2022 Volume 70 Issue 5 Pages 330-333
2022 Volume 70 Issue 5 Pages 330-333
Albumin, the most abundant protein in human serum, is applied to various diseases as a drug delivery carrier because of its superior blood retention, high biocompatibility, and a wide variety of drug binding abilities. Albumin is known to distribute widely in the blood and various interstitial fluids and organs. Different albumin receptors skillfully regulate the distribution characteristics of albumin in the body. Albumin receptors are a group of diverse proteins, such as FcRn, gp60, gp18, megalin, cubilin, SPARC, and CD36. Their tissue distributions in vivo are unique, with different albumin’s recognition sites. Therefore, the distribution of albumin in vivo is ingeniously controlled by these multiple albumin receptors. Reevaluation of these albumin receptors opens up new possibilities for applying albumin as a drug delivery carrier. If the tissue distributions of albumin receptors were known and the albumin recognition site of the receptor was identified, organ-specific active targeting would be possible. In this review, we would like to scrutinize what is currently known and share information to develop next-generation albumin carriers that focus on interactions with albumin receptors.
Human serum albumin (HSA) is present in human serum at a concentration of about 40 mg/mL, is a 66.5 kDa molecular weight serum protein and has a long half-life in the blood of about 20 d. Due to its nontoxic, nonimmunogenic and biocompatible properties, albumin has become a popular drug carrier, especially for biological proteins with short half-lives such as interferon-alpha and growth hormone via genetic fusion.1) Therefore, albumin has great potential for use as a superior biomaterial for drug delivery. Many researchers have developed new drug formulations and drug delivery systems using albumin.2–4)
However, albumin undergoes various post-translational modifications, such as oxidization and glycation in the circulating blood due to its prolonged plasma half-life. Some of these modifications have been reported to induce adverse effects such as oxidative stress or non-enzymatic glycation on vascular endothelial cells.5) To protect the living cells from these adverse effects, vascular endothelial cells possess a surveillance system comprising an albumin receptor called FcRn. This system uptakes all albumin into vascular endothelial cells via macropinocytosis, normal albumin is returned to the blood, and lysosomes directly degrade severely modified albumin.6)
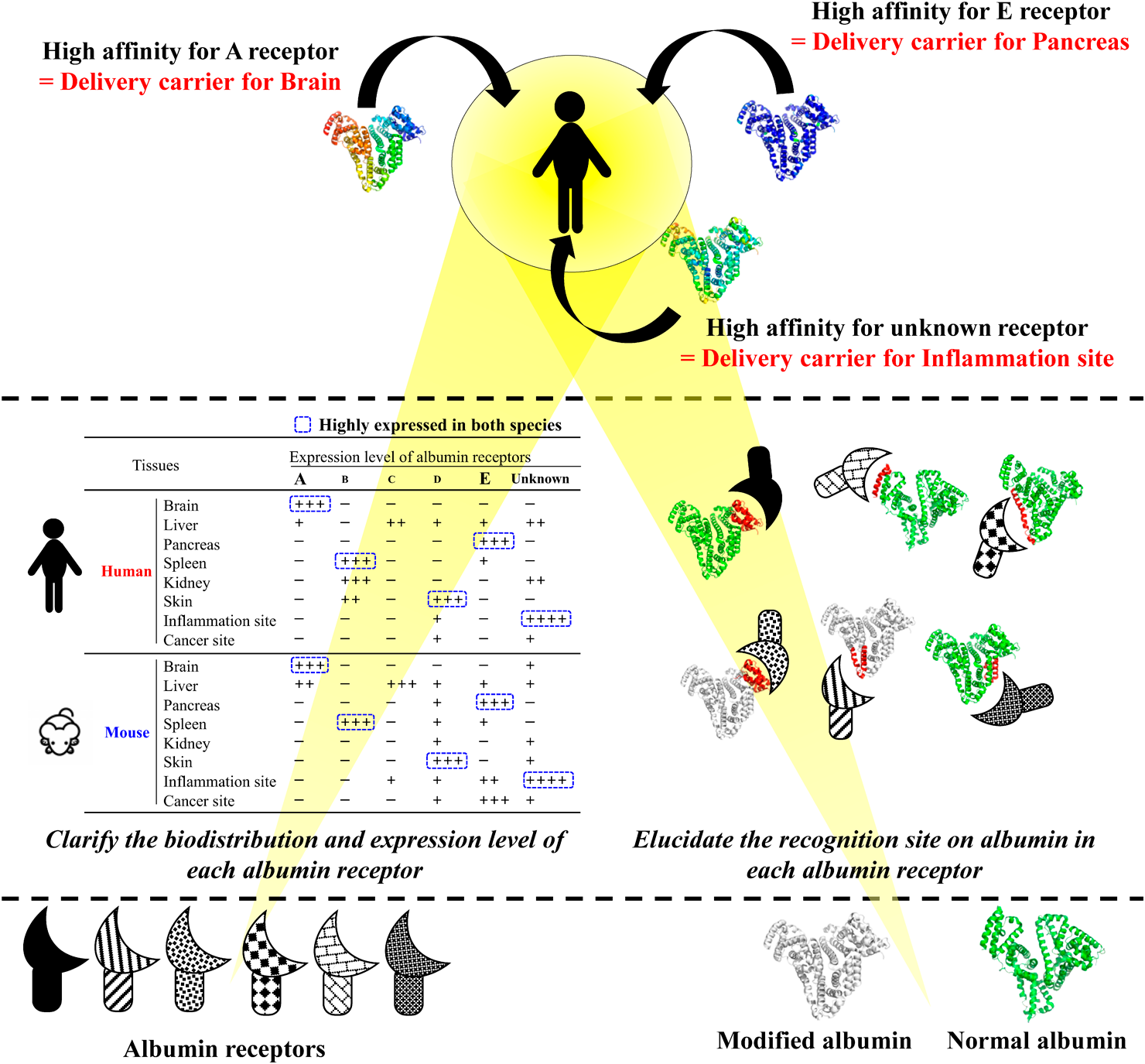
Furthermore, CD36, one of the scavenger receptors, recognizes oxidized and glycated albumin and excretes it into the urine.7) Although the detailed mechanisms are still unknown, albumin in the central bone marrow has been reported to be in an exceptionally highly reduced form. This observation suggests the existence of a novel albumin receptor that recognizes high-quality albumin and takes up albumin into the bone marrow.8) Table 1 summarizes the types of albumin receptors, their expression sites, and their substrate specificities, indicating that a wide variety of albumin receptors exist in various tissues and delicately control the biodistribution of albumin (Table 1).
