2022 Volume 70 Issue 10 Pages 699-706
2022 Volume 70 Issue 10 Pages 699-706
Chemically modified nucleic acids are essential for the therapeutic application of oligonucleotides. In this study, 6′-C-spiro-thymidine exhibiting a fixed torsion angle γ was designed, synthesized, and incorporated into oligonucleotides. The conformational analysis of the 6′-C-spiro-thymidine monomer revealed that its torsion angle γ was in the +synclinal range (approx. 60°), which is similar to that in a natural RNA duplex, as expected. On the other hand, the sugar conformation of the RNA duplex is known to be predominantly an N-type, whereas that of the synthesized monomer was an S-type. The results of the UV melting analysis demonstrated that the duplex-forming ability of 6′-C-spiro-thymidine was inferior to that of natural DNA. Contrarily, 6′-C-spiro-thymidine could enhance the stability of oligonucleotides toward nucleases. Particularly, the incorporation of 6′-C-spiro-thymidine on the 3′-ends of the oligonucleotides significantly increased the nuclease resistance of the oligonucleotides.
Oligonucleotide therapeutics, such as antisense oligonucleotides and small interfering RNAs (siRNAs), have recently made rapid progress as new therapeutic agents.1–4) Oligonucleotide therapeutics are generally modified with artificial nucleic acid analogs because natural DNAs and RNAs lack stability toward nuclease, as well as the ability to hybridize with complementary RNA. Thus, different chemically modified nucleic acids have been developed to address these shortcomings.5–8) Particularly, the chemical modification of oligonucleotides employing a phosphorothioate (PS) linkage is among the most widely utilized modification strategies for improving the nuclease resistance of oligonucleotide medicines. However, a negative feature of the PS linkage is that the incorporated sulfur atom introduces chirality in the phosphorus atom, thereby generating numerous diastereomers, which are very challenging to purify. Several 5′-alkyl-modified nucleosides, in which 5′-alkyl modification was employed as an alternative to PS modification, have been synthesized and applied to oligonucleotide medicines.9–11) Compared with the oligonucleotides bearing the PS linkage, those that were modified with 5′-alkyl nucleosides exhibited higher stability toward enzymatic degradation.
Further, restricting the sugar conformation of nucleotides to the N-type, which is a suitable conformation for forming A-type RNA duplexes, is a powerful strategy for enhancing the hybridizing ability of oligonucleotides. Locked nucleic acid (LNA)12,13) or 2′,4′-bridged nucleic acid (2′,4′-BNA)14,15) is an example of a chemically modified nucleic acid that comprises a constrained N-type sugar and can form a predominantly stable duplex with a target RNA. Additionally, LNA/2′,4′-BNA exhibits improved nuclease resistance probably owing to the steric hindrance resulting from the incorporated bridge structure. Moreover, LNA/2′,4′-BNA has already been employed in clinical trials because of such excellent properties.16) Furthermore, constraining the torsion angle γ, which is defined as the main chain O5′-C5′-C4′-C3′ torsion angle, is also a valuable strategy for improving the duplex-forming ability of oligonucleotides. The results of the structural analysis of duplexes have revealed that γ is generally in the +synclinal range (approx. 60°) in A-type RNA duplexes.17,18) For example, bicyclo-DNA19) and tricyclo-DNA20,21) are chemically modified nucleic acid analogs exhibiting fixed γ; they can thermally stabilize duplexes that are formed with complementary RNAs. Based on the above two strategies for restricting the conformation of nucleotides, tricyclic-LNA (TriNA) was designed and developed (Fig. 1). Its RNA-binding affinity was superior to that obtained by restricting the only the conformation.22)

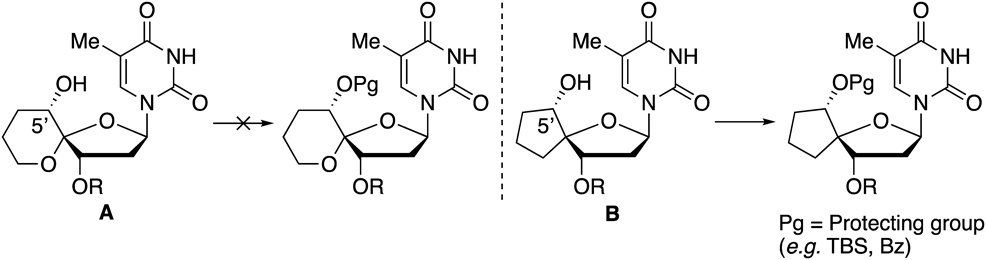
