2022 Volume 70 Issue 9 Pages 628-636
2022 Volume 70 Issue 9 Pages 628-636
Mini-tablets (MTs) contain a small amount of active pharmaceutical ingredients in one small tablet. MTs are advantageous because they can be fine-tuned according to the age and weight of pediatric patients and they are easy for children and the elderly to swallow. However, there are manufacturing concerns such as the difficulty in achieving both hardness and disintegration of a small tablet and it is difficult to keep the tablet weight and drug content consistent in MTs because the mold used for its production is special. In this study, we aimed to determine if an additive such as cellulose nanofibers (CNF), which has been studied in various fields in recent years, could be used to manufacture MTs without difficulties. In this study, an MT was manufactured using a rotary tableting press with a compression force of 2, 5, and 8 kN, and the weight variation, drug content variation, tensile strength, friability, disintegration time, and drug dissolution were evaluated. Of note, the tensile strength of MTs produced with a compression force of ≥5 kN was ≥1.3 MPa, which was comparable to that of an ordinary tablet with an 8 mm diameter and a hardness of ≥30 N. The disintegration time of the MT which was 20–30% CNF was ≤30 s at any compression force. MTs with CNF showed similar disintegration to MTs with other common disintegrants. Therefore, we found that CNF is a functional additive capable of manufacturing MTs by direct powder compression which has both strength and disintegration.
Compared to other dosage forms, tablets have various advantages such as being accurate in dosage, easy to carry, masking drug bitterness, and they can be modified to impart various functions such as sustained release and enteric properties.1) Such highly convenient tablets are manufactured by automatic operations, using a high-speed tableting press in many pharmaceutical companies to meet the high demand from clinical sites and markets. Therefore, the quality of tablets requires a high level of robustness that can be met in various manufacturing environments.2–4)
The tablet manufacturing methods include direct powder compression and granule compression, and further, granule compression includes a wet and dry method. Wet granule compression is most pharmaceutical companies’ first choice for obtaining high levels of drug content uniformity.5–7) This method makes it possible to adjust the hardness, disintegration, and dissolution of the final tablet by changing the granulation conditions.8,9) However, many processes are required to prepare granules by this method, and the number of variable factors increases depending on the number of processes. Therefore, the drawbacks of this method include difficulty in setting manufacturing conditions, and it takes time and money.10) In contrast, direct powder compression has fewer steps than the wet granule compression method, and it is relatively easy to set the manufacturing conditions. In addition, since the power energy, time, equipment, etc. required for the granulation process are not required, it leads to cost reduction and an increase in manufacturing efficiency. Because of these advantages, direct powder compression is a tablet manufacturing method that has been attracting attention in recent years and is used as the first choice in the United States.11) However, the pharmaceutical powder obtained by simply mixing the active pharmaceutical ingredient and additives is directly put into the tableting press and continuously compressed at high speed, and thus, the weight and the drug content variations tend to increase. This process also causes tableting damage often. Currently, research is being conducted to solve these problems. The formulation and manufacturing conditions are not the only factors being examined, as equipment improvements and the development of new additives are being carried out.12–19)
Orally disintegrating tablets (ODTs) were introduced in the 1990s, and are tablets that are more convenient for patients to take, and various types are now on the market.20–22) Since ODTs rapidly disintegrate and change shape due to saliva and water in the oral cavity. Thus, ODTs are easy for elderly people with reduced swallowing ability and children with a narrow pharynx to take. Further, even when many tablets are taken at the same time, the shape of the tablets is lost in the oral cavity, so that troubles such as aspiration can be avoided. Furthermore, ODTs can be taken by patients with restricted water intake such as those on dialysis and with an overactive bladder, and can be taken for sudden diarrhea and motion sickness. Accordingly, ODTs are a dosage form that is more patient-centric than ordinary tablets and it is expected to improve medication adherence by improving the QOL for many patients.23,24) For this reason, public demand for ODTs has increased, and many pharmaceutical companies have focused on adding ODT dosage forms to existing products.25–27) The ODT must be strong enough to withstand normal transport and handling such as dispensing and have excellent disintegration. In the past, special manufacturing methods and equipment were used for ODTs that required such trade-off properties. However, since then, attention has been focused on the development of new additives that have both binding and disintegration properties.17,18,28)
Tablets have problems other than the ease of administration. One such problem is that the dose of the active pharmaceutical ingredient to be prescribed cannot be finely adjusted. Unlike adults, children are highly sensitive to drugs and their dosages are calculated in detail based on their age and weight. However, with ordinary tablets, it is not possible to count the amount of drug corresponding to the dose, and it may be difficult to dispense according to pediatric patients. Therefore, to avoid these problems, mini-tablet (MTs), which have a smaller tablet size than ordinary tablets, have been attracting attention in recent years.29–31) Since the amount of active pharmaceutical ingredient in one MT is small, it is possible to adjust the number of tablets according to pediatric patients. In addition, the small tablet size makes it easy for elderly people to swallow, and it is expected to be advantageous for patients of other age groups as well. However, since the mold used for manufacturing MTs is small, it is difficult to maintain the tablet weight and drug content. Moreover, the small size makes it difficult to achieve both hardness and disintegration. Therefore, various studies on MT manufacturing are still underway.32–35)
Cellulose, which is the main component of the cell wall of plants, is a naturally abundant carbohydrate (polysaccharide), and cellulose fibers that are made from it have been used in paper and cotton for a long time. In recent years, cellulose nanofibers (CNFs), which are miniaturized cellulose fibers, have been attracting attention from various fields as a state-of-the-art, high-performance, biomass material.36) CNFs have a large specific surface area due to the fine fiber structure and have excellent properties such as high strength, high water retention, high elasticity, and high viscosity, while also being lightweight. Furthermore, since the CNF is a renewable resource that also has properties such as biodegradability, biocompatibility, and resistance to organic solvents, a wide range of research and applications are underway. In Japan, the Ministry of the Environment is promoting a CNF performance evaluation model aimed at reducing CO2.37) Currently tires, food thickeners, disposable diapers, etc. containing CNF have been put into practical use and their use in home appliances, building materials, transportation equipment, etc. is being considered as a further application of CNF. In the medical field, research on CNF application for wound treatment, bone graft scaffolding materials, artificial organs, and functional contact lenses is underway.38,39) In the pharmaceutical industry, CNFs are expected to be applied as drug delivery carriers, drug release control materials, and to improve the solubility of poorly soluble drugs by taking advantage of the enormous specific surface area derived from its characteristic structure.40–46) However, CNF is a new substance with few examples as a pharmaceutical additive, and thus, its effects upon addition to the pharmaceutical powder for tablet manufacturing are not fully understood.
