.
Generally, taste-masking methods can be categorized as physical, chemical, or physiological.15,16) Due to the intense bitterness of solifenacin succinate, taste-masking by a physiological method using sweeteners or flavors, or a chemical method which reduces adsorption of the drugs to the taste bud on the tongue by chemical interaction, are inadequate, so that a physical method capable of suppressing drug release in the oral cavity to below the bitterness threshold is required.
In our previous study, novel functional drug Particles Embedded in a Gelling–Swelling layer (PEGS) which are capable of achieving both taste-masking of unpalatable drugs and rapid drug elution were developed.17) PEGS have a three-layer structure consisting of a core drug layer, a gelling–swelling layer and an outer water-penetration control layer containing a water-insoluble polymer. When water reaches the gelling–swelling layer, pulverized fine gelling–swelling particles gellate to form a rigid layer, thereby preventing drug release. After a defined lag time, the increased volume of the gelling–swelling layer by water absorption rupture the outer water-penetration control layer, leading to rapid drug release.
In the present study, therefore, PEGS containing solifenacin succinate as a model drug were prepared and then this PEGS system was adapted for use in ODTs. In the development of tablets containing spherical, high-density, functional particles such as PEGS, achieving good content uniformity is often a problem. If the functional particles and other additives are granulated to address this problem, the functional particles may themselves be damaged. There is also a danger that, if the ODT is prepared with a low compression force in order to avoid damage to the functional particles, insufficient tablet hardness will become a problem.
In this study, the optimal formulation of an ODT containing PEGS with sufficient drug-release lag time for taste-masking in the oral cavity and the desired drug-release profile, is described. The ODT was prepared by a semi-direct compression method, a simple manufacturing method not involving a granulation process. The effects of varying the excipients and compression forces used on tablet hardness, disintegration time, content uniformity and the drug-release profile of ODTs containing PEGS were examined.
Solifenacin succinate (Yuki Gosei Kogyo Co., Ltd., Japan) was used as a model drug.
In the drug-coating process, microcrystalline cellulose spheres (Celphere® grade CP-102, Asahi Kasei Corp., Japan), hydroxypropylmethylcellulose (HPMC; TC-5® grade E, Shin-Etsu Chemical Co., Ltd., Japan) and talc (Fuji Talc Industrial Co., Ltd., Japan) were used as core particles, to bind the drug, and as the aggregation-preventing agent, respectively. Carboxymethylcellulose sodium (CMC-Na; Cellogen® grade F-SC, DKS Co., Ltd., Japan) and hydroxypropylcellulose (HPC; Nisso HPC® grade L, Nippon Soda Co., Ltd., Japan) were used to form the gelling–swelling layer. Ethylcellulose (EC; Ethocel® grade STD 7, DuPont de Nemours, Inc., U.S.A.) and HPMC were used to form the outer water-penetration control layer.
Microcrystalline cellulose (Ceolus® grade UF-702, PH-301, UF-711, PH-101, KG-802 and KG-1000, Asahi Kasei Corp.), mannitol (Pearlitol® grade 160 C, Roquette Japan Co., Ltd., Japan), granular mannitol (Granutol® grade R, Freund Corp., Japan), spherical mannitol (mannite Q, Mitsubishi Corp. Life Sciences Ltd., Japan), crospovidone (Kollidon® grade CL-SF, BASF Japan Ltd., Japan) and magnesium stearate (Taihei Chemical Industrial Co., Ltd., Japan) were used in the preparation of the ODTs.
Preparation of PEGSA solution containing solifenacin succinate was prepared in 6% (w/v) HPMC aqueous solution in which talc was dispersed. Microcrystalline cellulose spheres were placed in a tumbling fluidized bed granulating–coating machine (MP-01, Powrex Corp., Japan) and sprayed with the drug solution. After drying, the drug-coated particles were sieved through a 355-µm sieve to prevent aggregation. Pulverized CMC-Na, used as a gelling–swelling agent, was suspended in 4% (w/v) HPC ethanol solution. The drug-coated particles were put into a tumbling fluidized bed granulating–coating machine and the suspension was coated by spraying. Finally, the particles were coated with an outer layer containing a water-insoluble polymer by spraying with a 5% (w/v) polymer solution, prepared by dissolving EC and HPMC in a water–ethanol mixture (1 : 9, w/w). After drying, the particles were sieved through a 355-µm sieve to prevent aggregation. The particle size of the PEGS was measured using a laser-scattering particle size distribution analyzer (LA-950, Horiba, Ltd., Japan). The operating conditions for spray coating determined according to our previous report17) and they were shown in Table 1. The formulations of PEGS used in the experiments on drug release are shown in Table 2.