We have reported the syntheses and properties of a series of thymidine derivatives bearing a spiro-ring structure in the sugar moiety.23,24) Further, 5′R- and 5′S-spiro-DNA with sugar conformation and the torsion angle γ that were fixed by a simple 1,6-dioxaspiro[4.5]decane skeleton were recently developed25) (Fig. 1). The results of this study revealed that the sugar conformation of 5′S-spiro-DNA was the N-type and that γ in the 5′S-isomer was restricted to a suitable form (the +synclinal range) to hybridize with RNA. However, the spiro-DNAs exhibited several limitations during the chemical syntheses. For instance, laborious dimerization was necessary to incorporate the spiro-DNA into the oligonucleotides because of the poor reactivity of 5′-OH in spiro-DNA, as well as the lack of protection for the 5′-OH group by the 4,4′-dimethoxytrityl (DMTr) and levulinyl groups, which are commonly employed for the syntheses of automated oligonucleotides. Although the secondary alcohol on the 5′-position in spiro-DNA exhibited low reactivity, the nucleosides exhibiting the 1-oxaspiro[4.4]nonane skeleton (B), which was quite similar to the spiro-DNA (A), were readily acylated and silylated26,27) (Fig. 2). These results revealed that the poor reactivity of 5′-OH in spiro-DNA might be caused by the electron-withdrawing effect of the 6′-oxygen and/or the steric hindrance of the spiro ring containing 6′-oxygen. Therefore, the 5′-OH group in the 6′-carba-analog of spiro-DNA (6′-C-spiro-DNA, Fig. 1) might exhibit adequate reactivity for the protection reaction, which is necessary for synthesizing the oligonucleotide. In this study, 6′-C-spiro-DNA was synthesized, after which the duplex-forming ability and nuclease resistance of the modified oligonucleotides were evaluated.
The 6′-C-spiro-thymidine monomer (6) was synthesized from the 4′-C-allylthymidine derivative (2), which was prepared from thymidine in five steps28,29) (Chart 1). C5′-Allylation was achieved through the o-iodoxybenzoic acid (IBX) oxidation of compound 2, followed by its Lewis acid-mediated allylation employing allyltrimethylsilane, to yield the desired 5′S-isomer (3) as the sole product. The configuration of the 5′-carbon atom of 3 was determined after the construction of the 1-oxaspiro[4.5]decane skeleton. Diallylthymidine (3) underwent a ring-closing metathesis reaction in the presence of a Grubbs second generation catalyst to obtain the cyclized product (4) in good yield. This product (4) was converted into 6′-C-spiro-thymidine (5) via the hydrogenation of 4, followed by the removal of tert-butyldimethylsilyl (TBS) group by tetra-n-butylammonium fluoride (TBAF).

The X-ray crystallography of 6 revealed that the configuration of its 5′-carbon group exhibited the desired S-configuration (Fig. 3). The crystal structure revealed that the six-membered ring in 6 could exist in a chair conformation, with the 5′-OH group in the equatorial position. Torsion angle γ of 6 was 54°, which is similar to that of natural RNA. The sugar conformation of 6 was an S-type in the crystal structure, and this differed from that of the RNA duplex. Additionally, the sugar conformation of 6 in solutions was analyzed via 1H-NMR spectroscopy.30–32) The coupling constants of 6 were, as follows: JH1′H2′α and JH1′H2′β = 5.0 and 7.0 Hz, respectively, indicating that its sugar conformation in solutions was the same S-type as the conformation exhibited by the X-ray crystal structure. Conversely, the sugar conformation of 5′S-spiro-thymidine was predominantly the N-type.25) These results demonstrated that the conformational equilibrium of 5′S-spiro-thymidine was biased toward the N-type probably because of the anomeric effect from the 6′-oxygen atom.33,34) Contrarily, 6 exhibited an S-type sugar conformation because of the absence of a 6′-oxygen atom.

Chart 2 shows that the protection of the secondary alcohol on the 5′-position of 5 by the DMTr group was achieved by combining DMTrCl with silver nitrate.35) The phosphoramidite (9), which is a suitable building block for DNA synthesis, was synthesized via the treatment of 7 with TBAF to remove the TBS group, followed by the phosphitylation of 8. The modified oligonucleotides (ON2–4, ON7, and ON8; Table 1 and Fig. 6) were synthesized by extending the coupling time of compound 9 to 10 min (cf. 25 s for coupling natural DNA phosphoramidites). The 5′S-spiro-thymidine-modified oligonucleotides (ON5, ON9, and ON10) were prepared by using the dimer-phosphoramidite 1025) (Fig. 4). Since the monomer amidite 9 could be obtained, it became possible to synthesize ON3 with three consecutive modifications, which is difficult to synthesize by the dimer-phosphoramidite strategy.