| Receptor/protein | Tissue/cell | Type of albumin |
|---|---|---|
| gp60 | Endothelial cells | Normal |
| gp18 and gp30 | Endothelium, macrophages, some cancer cell surfaces | Modified |
| CD36 | Macrophages, kidneys | Modified |
| SPARC | Endothelial cells, skeletal muscle, tumor cells | Normal and modified |
| hnRNP | Breast cancer, melanoma cells | Normal |
| Calreticulin | Breast cancer, melanoma cells | Normal |
| FcRn | Endothelium, gut, liver, kidneys, lungs | Normal |
| Cubilin | Small intestine, placenta, kidneys | Normal and modified |
| Megalin | Thyrocytes, choroid plexus, proximal tubule cells | Normal and modified |
The most well-known albumin receptor, gp60 (also called Albondin), is widely expressed in the vascular endothelial cells except in the brain and participates in albumin’s internalization and transcytosis.9–15) The internalization and transcytosis of albumin by gp60 were induced via a caveolin-dependent pathway.16–19)
Macrophages and fibroblasts express gp18 and gp30 that strongly bind modified albumins, like maleic- or formaldehyde-modified albumins, than native or non-modified albumin.11,13,14,20,21) Albumin could be modified by oxidative stress and non-enzymatic glycation during normal aging or some diseases.16,22) The elimination of modified (or denatured) albumins from blood circulation occur more rapidly and more efficiently than native albumin, suggesting that modified albumin could be picked up by these albumin receptors from the pool of native albumins.22)
SPARC (also called osteonectin) is secreted by several cancer cells and is highly distributed in stromal area.23–25) SPARC specifically interacts with native albumin similarly to albondin, but modified albumin can also bind to SPARC.20) SPARC in the tumour stroma seems to enhance albumin accumulation within the tumour tissue.26,27) A preliminary clinical trial reported that SPARC-positive patients had a better clinical outcome, the response to paclitaxel-loaded albumin nanoparticle (Abraxane®) treatment positively correlated with SPARC expression.27) However, a phase III study demonstrated a negative relationship between SPARC expression and the response to Abraxane®. Consequently, further clinical and basic studies are needed to validate this relationship.
Some albumin-binding proteins have been found in human plasma membranes of cancer cells such as melanoma and breast carcinoma. These proteins were identified as the heterogeneous nuclear ribonucleoproteins (hnRNP) A1, A2/B1, A3 and C1, and calreticulin.28) Calreticulin functions as a chaperone in the endoplasmic reticulum, a modulator of apoptosis, and a mediator of platelet-collagen interactions.29) Although hnRNPs are overexpressed in the tumour region and used as biomarkers for the early detection of tumours,30) their functions are still unclear. It was not clear whether calreticulin and hnRNPs participate in albumin-mediated uptake in the tumour region.