| Drug layer | Gelling–swelling layer | Outer layer | |
|---|---|---|---|
| Batch size (g) | 200 | 200 | 200 |
| Inlet air temp. (°C) | 70 | 50 | 50 |
| Outlet air temp. (°C) | 35–40 | 30–35 | 30–35 |
| Fluidizing air flow rate (m3/min) | 0.40 | 0.40 | 0.40 |
| Spray rate (g/min) | 1–3 | 1–4 | 1–2 |
| Spray air pressure (MPa) | 0.50 | 0.50 | 0.50 |
| Layer | Function | Component | EC : HPMC | ||
|---|---|---|---|---|---|
| 7 : 3 | 8 : 2 | 9 : 1 | |||
| Drug layer | Core particle | Microcrystalline cellulose sphere | 12.5 | ||
| Active compound | Solifenacin succinate | 5 | |||
| Binder | HPMC | 5 | |||
| Anti-aggregation agent | Talc | 2.5 | |||
| Gelling–swelling layer | Gelling–swelling agent | CMC-Na | 5 | ||
| Binder | HPC | 5 | |||
| Outer layer | Water-insoluble polymer | EC | 2.45–9.8 | 2.8–11.2 | 3.15–12.6 |
| Pore former | HPMC | 1.05–4.2 | 0.7–2.8 | 0.35–1.4 | |
Each component is represented as a weight ratio (w/w).
In general, when ODTs are taken without drinking water, most of the drug particles in the formulation are conveyed from the oral cavity to the stomach within 1 min.18–20) It has been reported that the rate of saliva secretion when geriatric patients hold an ODT in the oral cavity is approximately 1 mL/min.21) It has also been suggested that the strong bitterness of solifenacin succinate can be tasted at a concentration in the oral cavity of ≥0.16 mM.22) Therefore, in this study, the goal of taste-masking of solifenacin succinate was to suppress the drug dissolution rate to ≤3% (≤0.16 mM) for up to 2 min. In order to simulate the environment of the oral cavity, tests were conducted at pH 6.8, close to the pH in the oral cavity. Drug elution of ≥85% at 30 min was used to represent a successful drug-release profile, which would allow sufficient drug efficacy in vivo.
CMC-Na with a high swelling ratio which has the potential to allow a relatively long drug-release lag time was used as the gelling–swelling agent in the PEGS.
In order to investigate the optimal formulation of the outer layer of the PEGS to achieve bitterness suppression of solifenacin succinate in the oral cavity, various PEGS were prepared with outer layers consisting of three different mixing ratios of EC, a water-insoluble polymer, to HPMC, a water-soluble polymer (7 : 3, 8 : 2, and 9 : 1 by weight). The outer layer was coated at between 10 and 40% (w/w) relative to the gelling–swelling-layer-coated particle.
Preparation of ODTs Containing PEGSODTs containing PEGS were prepared using a semi-direct compression method. The prepared PEGS, together with microcrystalline cellulose, crystalline mannitol and crospovidone, were blended in the weight ratios shown in Table 3. Magnesium stearate was then added and the mixture blended again. The mixture was then compressed in a rotary tablet press (Virgo, Kikusui Seisakusho, Ltd., Japan).
| Function | Component | mg |
|---|---|---|
| — | PEGS containing solifenacin succinate | 45.5 |
| Diluent | Microcrystalline cellulose | 37.5 |
| Diluent | Mannitol | 59.5 |
| Disintegrant | Crospovidone | 6 |
| Lubricant | Magnesium stearate | 1.5 |
| Total (mg) | 150 | |
ODTs containing different grades of microcrystalline cellulose (150 mg), 7.5 mm in diameter, were prepared at a compression speed of 30 rpm and 8 kN compression force.
ODTs containing different types of mannitol (150 mg), 7.5 mm in diameter, were prepared at a compression speed of 30 rpm and using six different compression forces (5, 6, 7, 8, 9, and 10 kN).