| Oligonucleotides | vs. ssRNA | vs. ssDNA |
|---|---|---|
| Tm (ΔTm) | Tm (ΔTm) | |
| 5′-d(GCGTTTTTTGCT)-3′ (ON1) | 46 °C | 50 °C |
| 5′-d(GCGTTTTTTGCT)-3′ (ON2) | 43 °C (−3 °C) | 45 °C (−5 °C) |
| 5′-d(GCGTTTTTTGCT)-3′ (ON3) | 38 °C (−8 °C) | 36 °C (−14 °C) |
| 5′-d(GCGTTTTTTGCT)-3′ (ON4) | 39 °C (−7 °C) | 38 °C (−12 °C) |
| 5′-d(GCGTTTTTTGCT)-3′ (ON5) | 45 °C (−1 °C) | 48 °C (−2 °C) |
a) Conditions: 10 mM sodium phosphate buffer (pH 7.2), 100 mM NaCl, and 4 µM each oligonucleotide. Sequences of the target ssRNA and ssDNA are 5′-r(AGCAAAAACGC)-3′ and 5′-d(AGCAAAAACGC)-3′, respectively. ΔTm: change in the Tm values compared with that of the natural DNA (ON1).

The duplex-forming abilities of ON2–4 modified with 6′-C-spiro-thymidine (6) were evaluated via UV melting experiments and compared with those of the corresponding natural DNA (ON1) and 5′S-spiro-thymidine-modified oligonucleotide (ON5). Figure 5 shows the melting data, and Table 1 presents the melting temperature (Tm) of the duplex and the changes in the Tm values (ΔTm) relative to the natural ON1. The ΔTm values of ON2 were −3 °C (vs. ssRNA) and −5 °C (vs. ssDNA), indicating that 6 reduced the stabilities of the DNA/RNA and DNA/DNA duplexes. The results for ON3 and ON4 exhibiting multiple modifications of 6 indicated that the Tm values decreased with the increasing modification numbers regardless of whether the modification positions were continuous or discontinuous. Comparing the duplex-forming abilities of ON2 and ON5, the Tm values of ON5 were higher than those of ON2. Additionally, while the duplex formed by ON5 and cDNA was slightly less stable than the natural DNA/DNA duplex, the thermal stability of the duplex formed with ON5 and RNA was comparable to that of the natural DNA/RNA duplex, and the results are similar to those of the previous study.25) As shown in Fig. 3, the results of the structural analysis of 6 demonstrated that its torsion angle γ was similar to that of the natural duplex. Similarly, it was also reported that the torsion angle γ of 5′S-spiro-thymidine, having an oxygen atom on 6′-position, was restricted to a suitable angle (in the +synclinal range) to form the stable duplex.25) Conversely, the sugar conformation of 5′S-spiro-thymidine was predominantly the N-type, which is the same as that in the RNA duplex, whereas the sugar conformation of 6 tended toward the S-type, indicating that the change in the sugar conformation of 6 from the S-type to the N-type in the process of forming the duplex might decrease the thermal stability of the duplex.

The nuclease resistance of the T-decamer oligonucleotides bearing 6 was investigated employing 3′-exonuclease (Crotalus adamanteus venom phosphodiesterase, CAVP) and compared with those of natural and PS-modified oligonucleotides (Fig. 6). The 6′-C-spiro-thymidine-modified oligonucleotide (ON7) exhibited a higher nuclease resistance compared with natural T-decamer (ON6) probably because of the steric effect of the bulky spiro ring. The nuclease resistance of ON7 was higher than that of the 5′S-spiro-thymidine congener (ON9), indicating that the presence of the 6′-oxygen atom in 5′S-spiro-thymidine might have accelerated the enzymatic cleavage of the phosphodiester bond on the 3′-position. Furthermore, ON8 and ON10 with spiro modifications on the 3′-ends were barely degraded by the nuclease and exhibited higher stability than ON11 with PS linkage, which is generally utilized for the chemical modification of oligonucleotide medicines to improve their in vivo stability. Although nuclease-resistant artificial nucleic acids are essential for increasing the in vivo stabilities of therapeutic oligonucleotides, a higher RNA-binding affinity of the oligonucleotide does not always correspond to higher therapeutic effects.36,37) Additionally, to enhance the RNA-binding ability of an oligonucleotide, it might be sufficient to combine 6 with LNA, which is known to enhance the stability of duplexes. These findings indicate that 6 might facilitate a valuable chemical modification to enhance the stability of therapeutic oligonucleotides.

Conditions: 1.0 × 10−2 µg/mL CAVP, 10 mM MgCl2, 50 mM Tris–HCl (pH 8.0), 7.5 µM each oligonucleotide at 37 °C.