In this study, the effects of the CNF concentration and the manufacturing conditions on the physical properties of MTs were evaluated by manufacturing MTs using the direct powder compression method. In addition, by optimizing the manufacturing conditions, the conditions for producing an orally disintegrating MT (OD-MT) with physical properties that can withstand general handling were examined. Finally, the usefulness of CNFs as a functional additive for MT production was evaluated.
Lactose hydrate (Lac, diluent, Tablettose® 80, Meggle, Wasserburg, Germany), CNF (ELLEX-P (alcohol-containing type), disintegrant, Daio Paper Corp., Tokyo, Japan), acetaminophen (APAP, model drug, Tyco Healthcare Japan, Tokyo, Japan), and Mg-St (vegetable-based, lubricant, FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) were used for preparing the pharmaceutical powder. Microcrystalline cellulose (MCC, CEOLUS® KG-1000, Asahi Kasei Corp., Tokyo, Japan), low-substituted hydroxypropyl cellulose (L-HPC, LH-21, Shin-Etsu Chemical Co., Ltd., Tokyo, Japan), crospovidone (CP, Kollidon® CL, BASF, Ludwigshafen, Germany), and croscarmellose sodium (CCS, KICCOLATE™ ND-2HS, Asahi Kasei Corp.) are commonly used additives, which were used as reference substances for CNF.
Preparation of the Pharmaceutical PowderThe pharmaceutical powder used for manufacturing the MTs was prepared using a V-type mixer (DV-5, Dalton, Tokyo, Japan). A total of 300 g of the powder was charged into the apparatus. The powder was premixed with Lac, CNF, and APAP (quantities in Table 1) for 10 min. The Mg-St was subsequently added followed by mixing for another 5 min. The obtained mixture was used as the pharmaceutical powder.
| Material | F1 | F2 | F3 | F4 | F5 | F6 |
|---|---|---|---|---|---|---|
| Lac | 94.5 | 84.5 | 74.5 | 64.5 | 54.5 | 44.5 |
| CNF | 0 | 10 | 20 | 30 | 40 | 50 |
| APAP | 5 | 5 | 5 | 5 | 5 | 5 |
| Mg-St | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
F: formulation number; Lac: lactose hydrate; CNF: cellulose nanofiber; APAP: acetaminophen; Mg-St: magnesium stearate.
The angle of repose was measured using the powder tester (PT-S, Hosokawa Micron Corp., Osaka, Japan) at 25 ± 2 °C and a relative humidity of 35 ± 10%. The pharmaceutical powder, which was previously passed through No. 22 sieve, was freely dropped onto a round table, and the powder heap that formed was photographed using a built-in charge-coupled device (CCD) camera. The angle of repose of the pharmaceutical powder was directly measured using the analysis function of the powder tester.
Manufacturing of MTs by Continuous TabletingThe MTs were manufactured on a rotary tableting press (VELA 5, Kikusui Seisakusho, Kyoto, Japan) by continuous tableting using direct powder compression method. The pharmaceutical powder was supplied via a gravity feeder and then compressed using a flat-face multiple-tip tooling (3 mm-diameter, 3 tips per die, Ichihashi Seiki Co., Ltd., Kyoto, Japan; Fig. 1) attached at one station on the turntable (10 rpm, compression force; 2/5/8 kN). The target weight of the MTs was 30 mg and they were collected after 2 min of tableting (Fig. 1).
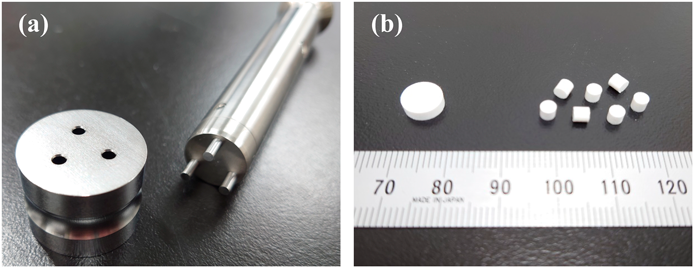
(a) This panel shows a set of punch and die, and (b) shows ordinary tablets (left) and MTs (right) containing equal amounts of active pharmaceutical ingredients by the same formulation.
Physical properties of MTs that were determined after storing in a desiccator for ≥24 h after tableting.
Weight variation was evaluated by weighing 10 randomly selected MTs on an electronic balance (TE214S, Sartorius, Göttingen, Germany) and calculating the coefficient of variation (% CV) of tablet weight.
The hardness of MTs was measured for 10 tablets per formulation using a load-cell type hardness tester (PC-30, Okada Seiko Co., Ltd., Tokyo, Japan). Tensile strength was calculated from hardness value using the formula:
 |
Friability was evaluated using a friability tester (TFT-120, Toyama Sangyo Co., Osaka, Japan). Thirty-five MTs (≅ approximately 1 g) were weighed after brushing them to remove adhered fine powder, and then placed in the friability tester with 5.5 g of 3 mm glass beads, and rotated 100 × at 25 ± 1 rpm. Whole MTs were reweighed after removing the attached fine powder generated during the test. Friability was calculated based on the weight reduction.