Measurement of Repose Angle and Bulk Density of Microcrystalline CelluloseThe repose angle and bulk density of the different grades of microcrystalline cellulose were measured using the powder tester Model PT-S (Hosokawa Micron Corp., Japan). Measurement conditions were as follows: repose angle: table diameter 8 cm; bulk density: cup volume 100 cm3.
Evaluation of Excipient Features Using Scanning Electron MicrographsEach excipient and mixture for tableting was viewed using a scanning electron microscope (TM3030 Plus, Hitachi High-Tech Corp., Japan).
Measurement of Tablet CharacteristicsThe hardness of the prepared ODTs was measured using a tablet hardness tester (MultiTest 50, Dr. Schleuniger Pharmatron AG, Switzerland).
The disintegration time of ODTs in a simulated human mouth environment was evaluated using a measuring device for oral disintegration (Tricorptester, Okada Seiko Co., Ltd., Japan).23) The disintegration test solution, imitating saliva, was composed of 1.44 g/L NaCl, 1.47 g/L KCl, and 0.3% polysorbate 80, and was kept at 37 °C in an incubator.
Preparation of a Mobile Phase for HPLC MethodDissolve 8.2 g of potassium dihydrogen phosphate in 2000 mL of water, add 8 mL of triethylamine, and then add phosphoric acid to adjust the pH to 7.0. To this solution, add 1500 mL of acetonitrile and 1500 mL of methanol.
HPLC Method ConditionsAn XBridge BEH C18 column (150 × 4.6 mm, 5 µm, Waters Corp., U.S.A.) was eluted with a mobile phase at a flow rate of 1.6 mL/min and the column temperature was 30 °C. Solifenacin was determined by UV detection at 220 nm. The injection volume was 10 µL and the run time was 7 min.
Measurement of Content UniformityFor content uniformity testing, one tablet was placed into each of ten 50 mL volumetric flasks. Add exactly 4 mL of the internal standard solution (0.1% (w/v) butyl 4-hydroxybenzoate mobile phase solution) and approximately 30 mL of mobile phase, shake well and sonicate until the tablet completely disintegrates, then add the mobile phase to 50 mL and use this as the sample solution.
Separately, 5 mg of solifenacin succinate was accurately weighed into a 50 mL volumetric flask and add exactly 4 mL of the internal standard solution. Add the mobile phase to make 50 mL and use this as the standard solution.
After filtration of the sample solution and the standard solution (Millex® syringe filters, 0.45 µm, type polytetrafluoroethylene (PTFE), Merck KGaA, Germany), the filtrate was injected for analysis and the amount of drug was measured by HPLC (Prominence, Shimadzu Corp., Japan).
Drug-Release StudyThe drug-release profiles of PEGS and ODTs containing PEGS were investigated using the Japanese Pharmacopoeia (JP) 17 paddle method at a rotation speed of 50 rpm, using 900 mL of JP 2nd test solution (pH 6.8) maintained at 37 ± 0.5 °C. A 20-mL aliquot of the eluate was taken at the specified times and the volume of study solution immediately restored with fresh test solution, preheated to 37 °C. After filtration (Millex® syringe filters, 0.45 µm, type PTFE, Merck KGaA), the amount of released drug was measured by HPLC (Prominence, Shimadzu Corp.) under the same conditions as content uniformity measurement. The quantity of particles used in each test was designed to be sufficient to contain 5 mg solifenacin succinate, based on a normal drug dosage. When the drug-release lag time was measured, sampling of the eluate was performed every 20 s for up to 2 min. Using the drug dissolution rate derived, the drug-release lag time (the time for the drug dissolution rate to reach 3%) was calculated by linear interpolation.