Here, 6′-C-spiro-thymidine (6) were synthesized and incorporated into oligonucleotides. The X-ray crystallographic analysis of 6 indicated that its torsion angle γ was 54°, which is similar to that of a natural RNA duplex; moreover, the sugar conformation of 6 was predominantly an S-type. Although the modification with 6 reduced the thermal stability of the duplex, the enzymatic degradation experiments revealed that its incorporation remarkably enhanced the nuclease resistance of the oligonucleotides. Particularly, the nuclease resistance of the 6′-C-spiro-thymidine-modified oligonucleotide at the 3′-ends was significantly higher than that of the oligonucleotide comprising a PS linkage, which has been employed in the practical applications of oligonucleotide-based medicines. We believe that the accumulation of properties, such as the duplex-forming ability and enzymatic stability through the development of different chemically modified nucleic acids, would contribute to the development of ideal tools for nucleic acid-based technologies.
All the moisture-sensitive reactions were conducted in well-dried glassware in an N2 atmosphere. The IR spectra were recorded on a JASCO FT/IR-4200 spectrometer. The 1H-, 13C-, and 31P-NMR spectra were recorded on JEOL JNM-AL300, JNM-ECS400, and JNM-ECA500 spectrometers. The chemical shifts were reported in part per million (ppm), relative to internal tetramethylsilane (δ = 0.00 ppm), residual CHCl3 (δ = 7.26 ppm), CHD2OD (δ = 3.31 ppm), or CHD2CN (δ = 1.94 ppm) for 1H-NMR. For 13C-NMR, the chemical shifts were reported in ppm, relative to chloroform-d1 (δ = 77.0 ppm), methanol-d4 (δ = 49.0 ppm), or acetonitrile (MeCN)-d3 (δ = 1.32 ppm). For 31P-NMR, the chemical shifts were reported in ppm, relative to 5% H3PO4 (δ = 0.0 ppm) as an external standard. The oligonucleotides were synthesized on a 0.2- and 1.0-µmol scale with an automated DNA synthesizer (Gene Design nS-8). The UV melting experiments were performed on SHIMADZU UV-1650PC and UV-1800 spectrometers that were equipped with Tm analysis accessory quartz cuvettes of 1-cm optional path lengths. The matrix-assisted laser desorption/ionization–time of flight (MALDI-TOF) mass spectra of all the new compounds were recorded on a JEOL SpiralTOF JMS-S3000 instrument. MALDI-TOF mass spectra of all the new oligonucleotides were recorded on a Bruker Daltonics Autoflex speed TOF mass spectrometer. For the column chromatography, silica gel PSQ-100B columns (Fuji Silysia Chemical Ltd., Aichi, Japan) were utilized. The progress of the reaction was monitored via analytical TLC on pre-coated glass sheets (Silica gel 60 F254 by Merck, Germany). For the HPLC, SHIMADZU CBM-20 A, DGU-20A3, LC-20AT, CTO-20 A, SPD-20 A, and FRC-10A were utilized. A SHIMADZU UV-1800 spectrometer was utilized for the UV absorbance measurement.
Syntheses of Phosphoramidite (9)1-[(5R)-3-O-tert-Butyldimethylsilyl-4,5-C-diallyl-β-D-2-deoxyribofuranosyl]thymine (3)IBX (2.66 g, 9.51 mmol) was added to a solution containing 229) (1.26 g, 3.17 mmol) in dry MeCN (30 mL) under N2 atmosphere, at room temperature. The reaction mixture was refluxed for 1.5 h, after which the suspension was cooled to room temperature and filtered with Celite®. The filtrate was concentrated in vacuo, and the obtained residue (1.34 g) was dissolved in dry CH2Cl2 (35 mL) in the N2 atmosphere. Next, allyltrimethylsilane (1.6 mL, 10 mmol) and BF3·OEt2 (0.64 mL, 5.10 mmol) were added to this solution at 0 °C, and the reaction mixture was stirred for 3 h at room temperature. After quenching with sat. NaHCO3 aq. at 0 °C, the whole mixture was diluted with CHCl3. Thereafter, the solution was washed with water and brine, dried over Na2SO4, and concentrated in vacuo. The residue (1.22 g) was purified via flash column chromatography (n-hexane–ethyl acetate (AcOEt) = 3 : 2) to yield 3 (865 mg, 63%, two steps from 2) as a white foam.