Disintegration time was measured using a disintegration tester (NT-1HM, Toyama Sangyo Co.) with distilled water at 37 ± 2 °C as the testing medium. One MT was placed inside a glass tube in the testing basket. For each MT formulation, disintegration time was measured six times, and their mean value was calculated.
Evaluation of Drug Content VariationDrug content variation was determined using 10 randomly selected MTs. One MT was vortexed (IMS-1000, Tokyo Rikakikai Co., Ltd., Tokyo, Japan) in a test tube for 5 min and sonicated in an ultrasonic bath (8510J-DTH, Branson, Danbury, CT, U.S.A.) for 1 h with 10 mL distilled water to completely dissolve the drug. The solution was then centrifuged for 5 min at 1600 × g at 20 °C (CF16RX II, Hitachi Koki Co., Ltd., Tokyo, Japan). The supernatant was passed through a 0.45 µm syringe filter (Millex HA, Merck Millipore Corp., Darmstadt, Germany) and diluted 50-fold with distilled water. The absorbance of the diluted solution was measured at 237.5 nm using an UV spectrophotometer (U-1900, Hitachi High-Technologies Corp., Tokyo, Japan) to determine the APAP weight per MT. To eliminate variation due to MT weight, the APAP content was corrected by considering weight of one MT to be exactly 30 mg. The % CV of the drug weight obtained was considered as the drug content variation. Acceptance value was calculated according to the method listed in the 18th Japanese Pharmacopoeia.47,48)
Evaluation of APAP DissolutionDissolution of APAP from the MT was evaluated by a dissolution test, using the paddle method (50 rpm) at 37 ± 0.5 °C. One MT containing 1.5 mg of APAP was used in a flow-cell system (dissolution tester, NTR-1000, Toyama Sangyo Co.; UV-absorption photometer, U-1900, Hitachi High-Technologies Corp.), and the absorbance was measured at a wavelength of 237.5 nm to determine the dissolution of APAP over time. To determine the APAP dissolution rate, the amount of APAP in the test medium was converted to a percentage, based on the prescribed APAP content (set at 100%). The similarity of the dissolution profile was evaluated by the similarity factor (f2), calculated as follows:
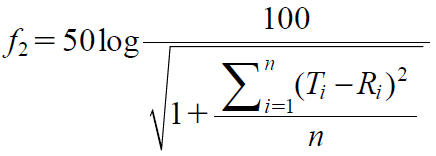 |
where n is the number of time points, Ti and Ri are the test and the reference sample amounts dissolved at each time point i, respectively. When f2 ≥ 50, it was determined that the two dissolution behaviors compared were equivalent.49–51)
Measurement of MT PorosityThe true density of CNF or the reference substances was measured by an automatic pycnometer (Ultrapyc1200e, Quantachrome, Boynton Beach, FL, U.S.A.) using helium gas. Tablets composed of only CNF or reference substances (200 mg/tablet) were prepared using a tabletop tableting press (Handtab-100, Ichihashi Seiki Co., Ltd.) fitted with an 8 mm diameter flat-face punch. Tablets of all substances were prepared by applying various compression forces for 5 s. After storage in a desiccator for >24 h, the tablet diameter and thickness were measured using a digital micrometer (PK-1025, Mitutoyo Corp., Kanagawa, Japan). Subsequently, the porosity of the tablet was calculated from volume of the tablet and true density of each substance.
Observation of CNF ShapeRaw CNF powder or its tablets were fixed using a conductive double-sided carbon tape on an aluminum stub, and an ion sputtering apparatus (E-1010, Hitachi High-Technologies Corp.) was used for platinum deposition for 10 s using 15 mA of discharge current under 10 Pa vacuum. Particle shape and surface morphology were observed with scanning electron microscopy (SEM, S-3400N, Hitachi High-Technologies Corp.) in the high vacuum mode with an acceleration voltage of 5 kV.
Preservation of Moisture Absorption in CNF TabletsTablets (200 mg/tablet) composed of only CNF or reference substances were prepared using a tabletop tableting press (Handtab-100, Ichihashi Seiki Co., Ltd.) with a compression force of 10 kN for 5 s. Tablets were then dried for ≥24 h by placing in a desiccator over saturated potassium chloride solution. Thereafter, the tablets were stored for 48 h at 20 °C and relative humidity of 85%, as per Japanese Industrial Standards (regulation No. JIS B7920). After storage under high humidity conditions, some of the tablets were then vacuum dried for 24 h in a desiccator vacuumed with a diaphragm pump (DA-41D, ULVAC, Inc., Kanagawa, Japan). The amount of water in the recovered tablet was measured using a moisture meter (MB120, OHAUS, Parsippany, NJ, U.S.A.) by heating for 30 min.
Optimization of OD-MT ManufacturingContour plots were developed using Minitab 21 (Minitab, LLC, Pine Hall Rd State College, PA, U.S.A.) to determine correlations between CNF concentration, compression force, and physical properties of the OD-MT for optimization of the manufacturing conditions.
