2023 Volume 71 Issue 1 Pages 41-51
2023 Volume 71 Issue 1 Pages 41-51
Globalization of pharmaceutical supply chains has expanded and manufacturers are required to manufacture products in compliance with the pharmacopoeial standards used in all exporting countries/regions to ensure product quality. International harmonization has been facilitated by the Pharmacopoeial Discussion Group consisting of the Japanese Pharmacopoeia, the United States Pharmacopeia, and the European Pharmacopoeia. However, since the pharmacopoeias have been developed individually under the regulatory framework of each country/region, differences exist between these pharmacopoeias. When using pharmacopoeias, an understanding of common pharmacopoeial rules is essential. Clarifying the similarities and differences in the General Notices of the pharmacopoeias widely referenced worldwide is considered valuable for those already using one or two of them to access the remaining pharmacopoeias. In this study, we compared the existence of items and the contents described in the General Notices of the three pharmacopoeias to clarify the differences. Investigation of the existence of items revealed that more than 70% of the 105 items in General Notices in the three pharmacopoeias were in the entire pharmacopoeias (for Japan, including Japanese laws and notifications). Furthermore, investigating contents revealed that approximately 20% of the 105 items have some differences such as numerical values and test conditions. However, it was shown that most of the items did not have major differences. It is expected that the three pharmacopoeias will be utilized simultaneously by understanding the similarities and differences shown in this study.
The globalization of pharmaceutical supply chains has expanded and pharmaceuticals are being manufactured using active pharmaceutical ingredients (API) and excipients manufactured in various countries. In 2021, for approximately 59% of generic drugs distributed in Japan, some or all of their API manufacturing processes were performed outside Japan, and imported APIs used directly for manufacturing were sourced from China, India, South Korea, and Italy, in that specific order, in terms of the number of companies.1) Similar trends were also observed in pharmaceuticals distributed overseas. In 2019, approximately 87 and 60% of API and drug product manufacturing sites for generics sold in the United States (US) market were located outside the US, primarily in India and China.2) The globalization of excipient supply chains has grown rapidly, and excipient supply chains are becoming more complex.3) Thus, APIs, excipients, and drug products manufactured abroad are being supplied worldwide.
Each country/region has its own pharmacopoeia to ensure the quality of the pharmaceuticals distributed in that country/region. The WHO describes that “a pharmacopoeia is a legally-binding collection, prepared by a national or regional authority, of standards and quality specifications for medicines used in that country or region.”4) In Japan, the Law on Securing Quality, Efficacy and Safety of Products including Pharmaceuticals and Medical Devices (PMD Act) defines the Japanese Pharmacopoeia (JP),5) and the JP should provide an official standard being required to assure the quality of medicines in Japan.6) In the US, Federal Food, Drug, and Cosmetic Act (FD&C Act) defines the United States Pharmacopeia (USP),7) and the mission of USP is to improve global health through public standards and related programs that help ensure the quality, safety, and benefit of medicines and foods.8) Standards in Europe are dictated by the European Pharmacopoeia (Ph. Eur.), and the purpose of the Ph. Eur. is to promote public health by the provision of recognized common standards for the quality of medicines and their components.9) Pharmacopoeias have a common objective of assuring the quality of medicines and contributing to the improvement of public health in their respective countries/regions; however, most of their contents have been developed individually by each pharmacopoeia. The JP consists of General Notices, General Rules for Crude Drugs, General Rules for Preparations, General Tests, Processes and Apparatus (abbreviated as “General Tests” hereafter), Monographs, and Reference Spectra, the USP consists of General Notices, Front Matter, General Chapters (including General Texts & Assays, General Information), Monographs, and Reagents, and the Ph. Eur. consists of Introduction, General Chapters (including General Notices, Methods of Analysis, Reagents, and General Texts), General Monographs, and Monographs6,8,10) (Table 1). Although the structure is not completely consistent between the three pharmacopoeias, the pharmacopoeias have a common principle, that is, General Notices list general rules which apply to the entire pharmacopoeia, followed by test methods used for judging compliance to the standards and individual monographs.11) Thus, General Notices serve as basic rules for each of the three pharmacopoeias.
| JP18a) | USP 2021 | Ph. Eur. 10.0 |
|---|---|---|
| General Notices General Rules for Crude Drugs General Rules for Preparations General Tests, Processes and Apparatus Monographs Infrared Reference Spectra Ultraviolet-visible Reference Spectra | General Notices and Requirements Front Matter General Chapters General Tests & Assays General Information Monographs Reagents | Introduction General Chapters 1. General Notices 2. Methods of Analysis 3. Materials for Containers and Containers 4. Reagents 5. General Texts General Monographs Monographs |
a) For the JP, the contents in the table are within the scope of the Ministry of Health, Labour and Welfare (MHLW) Ministerial Notification; however, the Ministerial Notification, Preface, General Information, and Appendix are also attached.