1H-NMR (CDCl3) δ: 0.11 (3H, s), 0.11 (3H, s), 0.92 (9H, s), 1.92 (3H, d, J = 1.0 Hz), 2.23–2.30 (2H, m), 2.34 (1H, dd, J = 14.5, 8.0 Hz), 2.42 (1H, dt, J = 13.5, 7.0 Hz), 2.50 (1H, dd, J = 13.0, 5.0 Hz), 2.60 (1H, dd, J = 14.0, 6.5 Hz), 3.63 (1H, ddd, J = 10.0, 4.0, 2.5 Hz), 4.64 (1H, dd, J = 7.0, 5.0 Hz), 5.02–5.30 (4H, m), 5.81–5.94 (2H, m), 6.13 (1H, t, J = 6.5 Hz), 7.54 (1H, d, J = 1.0 Hz), 8.00 (1H, s); 13C-NMR (CDCl3) δ: −5.0 (s), −4.2 (s), 12.7 (s), 18.0 (s), 25.8 (s), 36.1 (s), 36.4 (s), 41.2 (s), 73.3 (s), 73.5 (s), 85.4 (s), 90.7 (s), 110.8 (s), 118.4 (s), 118.8 (s), 134.2 (s), 135.2 (s), 137.2 (s), 150.5 (s), 164.2 (s); IR (KBr) cm−1: 3460, 3200, 3070, 2960, 2930, 2900, 2860, 1690, 1470, 1440, 1410, 1360, 1280, 1260, 1210; HRMS (MALDI) m/z 459.2286 (Calcd for C22H36N2NaO5Si: 459.2291).
1-[(2R,4S,5R,10S)-4-tert-Butyldimethylsilyloxy-10-hydroxy-1-oxaspiro[4.5]dec-7-en-2-yl]thymine (4)Grubbs second generation catalyst (12 mg, 0.015 mmol) was added to a solution 3 (1.31 g, 3.00 mmol) in dry CH2Cl2 (30 mL) under N2 atmosphere at room temperature, and the reaction mixture was refluxed for 4 h. The reaction mixture was cooled to room temperature and filtered with Celite®. The filtrate was concentrated in vacuo, and the residue (1.43 g) was purified via flash column chromatography (n-hexane–AcOEt = 3 : 2) to yield 4 (1.15 g, 94%) as a white foam.
1H-NMR (methanol-d4) δ: 0.08 (3H, s), 0.09 (3H, s), 0.90 (9H, s), 1.84 (3H, d, J = 1.0 Hz), 2.15 (1H, ddd, J = 13.5, 6.0, 3.0 Hz), 2.18–2.32 (4H, m), 2.38 (1H, ddd, J = 13.5, 8.0, 5.5 Hz), 3.77 (1H, dd, J = 9.5, 6.0 Hz), 4.55 (1H, dd, J = 5.5, 3.0 Hz), 5.54 (2H, s), 6.24 (1H, dd, J = 8.0, 6.0 Hz), 8.06 (1H, d, J = 1.0 Hz); 13C-NMR (methanol-d4) δ: −5.0 (s), −4.6 (s), 12.6 (s), 18.9 (s), 26.3 (s), 33.2 (s), 33.5 (s), 42.2 (s), 70.4 (s), 75.1 (s), 85.2 (s), 89.3 (s), 111.5 (s), 125.3 (s), 125.3 (s), 138.6 (s), 152.4 (s), 166.4 (s); IR (KBr) cm−1: 3390, 3180, 3030, 2950, 2930, 2900, 2860, 1680, 1470, 1430, 1360, 1330, 1280, 1260, 1210; HRMS (MALDI) m/z 431.1973 (Calcd for C20H32N2NaO5Si: 431.1978).
1-[(2R,4S,5R,6S)-4-tert-Butyldimethylsilyloxy-6-hydroxy-1-oxaspiro[4.5]decan-2-yl]thymine (5)10% Pd/C (268 mg) was added to 4 (1.15 g, 2.81 mmol) in tetrahydrofuran (THF) (30 mL). After stirring the reaction mixture under H2 atmosphere for 1 h at room temperature, the mixture was filtered with Celite®, after which the filtrate was concentrated in vacuo. The residue (1.14 g) was purified via flash column chromatography (n-hexane–AcOEt = 1 : 1) to yield 5 (1.00 g, 87%) as a white foam.
1H-NMR (CDCl3) δ: 0.08 (3H, s), 0.08 (3H, s), 0.90 (9H, s), 1.16–1.33 (3H, m), 1.67–1.76 (2H, m), 1.78–1.87 (2H, m), 1.93 (3H, d, J = 1.0 Hz), 2.15–2.24 (2H, m), 2.43 (1H, dt, J = 13.5, 7.0 Hz), 3.49 (1H, ddd, J = 11.5, 7.0, 4.5 Hz), 4.58 (1H, dd, J = 7.0, 5.0 Hz), 6.06 (1H, t, J = 6.5 Hz), 7.53 (1H, d, J = 1.0 Hz), 8.02 (1H, s); 13C-NMR (CDCl3) δ: −4.9 (s), –4.6 (s), 12.7 (s), 18.1 (s), 21.1 (s), 24.2 (s), 25.9 (s), 29.5 (s), 32.3 (s), 40.3 (s), 72.4 (s), 72.6 (s), 85.2 (s), 88.4 (s), 111.0 (s), 137.6 (s), 150.6 (s), 164.0 (s); IR (KBr) cm−1: 3430, 3170, 3060, 2940, 2860, 2710, 1680, 1470, 1400, 1360, 1290, 1260, 1210; HRMS (MALDI) m/z 433.2129 (Calcd for C20H34N2NaO5Si: 433.2135).