Statistical AnalysisAll data are expressed as the mean ± standard deviation (S.D.). Statistical analysis was performed using the Kruskal–Wallis test or the Mann–Whitney U test, using Minitab 21. p < 0.01 or p < 0.05 were the criteria for statistical significance. Correlational analysis was performed using Excel Multivariate Analysis V7 (Esumi Co., Ltd., Tokyo, Japan). Correlations among factors were determined based on the coefficient of determination (R2) that was calculated in relation to the correlation coefficient (R), where R2 ≥ 0.49 (|R| ≥ 0.7) indicates high correlation, while 0.49 > R2 ≥ 0.16 (0.7 > |R| ≥ 0.4) indicates moderate correlation.52)
The CNF particles had an irregular and distorted flat plate shape, and pores with a diameter of <1 µm were present on the particle surface, which is consistent with previous reports.53) The average particle size was 290.75 ± 13.03 µm, and its bulky nature was inferred from the small tap density value (0.36 ± 0.001 g/cm3). The angle of repose was 49.7 ± 0.265° and the compressibility was 27.8 ± 0.29%; it was classified as “not very flow (poor),” according to Carr’s flowability index.54) The amount of water retained by CNF was 68.31 ± 0.87% (w/w). As such, CNF could retain a water weight that was about twice its weight.
Manufacturing of MTsMTs, which have a smaller weight than ordinary tablets, also have a lower drug content per tablet. Therefore, many MTs are necessary to obtain the same medicinal effect as ordinary tablets. Figure 1b shows the shape of an ordinary tablet with an 8 mm diameter (and 3 mm thickness) and an MT with a 3 mm diameter (and 3 mm thickness) containing the same amount of APAP, prepared according to F1 in Table 1. The tablet weight used in this study is 200 mg for ordinary tablets and 30 mg for MTs. Therefore, seven MTs will contain almost the same amount of APAP that is in one ordinary tablet. By changing one ordinary tablet to seven MTs, the specific surface area of the tablets will increase about 1.6 times, so we expected that the disintegration and the dissolution of the active pharmaceutical ingredient would be improved. Therefore, we evaluated the physical properties of MTs to which various amounts of CNF were added.
The Effect of CNF Addition on the Physical Properties of MTsFigure 2 shows the relationship between the angle of repose of pharmaceutical powders with different CNF concentrations and the weight variations, along with drug content variations in the tablets manufactured via continuous tableting. During continuous manufacturing of MTs using a rotary tableting press, the flowability of the pharmaceutical powder is an important factor for smooth powder supply to the die. Therefore, MTs of each formulation was manufactured. The weight variation, drug content variation, and the acceptance value of the drug content were obtained for each tablet, and the relationship with the angle of repose, which is an index of the flowability of the pharmaceutical powder, was evaluated.
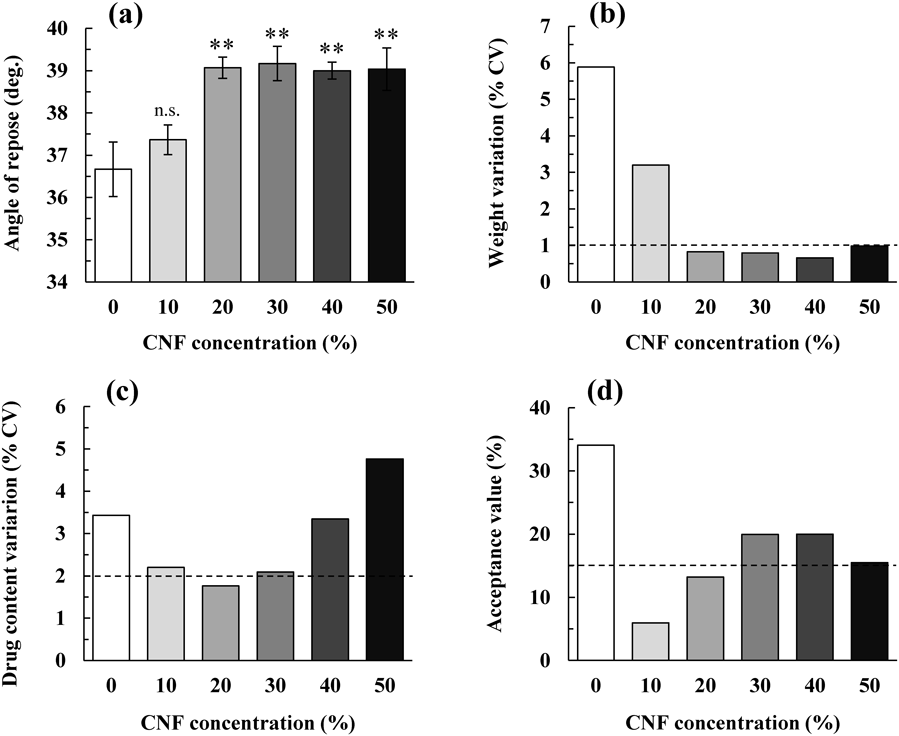
(a) Differences between the angle of repose of pharmaceutical powders were assessed from 0% CNF. n.s.: not significant, **: p < 0.05. Data are expressed as the mean ± S.D. (n = 3). (b) Weight variation (n = 10), (c) drug content variation (n = 10), and (d) acceptance value. The dotted line in the panels indicates the target value.
The angle of repose of the pharmaceutical powder increased as the CNF concentration increased in the range of ≤20% CNF, but it remained almost constant at >20% CNF (Fig. 2a). Since CNF is a bulky particle with large internal voids, it was presumed that electrostatic adhesion with other additive particles was generated, and the flowability under unloaded conditions was reduced.
As shown in Fig. 2b, the weight variation of MT was reduced upon the addition of CNF, and the target weight variation of ≤1% was obtained by adding ≥20% CNF. The weight variation of tablets showed a strong negative correlation with the angle of repose (R2 = 0.95). When CNF was added, the weight variation of the tablets became smaller, even though the flowability of the pharmaceutical powder was low. It was hypothesized to be because other additives with a large particle size electrostatically attached to the CNF and moved together so that a certain amount of powder was filled in the small holes of the die that was used for MT manufacture.