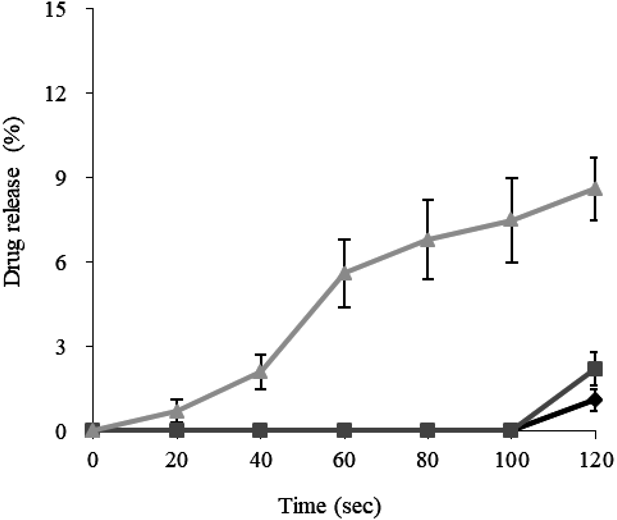
The results of the drug-release lag time experiments are shown in Fig. 1. The drug-release lag time from PEGS with an outer layer with a mixing ratio of EC : HPMC of 7 : 3 hardly changed, even when the amount of coating was increased. The water-penetration rate through an outer layer which included 30% pore former was too rapid, so that the inner gelling–swelling layer swelled rapidly, rupturing the outer layer and allowing drug release. If an outer layer with a mixing ratio of EC : HPMC of 8 : 2 was used, a slight lag time was observed as the outer layer thickness increased, but even 40% coating did not provide sufficient lag time. Although increasing the quantity of outer layer still further might achieve sufficient lag time, this would result in an unacceptable increase in both production time and particle size of the PEGS. Suppression of the initial drug-release from PEGS coated with an outer layer in which the mixing ratio of EC : HPMC was 9 : 1, was successful and a drug-release lag time of 2 min or more could be achieved by coating with 30% outer layer.

Data are expressed as mean ± standard deviation (S.D.) (n = 6). Mixing ratio of EC : HPMC in the outer layer: ♦, 7 : 3; ■, 8 : 2; ▲, 9 : 1.
Figure 2 shows the drug-release profile from PEGS with a 30% coating of the 9 : 1 outer layer. After a sufficient lag time, the drug was released rapidly, showing a drug dissolution rate of about 90% at 30 min, thus reaching the target drug-release profile. The particle size of the PEGS was about 250 µm, which is a suitable size for use in ODTs from the standpoint of texture and to avoid destruction in the mouth from chewing.24) The particle size distribution of PEGS was sharp as shown in Fig. 3. In subsequent experiments, this PEGS was used in the development of suitable formulations of ODTs. Actually, the relationship between the particle size of PEGS and drug release profile is very interesting and we would like to pursue further research in future study.
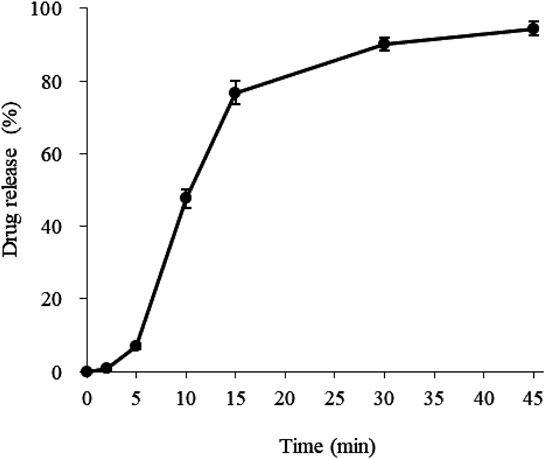
The JP paddle method was used at a rotation speed of 50 rpm, using 900 mL of JP 2nd test solution (pH 6.8) at 37 ± 0.5 °C. Data are expressed as mean ± S.D. (n = 6).

For the preparation of ODTs containing PEGS, a semi-direct compression method was selected, in which only simple physical blending of PEGS with excipients was performed before the mixture was compressed. Under normal circumstances, in order to improve the fluidity of a powder and obtain the desired content uniformity, a granulated powder would be prepared, by subjecting an active compound and additives to wet or dry granulation. However, when a tablet contains functional particles such as PEGS, there is a risk that these particles may be damaged due to the addition of solvents in wet granulation or compaction with other excipients in dry granulation, resulting in loss of function. Therefore, a semi-direct compression method, without a granulation process, was selected for the preparation of ODTs containing PEGS. However, as there may be a problem ensuring content uniformity in continuous tableting using a rotary tableting machine, we investigated the use of different additives to be blended into the ODTs together with the PEGS.