1-[(2R,4S,5R,6S)-4,6-dihydroxy-1-oxaspiro[4.5]decan-2-yl]thymine (6)TBAF (1 M in THF, 0.78 mL, 0.78 mmol) was added to a solution containing 5 (100 mg, 0.24 mmol) in dry THF (5 mL) under N2 atmosphere at 0 °C. The reaction mixture was stirred for 24 h at room temperature, after which it was concentrated in vacuo. The residue (516 mg) was purified by flash column chromatography (CHCl3–MeOH = 10 : 1, followed by MeCN 100%) to yield 6 (30 mg, 42%) as a white foam.
1H-NMR (methanol-d4) δ: 1.28–1.39 (2H, m), 1.47–1.86 (6H, m), 1.88 (3H, d, J = 1.0 Hz), 2.22–2.31 (2H, m), 3.55 (1H, dd, J = 11.5, 4.5, Hz), 4.51 (1H, dd, J = 5.0, 7.0 Hz), 6.23 (1H, t, J = 6.5 Hz), 8.16 (1H, d, J = 1.0 Hz); 13C-NMR (methanol-d4) δ: 11.6 (s), 11.7 (s), 21.5 (s), 24.5 (s), 29.6 (s), 31.8 (s), 40.2 (s), 47.7 (s), 47.9 (s), 48.0 (s), 48.4 (s), 72.4 (s), 83.9 (s), 138.2 (s), 151.7 (s), 165.7 (s); IR (KBr) cm−1: 3480, 3170, 3060, 2960, 2910, 2870, 2790, 1860, 1660, 1510, 1470, 1400, 1380, 1350, 1340, 1280, 1260, 1240, 1210; mp: 209 °C; HRMS (MALDI) m/z 319.1264 (Calcd for C14H20N2NaO5: 319.1270).
1-[(2R,4S,5R,6S)-4-tert-Butyldimethylsilyloxy-6-(4,4′-dimethoxytrityloxy)-1-oxaspiro[4.5]decan-2-yl]thymine (7)DMTrCl (2.50 g, 7.35 mmol) and AgNO3 (1.88 g, 7.35 mmol) were added to a solution of 5 (1.00 g, 2.45 mmol) in the mixed solvent of dry pyridine (5 mL) and dry THF (20 mL) under an N2 atmosphere at room temperature. The reaction mixture was stirred for 3 h at 80 °C. Thereafter, the mixture was filtered by Celite®, and the filtrate was diluted with CHCl3. The solution was washed with water and brine, dried over Na2SO4, and concentrated in vacuo. The residue (4.40 g) was purified via flash column chromatography (n-hexane–AcOEt = 7 : 3) to yield 7 (1.55 g, 88%) as a yellow foam.
1H-NMR (MeCN-d3) δ: −0.17 (3H, s), −0.04 (3H, s), 0.62–0.73 (1H, m), 0.80 (9H, s), 0.95–1.06 (1H, m), 1.34–1.51 (4H, m), 1.58–1.68 (4H, m), 1.73–1.81 (1H, m), 2.20–2.27 (1H, m), 2.41–2.63 (1H, m), 3.01 (1H, dd, J = 11.5, 4.0 Hz), 3.75 (3H, s), 3.76 (3H, s), 4.54 (1H, dd, J = 7.0, 5.0 Hz), 6.12 (1H, t, J = 6.0 Hz), 6.83–6.88 (4H, m), 7.19–7.23 (1H, m), 7.26–7.34 (4H, m), 7.35–7.40 (2H,m), 7.45–7.49 (2H, m), 8.25 (1H, d, J = 1.0 Hz), 9.31 (1H, s); 13C-NMR (MeCN-d3) δ: −4.9 (s), −4.3 (s), 12.5 (s), 18.4 (s), 21.9 (s), 25.0 (s), 26.0 (s), 31.4 (s), 31.6 (s), 42.3 (s), 55.9 (s), 73.0 (s), 77.3 (s), 84.1 (s), 87.7 (s), 90.2 (s), 110.7 (s), 113.8 (s), 127.8 (s), 128.6 (s), 129.0 (s), 131.2 (s), 131.4 (s), 136.8 (s), 137.4 (s), 137.7 (s), 147.7 (s), 151.3 (s), 159.7 (s), 164.9 (s); IR (KBr) cm−1: 3170, 3010, 2930, 2860, 1690, 1610, 1580, 1510, 1460, 1410, 1360, 1340, 1290, 1250, 1220; HRMS (MALDI) m/z 735.3436 (Calcd for C41H52N2NaO7Si: 735.3411).