The Pharmacopoeial Discussion Group (PDG) was established in 1989 to harmonize testing procedures and acceptance criteria among pharmacopoeias, thereby reducing the burden on manufacturers and pharmaceutical companies to perform analytical procedures. The PDG has been working towards harmonizing excipient monographs and general chapters between the three pharmacopoeias.12) The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) was founded by the regulatory authorities and pharmaceutical industry in Japan, the US, and Europe in 1990 to achieve greater harmonisation worldwide to ensure that safe, effective, and high quality medicines are developed, and registered and maintained in the most resource efficient manner whilst meeting high standards.13) Accordingly, the ICH has developed guidelines on scientific and technical aspects of pharmaceutical registration. In addition to international harmonization of pharmaceutical quality assurance practice by the PDG and ICH, pharmacopoeias have been used not only in their own country/region but also worldwide. Currently, the USP is utilized in over 140 countries worldwide and integrated into the laws of more than 40 countries.14) Similarly, the Ph. Eur. is legally binding in 39 European countries and applied in more than 120 countries worldwide.15) The JP is officially recognized in various countries, including Brazil, Singapore, South Africa, South Korea, Taiwan, and Thailand.16,17) Furthermore, the JP is actively incorporating concepts of quality assurance based on the latest knowledge in the field, promoting the dissemination of the JP as a reference pharmacopoeia in other countries and regions, especially in Asian countries, and aiming to contribute to improving the quality of medicines distributed globally.18,19)
With the globalization of medicine supply chains, manufacturers are required to manufacture products in compliance with the pharmacopoeial standards used in all exporting countries/regions; thus, the need to confirm and ensure compliance with the three widely used pharmacopoeias, i.e., the JP, USP, and Ph. Eur., will increase substantially. To date, several articles have compared the legal position and structure of the three pharmacopoeias, as well as the monographs and general chapters.11,20) However, to the best of our knowledge, a comparison of the contents of the General Notices of the three pharmacopoeias has not yet been presented. This study compared the existence of items and the contents in the General Notices of the three pharmacopoeias comprehensively and presented their similarities and differences.
Before investigating the existence of items and the contents of General Notices between the three pharmacopoeias, we compared the structures of General Notices in the three pharmacopoeias. Consequently, each of them was found to be unique (Table 2, Supplementary Table 1). For the JP, only the general notice number was presented without an item heading; therefore, the content summarized by the authors is presented after the General Notices number. It should be noted that details of contents such as numerical values in the USP and Ph. Eur. were not recorded in the following results so as not to infringe their copyrights.
| USP 2021 | Ph. Eur. 10.0 |
|---|---|
| 1. Title and Revision 2. Official Status and Legal Recognition 2.10. to 2.30. 3. Conformance to Standards 3.10. to 3.20. 4. Monographs and General Chapters 4.10. to 4.20. 5. Monograph Components 5.10. to 5.80. 6. Testing Practices and Procedures 6.10. to 6.80.30. 7. Test Results 7.10. to 7.20. 8. Terms and Definitions 8.10. to 8.240. 9. Prescribing and Dispensing 9.10. to 9.20. 10. Preservation, Packaging, Storage, and Labeling 10.10. to 10.20. | 1.1. General Statements Quality systems, Alternative methods, etc. 1.2. Other Provisions Applying to General Chapters and Monographs Quantities, Apparatus and procedures, etc. 1.3. General Chapters Containers 1.4. Monographs Titles, Relative atomic and molecular masses, etc. 1.5. Abbreviations and Symbols 1.6. Units of the International System (SI) Used in the Pharmacopoeia and Equivalence with Other Units International system of units (SI), Notes |
See Supplementary Table 1 for items in the Japanese Pharmacopoeia 18th Edition (JP18).
Figure 1 and Supplementary Table 1 show the results of classifying 49 of the JP General Notices based on the existence of descriptions in the USP General Notices and Ph. Eur. General Notices. Supplementary Table 1 also provides the location of each item (in case of multiple locations, a representative example is provided) and the contents of the USP and Ph. Eur. Practical solutions or why the difference does not raise a problem are also included for differences observed between the three pharmacopoeias or two of them when the contents were present in two of them, except the JP General Notices Number (JP GN) related to editing of the pharmacopoeia, such as JP GN 1,2 (Official name of the pharmacopoeia), JP GN 3 (Positioning of monographs), JP GN 4 (Monograph placement of crude drugs and related drugs), and JP GN 7 (Expression of monograph titles). Furthermore, regarding the JP General Notices which are not present in the entire USP or Ph. Eur., although we also investigated the “Submission Guideline for Chemical Medicines,”21) “European pharmacopoeia style guide (2017)”22) and “Technical guide for the elaboration of monographs on medicinal products containing chemically defined active substances (2020)”23) which correspond to the JP drafting guidelines for the USP and Ph. Eur., there were no items that were present only in these documents.