Microcrystalline cellulose is a diluent normally added to tablets to improve tabletability and disintegration. The effects of various types of microcrystalline cellulose, graded by shape with the repose angle an indicator of fluidity, on the content uniformity of ODTs containing PEGS were investigated. The results are shown in Table 4. PEGS themselves have a relatively high density (bulk density 0.78 g/cm3) and a spherical shape with good fluidity (repose angle 36°). In general, blended powders with this degree of fluidity hardly segregate, and exhibit good content uniformity. However, when UF-702, a microcrystalline cellulose with an almost spherical shape, was added, the ODTs containing PEGS exhibited very low content uniformity, despite having the same repose angle as the PEGS. This is presumed to be due to the difference in bulk density (the actual weight of the particles), which causes particle segregation in the blended powder between the time it is added to the rotary tableting press and the time it is compressed into ODTs. In addition, differences in the centrifugal force applied to the particles in the rotary tableting machine may cause the separation of the blended powder.
| Grade | Repose angle (˚) | Bulk density (g/cm3) | Content uniformity | Tablet hardness (N)b) | |
|---|---|---|---|---|---|
| Assay (%)a) | S.D. (%) | ||||
| UF-702 | 34 | 0.29 | 98.9 | 9.87 | 21 |
| PH-301 | 40 | 0.42 | 99.1 | 6.55 | 19 |
| UF-711 | 43 | 0.23 | 101.7 | 7.41 | 31 |
| PH-101 | 45 | 0.30 | 99.4 | 5.05 | 30 |
| KG-802 | 50 | 0.22 | 100.2 | 2.29 | 37 |
| KG-1000 | 58 | 0.14 | 100.4 | 2.43 | 45 |
a) Data expressed as means (n = 10); b) Data expressed as means (n = 3).
The content uniformity of ODTs containing PEGS tended to improve when microcrystalline cellulose with a larger repose angle (poor fluidity) was used. As shown in Fig. 4 and Table 4, the larger the repose angle of microcrystalline cellulose the more fibrous the particle shape becomes. Scanning electron micrographs of mixed powders produced using microcrystalline cellulose of different shapes are shown in Fig. 5. When a more fibrous microcrystalline cellulose, such as KG-802 or KG-1000, is used, the microcrystalline cellulose, PEGS and mannitol became intermeshed, leading to an improved content uniformity of the ODTs containing PEGS. This is due to an increase in the interactions between the particles and the collective movements of the blended vehicle. As the more fibrous microcrystalline cellulose has a high plasticity and imparts a relatively high hardness to the tablet even at a low compression forces, it was selected for use in the final ODTs containing PEGS.
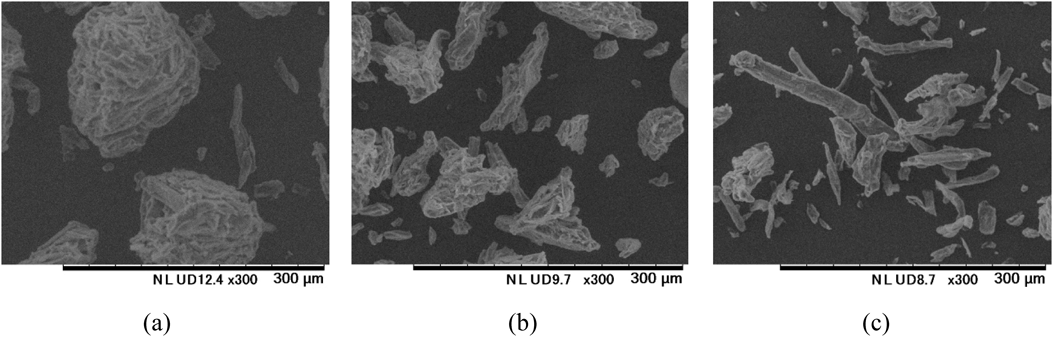
(a) UF-702 (spherical lumps), (b) PH-101 (between lumps and fibers), (c) KG-802 (fibrous).

(a) UF-702 (spherical lumps), (b) PH-101 (between lumps and fibers), (c) KG-802 (fibrous). The arrows indicate PEGS.
An ODT must have a rapid disintegration in the oral cavity but be sufficiently resistant to mechanical forces. Therefore, the criteria for ODTs containing PEGS include having a disintegration time in the oral cavity of ≤30 s and a tablet hardness of ≥40 N.25) In addition, to prevent damage to the PEGS during formulation, the ODTs need to be produced at a relatively low compression force. The selection of excipients is therefore crucial.