1-[(2R,4S,5R,6S)-6-(4,4′-Dimethoxytrityloxy)-4-hydroxy-1-oxaspiro[4.5]decan-2-yl]thymine (8)TBAF (1 M in THF, 2.4 mL, 2.4 mmol) was added to a solution of 7 (1.54 g, 2.16 mmol) in dry THF (20 mL) under N2 atmosphere at 0 °C. The reaction mixture was stirred for 12 h at room temperature, after which it was concentrated in vacuo. The residue (2.31 g) was purified by flash column chromatography (CHCl3–AcOEt = 2 : 1) to yield 8 (992 mg, 77%) as a white foam.
1H-NMR (MeCN-d3) δ: 0.58–0.74 (1H, m), 0.93–1.04 (1H, m), 1.32–1.47 (4H, m), 1.56–1.72 (5H, m), 2.18–2.25 (1H, m), 2.35–2.44 (1H, m), 2.83–2.99 (2H, m), 3.73 (3H, s), 3.74 (3H, s), 4.34–4.54 (1H, m), 6.05 (1H, dd, J = 6.5, 5.5 Hz), 6.80–6.86 (4H, m), 7.15–7.21 (1H, m), 7.22–7.27 (2H, m), 7.30–7.39 (4H, m), 7.43–7.48 (2H, m), 8.21 (1H, d, J = 1.0 Hz), 9.22 (1H, s); 13C-NMR (MeCN-d3) δ: 12.7 (s), 21.7 (s), 24.9 (s), 30.7 (s), 31.3 (s), 41.3 (s), 55.8 (s), 71.3 (s), 76.8 (s), 84.0 (s), 87.7 (s), 89.6 (s), 110.6 (s), 113.8 (s), 113.8 (s), 127.7 (s), 128.6 (s), 128.9 (s), 131.1 (s), 131.3 (s), 136.9 (s), 137.5 (s), 137.9 (s), 147.7 (s), 151.5 (s), 159.5 (s), 159.6 (s), 165.2 (s); IR (KBr) cm−1: 3400, 3180, 3060, 3010, 2940, 2860, 2840, 1700, 1610, 1580, 1510, 1460, 1410, 1370, 1280, 1250, 1220; HRMS (MALDI) m/z 621.2571 (Calcd for C 35H38N2NaO7: 621.2577).
1-[(2R,4S,5R,6S)-4-(2-Cyanoethoxy-N,N-diisopropylaminophosphinoxy)-6-(4,4′-dimethoxytrityloxy)-1-oxaspiro[4.5]decan-2-yl]thymine (9)Diisopropylethylamine (0.18 mL, 1.1 mmol) and 2-cyanoethyl N,N-diisopropylchlorophosphoramidite (87 µL, 0.39 mmol) were added to a solution of 8 (157 mg, 0.26 mmol) in dry CH2Cl2 (3 mL) under N2 atmosphere at 0 °C. The reaction mixture was stirred for 2 h at room temperature. After quenching with sat. NaHCO3 aq. at 0 °C, the whole mixture was diluted with CHCl3. Thereafter, the solution was washed with water and brine, dried over Na2SO4, and concentrated in vacuo. The residue (310 mg) was purified via flash column chromatography (n-hexane–AcOEt = 2 : 1) to yield 9 (186 mg, 88%) as a white foam.
1H-NMR (MeCN-d3) δ: 0.58–0.74 (1H, m), 1.03–1.16 (14H, m), 1.36–1.52 (4H, m), 1.56–1.70 (4H, m), 1.71–1.81 (1H, m), 2.35–2.60 (4H, m), 3.00–3.12 (1H, m), 3.50–3.55 (3H, m), 3.75–3.76 (6H, m), 4.77–4.92 (1H, m), 6.09–6.18 (1H, m), 6.84–6.88 (4H, m), 7.19–7.23 (1H, m), 7.26–7.30 (2H, m), 7.34–7.43 (4H, m), 7.48–7.51 (2H, m), 8.22–8.28 (1H, m), 9.71 (1H, s); 31P-NMR (MeCN-d3) δ: 149.0 (s), 149.2 (s); HRMS (MALDI) m/z 821.3732 (Calcd for C44H55N4NaO8P: 821.3655).