When CNF was not added, the drug content variation and acceptance value were very large (Figs. 2c, d). When the CNF concentration was 10–30%, the drug content variation was about 2%, and notably, when the CNF concentration was 20%, it was <2% (Fig. 2c). As shown in Fig. 2d, a CNF concentration of 10–20% was necessary to reach an acceptance value of ≤15%. There was almost no correlation between the drug content variation (R2 < 0.01) or the acceptance value (R2 = 0.12) and the angle of repose. The detailed mechanism by which the addition of CNF improves drug content uniformity has not yet been elucidated and was left for future research. However, it was speculated that the drug content uniformity could be ensured, even in the low drug content formulation, by adding about 10–20% CNF to the pharmaceutical powder.
Figure 3 shows the effect of the addition of CNF on the tensile strength and friability of MTs. Assuming a tablet of 8 mm (diameter) × 3 mm (thickness) as an ordinary tablet, the tensile strength of a tablet with a hardness of 30–80N was 1.33–3.62 MPa. Therefore, to obtain the same strength as an ordinary tablet, this value of tensile strength was set as a target for MT manufacturing. When the compression force was 2 kN, the tensile strength was <1.33 MPa regardless of the CNF concentration. However, with a compression force of 5 or 8 kN, tablets with a tensile strength of 1.33 to 3.62 MPa could be manufactured. At this time, even if the CNF concentration was altered, no significant change was observed in the tensile strength compared to the tablet with no CNF added (p ≥ 0.01). In contrast, the friability of MTs manufactured with a compression force of 2 kN showed a large value of >1% at any CNF concentration. However, the friability when compressed at 5 or 8 kN was approximately ≤1%. In this case, no significant difference was observed due to alterations in the CNF concentration (p ≥ 0.01). It was expected that when CNF was added to the pharmaceutical powder, these particles would be deformed or broken due to the high compression force during tableting, and the CNF nanofibers would form a network structure throughout the MT, thus increasing the tablet strength. However, since CNF having a large particle size (particle size of approximately 300 µm) with a large ratio for the tablet size (diameter of 3 mm) was used in this experiment, it is presumed that it was difficult to disperse the CNF in the MT completely and uniformly. Therefore, the tablet strength did not increase remarkably with the change in the addition amount, and no significant differences were observed in the tablet strength.
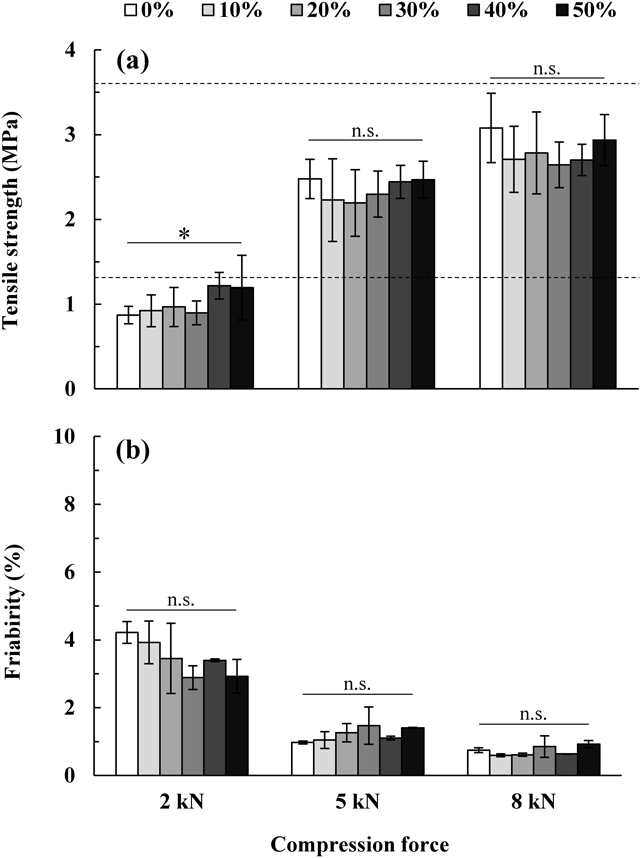
Panel (a) shows tensile strength of n = 10, and (b) shows friability of n = 3. Statistical analysis for each compression force was performed using the Kruskal–Wallis test. n.s.: not significant, *: p < 0.01. Data are expressed as the mean ± S.D.
Figure 4 shows the relationship between the CNF concentration and the disintegration time of MTs. When CNF was not added, MTs had a disintegration time of ≥3 min at any compression force. The addition of ≤20% CNF decreased the disintegration time at any compression force, but a further increase in the CNF concentration increased the disintegration time (p < 0.01). The improvement in disintegration with the addition of <20% CNF was considered to be because the CNF, which was deformed or broken by compression, formed a narrow path through which water passed inside the tablet. However, when >20% CNF was added, excessive CNF may have formed a strong three-dimensional network structure inside the MT, resulting in delayed disintegration. Formulations with 20–30% CNF showed disintegration at ≤30 s at any compression force. It was speculated that the excellent disintegration properties of CNF were due to the nano-sized fibers possessed by CNF, that form fine headraces inside the tablet.