FcRn is widely expressed in numerous tissues, including the gut, liver, kidneys, and lungs.31) FcRn protects albumin from degradation by interacting with albumin in endosomes (pH <6.5) in a pH-dependent manner, recycling albumin to the extracellular space (pH 7.4), thereby extending the half-life of serum albumin.32–36)
Cubilin is expressed in the small intestine, placenta and kidneys,37) involved in vitamin B12 absorption.38) Additionally, cubilin participates in the transcytosis of many ligands, including albumin.39) Megalin is mainly expressed in the thyrocytes, choroid plexus, proximal tubule cells, more widely distributed than cubilin.37) Cubilin strongly binds to megalin, resulting in that megalin contributes to the internalization of ligand complexes as a co-receptor.40) In particular, cubilin and megalin have an essential role in the reabsorption of albumin via the uptake by the kidneys.41,42)
Taken together, we believe that the production of albumin with a controlled affinity for these albumin receptors is an extremely attractive research approach that expands the potential of albumin as a drug delivery system (DDS) carrier.
Bern et al. produced albumin mutants with enhanced binding affinity for FcRn and succeeded in extended blood retention of FcRn.43) X-ray crystallographic analysis of the albumin-FcRn complex found that the interaction site was the C-terminal of HSA. Therefore, the binding affinity with FcRn using C-terminal mutants and albumin variants was evaluated and succeeded in producing E505Q/T527M/K573P mutants that bind more strongly to FcRn. The E505Q/T527M/K573P mutants have an extended half-life in animal models.43) In addition, Zhang et al. found that palmitic acid-modified bovine serum albumin (PAB) has significant scavenger receptor-A (SR-A) targeting ability in vitro and in vivo. Using this PAB carrier, they have succeeded in developing an efficient treatment of rheumatoid arthritis.44)
We recently reported novel evidence for the delivery of Abraxane.45) Abraxane is an anticancer preparation using albumin as a solubilizing agent for paclitaxel (PTX) and behaves as albumin-bound PTX after administration. Since albumin is a high molecular weight protein, it is subject to enhanced permeability and retention effect (EPR). In addition, albumin is actively delivered to tumour cells by albumin receptors. Specifically, the transcytosis mechanism mediated by Gp60 on the surface of vascular endothelial cells and the PTX uptake mechanism of cancer cells mediated by SPARC in the tumour stroma is well-known. Another paper showed that cancer cells induce albumin uptake as an amino acid source.46) We call this phenomenon the “Endogenous Albumin Transport (EAT) System.”47) More importantly, the expression of Gp60 and SPARC is high in intractable pancreatic cancer with poor blood vessel density, so EAT System is more likely to be activated in intractable cancers such as pancreatic cancer.
However, there is a major concern about whether carrier-mediated transport using HSA receptors would occur in the presence of large amounts of endogenous albumin (about 40 mg/mL). At standard doses, the Cmax of intravenously administered Abraxane is about 20 µg/mL for PTX and 0.16 mg/mL for albumin. The resulting albumin concentration was about 0.4% of endogenous albumin, sufficient for the competitive inhibition. To investigate this concern, we have performed basic studies with a conclusion shown in Fig. 1. Our data indicated that Abraxane-derived HSA was taken up into endothelial cells or tumour cells via a mechanism different from normal endogenous albumin.45,47) This result suggested that modified HSA underwent transcytosis on vascular endothelial cells without competing with endogenous albumin. These novel pieces of evidence shed light on the mechanisms of tumour delivery of Abraxane and provide a foundation for the development of a novel albumin drug delivery strategy via albumin receptors (Fig. 1).

Various sites of albumin molecules may be diversely modified by numerous factors such as oxidation or/and glycation. For instance, we recently found that some extra sulphur atoms are attached to the S–S bond of albumin (S–Sn–S, n > 1),48) resulting in the formation of polysulfide bridges. These excess sulphurs, called super sulphur species, are essential for the antioxidant activity and drug binding properties of albumin and are regarded as one of the criteria of the “quality” of albumin. The active sulphur of albumin decreases in the pathological conditions of oxidative stress. This active sulphur-mediated change in the “quality” of albumin can contribute to the change in affinity with the albumin receptor. In the near future, when the distribution of albumin receptors in the whole body, their expression patterns during pathological conditions, and the interaction characteristics between albumin receptors and albumins in various conditions such as modified (mutated) are clarified, a new horizon would be opened for the development of the next generation of albumin DDS strategy involving interaction with albumin receptors (Fig. 2).
This work was supported, in part, by Grants-in-Aid from the Japan Society for the Promotion of Science (JSPS), a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology (KAKENHI KIBAN (B) 21H02645), Japan.
The authors declare no conflict of interest.