Mannitol is frequently used as an excipient in ODTs as it has low reactivity, slight sweetness and is water-soluble.26–28) Its disadvantages include poor tabletability and fluidity, and it may cause powder caking.29) Various forms of mannitol are commercially available which aim to solve these problems and it has been reported that these particle-engineered mannitol can impart high hardness or rapid disintegration to ODTs.30–34) The adoption of these mannitols was also assumed to be effective for achieving the target hardness and disintegration time for ODTs containing PEGS.
Therefore, in addition to common crystalline mannitol (Fig. 6a), we used a granulated mannitol prepared for direct compression using fluidized bed granulation with improved tabletability28) (Fig. 6b), and a spherical mannitol with a hollow structure prepared by spray-drying with reduced caking and improved fluidity29) (Fig. 6c) in experiments to investigate the effects of mannitol on the hardness and oral disintegration times of ODTs containing PEGS.
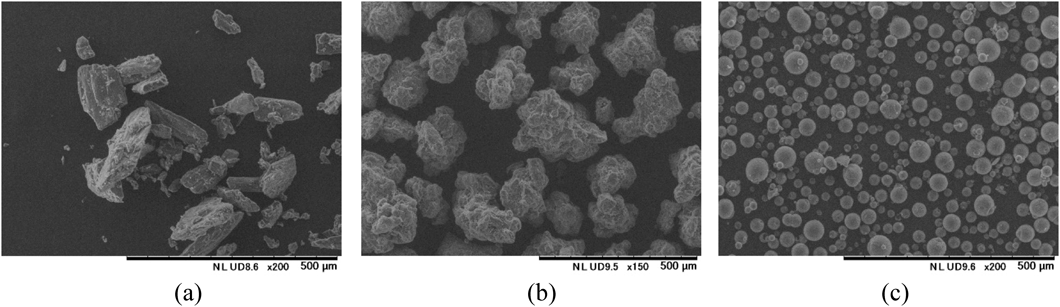
(a) Crystalline mannitol. (b) Granulated mannitol. (c) Spherical mannitol.
The tabletability of ODTs containing PEGS is shown in Fig. 7. There was an increase in tablet hardness in proportion to the compression force when any mannitol was used. When granulated mannitol and spherical mannitol were used, tablet hardness of ≥40 N could be achieved at a low compression force (5 kN), indicating good plasticity. When spherical mannitol was used, the ratio of tablet hardness to compression force became too large. The hollow spherical mannitol particles are relatively brittle,29) so the ratio of fine powder contained in the tablet may increase as compression force increases, which causes contact-friction to fill the gaps between the spherical PEGS and the other excipients.
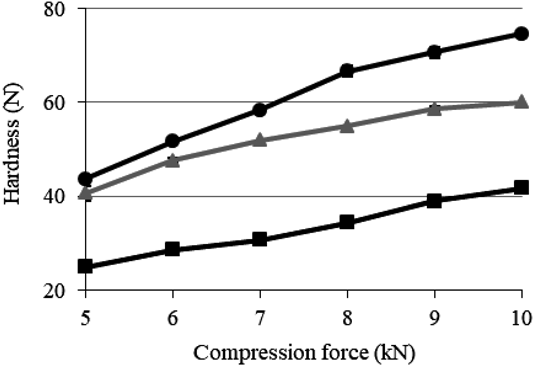
Data are expressed as mean ± S.D. (n = 3). Different mannitols included in ODTs containing PEGS: ■, crystalline mannitol; ▲, granulated mannitol; ●, spherical mannitol.
The results of the oral disintegration time experiments of ODTs containing PEGS in a simulated human mouth environment are shown in Fig. 8. When crystalline and granulated mannitol were used, disintegration times were delayed as hardness increased. When spherical mannitol was used, it showed a rapid disintegration time of about 20 s, even though the hardness of the tablet compressed at 10 kN was ≥70 N. It is speculated that the hollow structure of the spherical mannitol remaining in the tablet results in higher tablet porosity and that the relatively fine particle size (about 35 µm) increases blending uniformity, while the disintegrant (crospovidone) provides water-conducting properties.

Data are expressed as mean ± S.D. (n = 3). Different mannitols included in ODTs containing PEGS: □, crystalline mannitol; △, granulated mannitol; ○, spherical mannitol.
In conclusion, when designing an ODT containing PEGS, it is advisable to use a spherical mannitol as this imparts high hardness at a low compression force and confers a relatively fast disintegration time to the tablet.