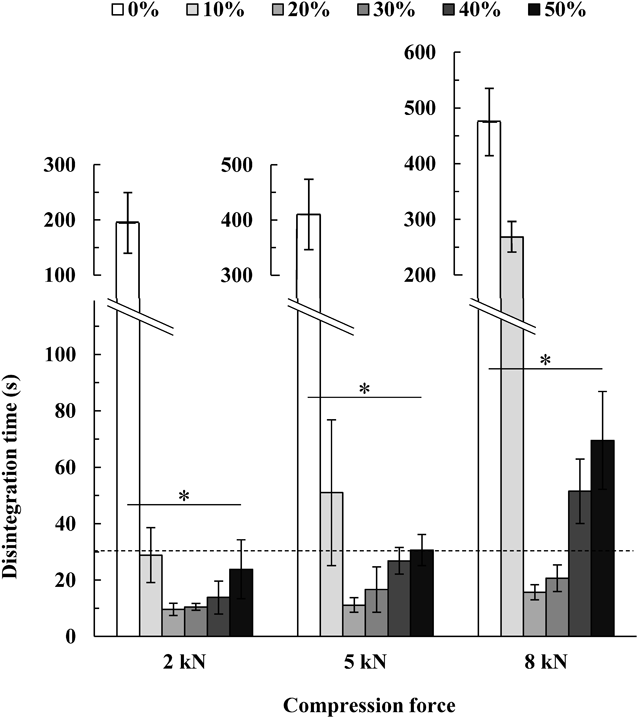
Statistical analysis was performed for each compression force using the Kruskal–Wallis test. *: p < 0.01. Data are expressed as the mean ± S.D. (n = 6).
Figure 5 shows the change in the APAP release profile of MTs with different CNF concentrations. The rate of APAP dissolution from MTs increased with increasing CNF concentration within the range of ≤20% CNF. However, the dissolution behavior of MTs containing ≥20% CNF was not significantly different from that of MTs containing 20% CNF at any compression force.

The compression forces used were (a) 2 kN, (b) 5 kN, and (c) 8 kN. Data are expressed as the mean ± S.D. (n = 3).
The similarity of APAP dissolution profiles from MTs of other formulations was evaluated by calculating the similarity factor, f2, based on MTs containing 20% CNF (Table 2). An f2 of ≤30% CNF and 20% CNF were each f2 ≥ 50, indicating that their dissolution profiles were similar.49–51) From the above, it was inferred that the optimum value of CNF concentration was about 20% and that excessive addition of CNF deteriorated the properties of MTs. The reason why the optimum value was 20% is not clear, but it is presumed that the particle size and the porosity in the CNF particles contribute, so we would like to focus on these points in the future.
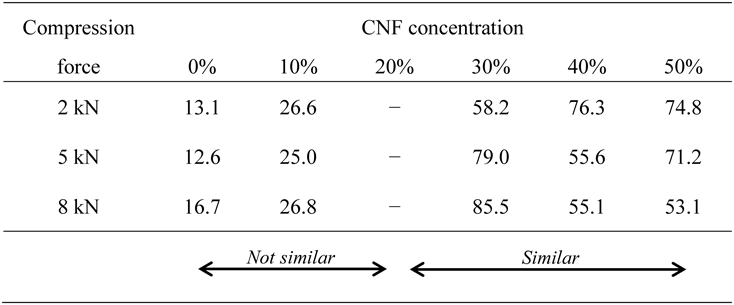 |
CNF: cellulose nanofiber; APAP: acetaminophen; MT: mini-tablet. These data represent n = 3.
MTs were manufactured by adding L-HPC, CP, CCS, and MCC, which are commonly used as disintegrants or binders, instead of CNF. Their average particle sizes were CNF: 290.75 ± 13.03 µm, L-HPC: 49.55 ± 1.07 µm, CP: 62.44 ± 2.80 µm, CCS: 45.89 ± 1.59 µm, and MCC: 52.77 ± 1.01 µm.
Figure 6 shows the tensile strength of MTs containing 20% of reference substances. The hardness of MTs to which L-HPC, CP, or CCS were added could not be measured because the strength was insufficient when the compression force was 2 kN. To obtain the target tensile strength of these MTs, it was necessary to compress the tablets at 8 kN. In contrast, MTs with CNF added reached the target tensile strength at a compression force of 5 kN. In brief, it was shown that CNF has a higher binding ability than the additives that are commonly used as a disintegrant, although it is not as good as MCC when used as a binder.

The content of CNF or reference substances was 20%, and the weight of the MTs was 30 mg. Statistical analysis of CNF-containing MTs and reference substance-containing MTs at each compression force was performed using the Mann–Whitney U test. n.d.: no tableting, *: p < 0.01. Data are expressed as the mean ± S.D. (n = 10).
To clarify the reason why the tensile strength of MTs with CNF added was higher than that of other disintegrants, model tablets consisting of each pure substance were prepared with various compression forces, and the porosity of the tablets was calculated (Fig. 7). The model tablets with CNF had a smaller porosity than the tablets with reference substances at any compression force. In addition, the porosity of the CNF tablets became about 10–15% with a compression force of 5 kN, and no change in the porosity was observed even when the compression force was further increased. In brief, it was inferred that the plastic deformation was sufficient, even with a low compression force of 5 kN. Figure 8 shows the shape of the CNF particles and the surface morphology of the model tablet with CNF compressed at 5 kN. The distorted CNF particles (Fig. 8a) resulted in a relatively smooth tablet surface due to the application of compression force (Fig. 8b). Thus, CNF could be easily deformed plastically. It is considered that this property contributes to ensuring the strength of MTs.
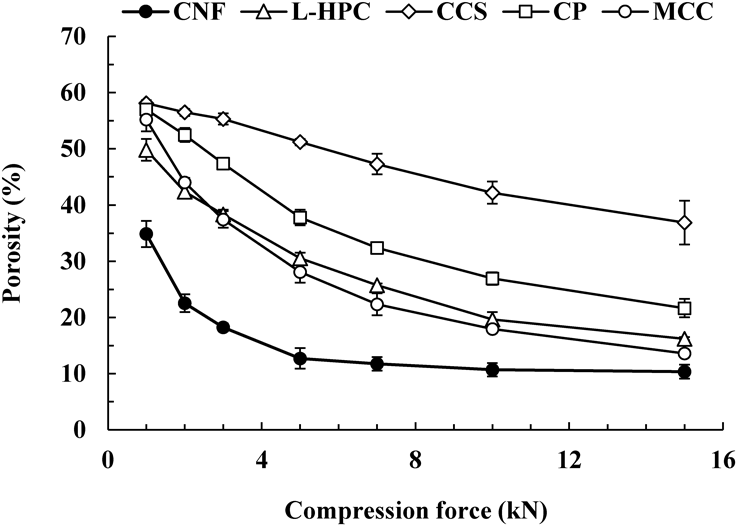
A model tablet with a weight of 200 mg was produced by compression for 5 s with various compression forces. Data are expressed as the mean ± S.D. (n = 5).