Crystal GrowthFor the X-ray crystallography, 6 was crystallized via vapor diffusion, after which it was dissolved in methanol in the inner vial, which was left open. Next, AcOEt was added to the outer vial, which was capped tightly. The vapors of the outer solvent slowly mixed with the inner solvent, and the crystals of 6 (colorless needle crystals) were produced in the inner vial.
X-Ray CrystallographyA suitable crystal of 6 was carefully selected under an optical microscope and glued to thin glass fibers and mounted on the goniometer in liquid N2 flow. The X-ray diffraction data were collected on a Rigaku R-AXIS XtaLAB P100 diffractometer Mo-Kα radiation. The structure was resolved via the direct method with the SIR-2014 program and refined with the SHELXL program. Further, the structural model was drawn with the ORTEP-3 program. Further information on the crystal structure determinations has been deposited with the Cambridge Crystallographic Data Center (2181435). The data can be obtained free of charge via http://www.ccdc.cam. ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk).
Syntheses of the OligonucleotidesPhosphoramidite (9) was dissolved in dry THF to a final concentration of 0.1 M. Phosphoramidite (10), dT-phosphoramidite (Sigma), Bz-dC-phosphoramidite (Sigma), and iBu-dG-phosphoramidite (Sigma) were dissolved in dry MeCN to a final concentration of 0.1 M. The oligonucleotides (ON1–11) were synthesized on a 0.2-µmol scale employing an automated DNA synthesizer (Gene Design nS-8 Oligonucleotides Synthesizer) with a 0.25 M Activator 42® in MeCN as an activator. The modified phosphoramidites (9 and 10) were incorporated into the oligonucleotides at a prolonged coupling time, 10 min (cf. 25 s for the coupling of natural phosphoramidites). The oligonucleotides, which were synthesized in the trityl-on mode, were cleaved from the controlled pore glass (GPG) resin via the treatment with 28% aqueous NH3 for 1.5 h at room temperature. All the protecting groups of the oligonucleotides were removed by treatment with 28% aqueous NH3 for 16 h at 55 °C (for ON1–5) and for 8 h at room temperature (for ON8 and ON 10). NH3 was removed in vacuo. The crude oligonucleotides were purified employing a Sep-Pak® Plus C18 cartridge (Waters, U.S.A.) with the removal of the DMTr group during purification employing 1% (v/v) aqueous trifluoroacetic acid. The separated oligonucleotides were further purified via reversed-phase HPLC (Waters XBridge® OST C18 column 2.5 µm, 10 × 50 mm) employing a 0.1 M triethylammonium acetate (TEAA) buffer (pH = 7.0) and MeCN as the eluent. The compositions of the new oligonucleotides (ON2–4, ON7, and ON8) were confirmed by MALDI-TOF/mass spectrometry.
UV Melting ExperimentsFor the UV melting experiments employing the duplexes that were formed by ON1–5, the oligonucleotides were dissolved in 10 mM sodium phosphate buffer (pH 7.2) containing 100 mM NaCl to obtain a final concentration of 4.0 µM per strand. The samples were annealed at 100 °C, followed by gradual cooling to room temperature. The melting profiles were recorded at 260 nm from 15 to 75 °C at a scan rate of 0.5 °C/min. The two-point average method was employed to obtain the Tm values, and the final values were determined by averaging three independent measurements, which were accurate within a 1 °C range.
Enzymatic Degradation ExperimentsThe enzymatic degradation experiments were performed employing 1.0 µg/mL CAVP, 10 mM MgCl2, 50 mM Tris–HCl (pH 8.0), and 7.5 µM each oligonucleotide (ON6–11) at 37 °C. Thereafter, the cleavage reaction was performed at 37 °C. A portion of each reaction mixture was removed at timed intervals and heated for 5 min at 100 °C to deactivate the phosphodiesterase. Aliquots of the timed samples were analyzed via reversed-phase HPLC [gradient: 5–15% MeCN in TEAA buffer (0.1 M, pH 7.0) for 20 min, flow rate: 1.0 mL/min, column temperature: 50 °C] to evaluate the amount of intact oligonucleotide remaining. The percentage of intact oligonucleotide in each sample was calculated and plotted against the digestion time to obtain the degradation curve in time.
This work was partially supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant Number JP20K15401) and the Japan Agency for Medical Research and Development (AMED) (Grant Numbers JP19am0401003, JP21ae0121022, JP21ae0121023, and JP21ae0121024). We would like to thank Dr. Shuichi Suzuki, Graduate School of Engineering Science, Osaka University for the X-ray diffraction experiment.
The authors declare no conflict of interest.
This article contains supplementary materials.