Effect of Compression Force on the Drug-Release Profile of ODTs Containing PEGSTo investigate the in vitro drug-release profiles of ODTs containing PEGS, two different types of ODT were prepared, containing granulated or spherical mannitol, at different compression forces (6, 8, and 10 kN). The drug dissolution profiles (for up to 2 min, mimicking the time required for taste-masking in the oral cavity) of these two types of ODT are shown Figs. 9 and 10. It was found that drug release from ODTs made using granulated mannitol was affected by the compression force, with the result that PEGS in an ODT compressed at 10 kN could not maintain the drug-release lag time required for taste-masking. This is thought to be due to damage to the outer layer of the PEGS caused by the high compression force used, resulting in an increase in water penetration. ODTs containing PEGS prepared using spherical mannitol maintained sufficient drug-release lag time for taste-masking of solifenacin succinate, regardless of changes in compression force. In addition, the drug-release profile following the required lag time was comparable to that from PEGS alone, and was not affected by compression force (Fig. 11). It is suggested that the hollow structure and brittleness of the spherical mannitol used may relieve the pressure on PEGS by breaking itself, thereby preventing damage to the PEGS.
The JP paddle method was used at a rotation speed of 50 rpm, using 900 mL of JP 2nd test solution (pH 6.8) at 37 ± 0.5 °C. Data are expressed as mean ± S.D. (n = 6). Compression force: ♦, 6; ■, 8; ▲, 10 kN.

The JP paddle method was used at a rotation speed of 50 rpm, using 900 mL of JP 2nd test solution (pH 6.8) at 37 ± 0.5 °C. Data are expressed as mean ± S.D. (n = 6). Compression force: ♦, 6; ■, 8; ▲, 10 kN.

The JP paddle method was used at a rotation speed of 50 rpm, using 900 mL of JP 2nd test solution (pH 6.8) at 37 ± 0.5 °C. Data are expressed as mean ± S.D. (n = 6). ●, PEGS. Compression force: ♦, 6; ■, 8; ▲, 10 kN.
ODTs containing drug particles with functions such as enteric coating and taste-masking have been widely developed.6,11,35–37) Shimizu et al. have reported on the development of ODTs containing enteric-coated drug particles with a 7-layer structure to prevent damage to the enteric layer during the compression process.24) Their ODTs were prepared by blending strong functional particles with inactive granules prepared by wet granulation and tableting. Mizumoto et al. have reported on a novel ODT consisting of taste-masked drug particles and low-compressibility saccharides which were granulated with high-compressibility saccharides.25) The crystal change of high-compressibility saccharides from an amorphous to a crystal state by a humidity conditioning process after compression, resulted in increased tablet hardness caused by strengthening adhesion between particles.36)
Most ODTs containing functional spherical drug particles, as described above, require complicated manufacturing processes and designs to enable resolution of problems such as particle damage, content uniformity, hardness and disintegration time. The semi-direct compression method used to produce the ODTs containing PEGS described here is relatively simple, mixing commercially available additives with PEGS followed by tableting. By selecting appropriate additives and manufacturing processes, it was possible to prepare a hard, fast-disintegrating ODT containing PEGS without losing the required taste-masking function.
In this study, PEGS capable of both taste-masking and rapid drug elution were prepared using a method described in a previous paper. This PEGS system was then adapted for use in ODTs. PEGS with a 2-min drug-release lag time and subsequent rapid drug release could be prepared using CMC-Na with a high swelling ratio and by adjusting the composition and thickness of the outer layer. For the preparation of ODTs containing PEGS, a semi-direct compression method was selected as it was considered that the PEGS would be damaged by granulation methods. By the selection of appropriate diluents, typical problems associated with this method, such as content uniformity, tablet hardness and disintegration time, could be avoided. This optimalization of PEGS and ODT formulations allowed the production of solifenacin succinate ODTs with sufficient drug-release lag time for taste-masking in the oral cavity but with subsequent rapid drug release. In this study, PEGS with a size of about 250 µm and a sharp distribution was prepared, on the other hand, the relationship between the particle size of PEGS and drug release profile is very interesting and further studies are required to clarify this.
Appreciation is due to Mr. T. Hayashida and Mr. Y. Nakano for their valuable suggestions in developing this formulation. Acknowledgements also go to Ms. K. Mori and Ms. S. Narimura for assistance in performing assays.
This study was designed and funded by Nipro Corporation.