The compression force on the model tablet was 5 kN. (a) CNF raw material; (b) model tablets made from CNF.
Figure 9 shows the disintegration time of MTs with CNF added and MTs with reference substances added. Tablets containing the binder, MCC, took more than 3 min to disintegrate at any compression force. In contrast, MTs containing L-HPC, CP, and CCS, which are typical disintegrants, disintegrated within 30 s. MTs containing CNF showed a disintegration time similar to that of the latter, and they were shown to have excellent disintegration properties that could be defined as an ODT.
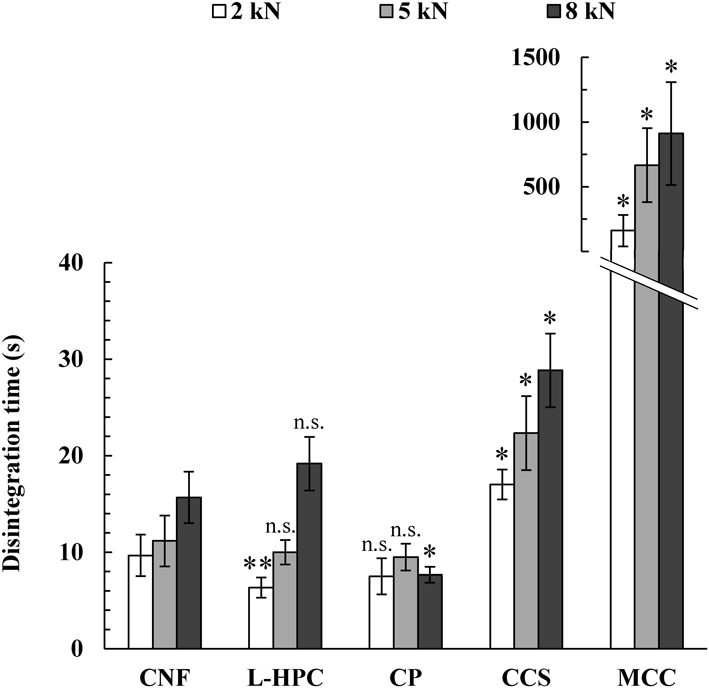
The content of CNF or reference substances was 20%, and the weight of MTs was 30 mg. Statistical analysis of CNF-containing MTs and reference substance-containing MTs at each compression force was performed using the Mann–Whitney U test. n.s.: not significant. *: p < 0.01, **: p < 0.05. Data are expressed as the mean ± S.D. (n = 6).
To explain the disintegration mechanism of CNF, model tablets consisting of each pure substance were prepared and the water content was measured after storage under high humidity and then vacuum dried (Fig. 10). Like other disintegrants, CNF absorbed large amounts of water under high humidity. Moreover, even if the CNF was sufficiently dried, it still retained a relatively large amount of water, suggesting that the interaction with water molecules was strong. It was hypothesized that CNF easily conducted water because the pores are formed inside of the particles by the entanglement of the nano-sized fibers and the surface area is extremely large.
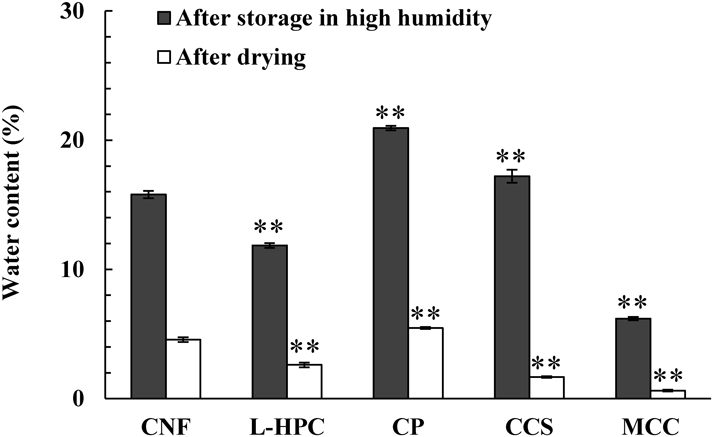
The statistical analysis for CNF was performed using the Mann–Whitney U test. **: p < 0.05. Data are expressed as the mean ± S.D. (n = 5).
Next, the volume of the model tablet before and after moisture absorption was measured and the ratio of volumetric expansion due to moisture absorption was calculated (Table 3). CNF showed the same volumetric expansion as L-HPC and CCS, but not as much as CP, which is a substance that easily swells. It was speculated that MTs containing CNF showed rapid disintegration because the water was guided to the inside of MTs through the pores inside the particles of CNF, and the water loosened the fibrous cellulose, expanding the particles.
| Volume before moisture absorption ± S.D. (mm3)a) | Volume after moisture absorption ± S.D. (mm3)a) | Ratio of volumetric expansion (%)b) | |
|---|---|---|---|
| CNF | 149.95 ± 1.06 | 213.05 ± 2.06 | 42.08 |
| L-HPC | 175.55 ± 1.81 | 223.62 ± 2.28 | 27.38 |
| CP | 241.78 ± 2.17 | 517.23 ± 21.39 | 113.92 |
| CCS | 200.66 ± 6.72 | 295.36 ± 5.93 | 47.20 |
| MCC | 167.35 ± 2.42 | 191.11 ± 0.59 | 14.20 |
CNF: cellulose nanofiber; L-HPC: low-substituted hydroxypropyl cellulose; CP: crospovidone; CCS: croscarmellose sodium; MCC: microcrystalline cellulose. a) Data are expressed as the mean ± S.D. (n = 5). b) Data are representative of n = 5.
These results showed that CNF addition to MTs gives the tablet enough strength to withstand handling and enough disintegration to be handled as an ODT (OD-MT). In brief, this suggested that CNF may be a multifunctional additive that satisfies both the strength and disintegration of OD-MTs.
Optimization of Manufacturing Conditions for OD-MTs with CNF AddedTo optimize the manufacturing conditions of OD-MTs, the effects of the CNF concentration and the compression force on the physical properties of MTs were evaluated three-dimensionally. Figure 11 is a contour plot showing the relationship between the CNF concentration, the compression force, and the tensile strength (Fig. 11a), disintegration time (Fig. 11b), or drug content variation (Fig. 11c) of MTs. In Fig. 11a, the target tensile strength of 1.3–3.6 MPa, is shown as a white region.55,56) Similarly, the target disintegration time was <30 s and shown as the white region in Fig. 11b.57) Accordingly, Fig. 11c shows the target range of <2% drug content variation in white.47,48) Next, the contour plots of Fig. 11a–c were superimposed to obtain the tablet quality required for the actual product. Then, the range of CNF concentration and compression force was optimized to achieve all the target physical characteristics of the tablet (Fig. 11d). A quadrangle can be drawn that fits within the target range, and each vertex of the quadrangle will correspond to the limit condition for achieving the target physical properties of OD-MTs. In brief, it is considered that the space represented by this quadrangle suggests the optimized conditions for OD-MT manufacturing. Figure 11d shows that the optimum conditions are a CNF concentration of 13–29% and a compression force of 2.7–4.7 kN. This data suggested that it is possible to manufacture OD-MTs with the target product quality, even if the CNF concentration and the compression force are freely changed within this set range.
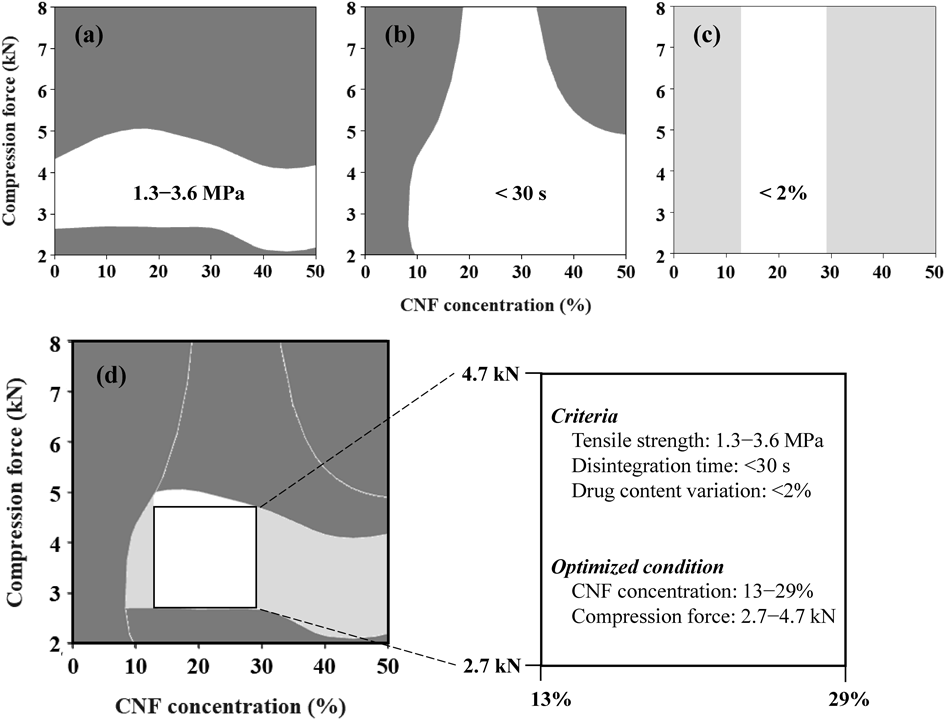
Contour plots showing the relationship between the CNF concentration, compression force, and (a) tensile strength, (b) disintegration time, or (c) drug content variation are shown. Panel (d) shows the CNF concentration and compression force ranges needed to obtain the target physical properties of OD-MTs.
When CNF was added to the pharmaceutical powder, MTs with a diameter of 3 mm could be manufactured by direct powder compression. When evaluating the physical properties of the manufactured MTs, it was possible to significantly reduce the disintegration time while maintaining the strength of the MTs by adding CNF. Fibrous CNF, which has a high affinity for water, is widely distributed inside the MT to increase its strength, but it is speculated that the CNF itself swells and conducts water when in contact, so it has excellent disintegration properties. Thus, CNF has enabled the production of MTs by direct powder compression, which has both tablet strength and disintegration. A formulation containing 20–30% CNF showed rapid disintegration and could be manufactured as MTs functioning as an ODT (OD-MT). Currently, the Environment Agency of Japan views the development and manufacturing of products that utilize CNF as important. Thus, it is conceivable that the establishment of a method for utilizing CNF as an additive for pharmaceutical formulations will contribute to society due to environmental considerations. The data in this manuscript shows the multifunctionality of CNF as an additive, and in turn, it was possible to produce MTs having the physical properties required for a pharmaceutical product.
The authors thank Daio Paper Corp. for providing the cellulose nanofiber.
The authors declare no conflict of interest.