The headings after the JP General Notices number are summary of the contents by the authors, and these headings are not present in the JP General Notices. JP GN: the Japanese Pharmacopoeia General Notice Number.
As listed in Fig. 1, Class A consisted of 27 out of 49 JP General Notices (55%). This clarifies that more than half of the JP General Notices were in both USP General Notices and Ph. Eur. General Notices. These were regarding the official names of the pharmacopoeia, positioning of the monographs, judgment of the compliance to the monographs, temperature for testing and storage, containers, labeling, and other basic information and definitions used in the pharmacopoeia.
Further investigation of the contents revealed that more than half of the items in Class A were similar between the three pharmacopoeias (Supplementary Table 1). For example, the three pharmacopoeias state that some tests specified in monographs may be omitted as occasion demands as shown in JP GN 13 (Omission of compendial tests). The three pharmacopoeias mention that the term “solution” indicates a solution in water where the name of the solvent is not stated as shown in JP GN 22 (Expression of solvents).
However, as shown in Supplementary Table 1, the contents of 10 out of 27 JP General Notices (JP GN 5: Conformance to standards, JP GN 8: Atomic masses, JP GN 16: Expression of temperatures for tests or storage, JP GN 21: Water in compendial tests, JP GN 26: Temperature for compendial tests, JP GN 30: Solubility, JP GN 37: Constant mass, JP GN 44: Tight containers, JP GN 47: Labeling requirements on contents or potency, and JP GN 48: Labeling requirements on origin, numerical value or physical properties) differed between the three pharmacopoeias. In JP GN 5 (Conformance to standards), while all the three pharmacopoeias judged the conformance not solely based on the provisions of the monograph but also including the General Notices and the General Tests, there were differences in terms of storage sections whether it is “an information to be used as a reference” or “a requirement.” As described in JP GN 16 (Expression of temperatures for tests or storage), all the three pharmacopoeias define temperature ranges for room temperature and cold places, but the ranges differ between the three pharmacopoeias. However, the ranges in the JP covered those in the USP and Ph. Eur. Moreover, the JP General Notices include the definitions for cold water, lukewarm water, warm water, and hot water, which are neither listed in the USP nor Ph. Eur. However, the JP does not define temperature for refrigerator or freezer, which are listed in the USP and Ph. Eur. Regarding the use of water, JP GN 21 (Water in compendial tests) mentions that water suitable for performing the relevant test should be used. However, the USP and Ph. Eur. describe that water complying with the requirement of monograph Purified Water should be used unless otherwise specified. In the case of JP GN 26 (Temperature for compendial tests), all the three pharmacopoeias specify the temperature for compendial tests; however, that of the USP, when it is not specified, was different from that of the JP and Ph. Eur. However, it is within the range of the JP and Ph. Eur., if performed at the specified temperature in the USP. As for JP GN 30 (Solubility), all three pharmacopoeias have the same definitions for solubility; however, the measurement temperature of the USP was different from that of the JP and Ph. Eur. The temperature in the USP is within the range of the JP and Ph. Eur. definitions. Additionally, the JP specified dissolution method and period, which were not described in the USP or Ph. Eur. As for JP GN 37 (Constant mass), although all three pharmacopoeias define constant mass, the JP specifies values to be considered as constant mass depending on type of balance, which the USP and Ph. Eur. do not. However, the value in the USP and Ph. Eur. is within the range in the JP. Moreover, the JP specifies one hour of drying or ignition period. Regarding JP GN 44 (Tight containers), as the definition of a well-closed container in the Ph. Eur. is similar to the definition of a tight container in the JP and USP, JP GN 43 (Well-closed containers) is shown as Not Listed, and JP GN 44 (Tight containers) is shown as Listed for the Ph. Eur. (Supplementary Table 1). The Ph. Eur. does not mention protecting the contents from efflorescence, deliquescence, or evaporation, which the JP and USP do. In JP GN 8 (Atomic masses), the JP specifies the version of international table of atomic weights of the elements and also mentions that molecular weights are displayed to the second decimal place. The USP and Ph. Eur. specify use of the latest version of the international table. As for JP GN 47 (Labeling requirements on contents or potency) and JP GN 48 (Labeling requirements on origin, numerical value or physical properties), the JP and USP list labeling items but the Ph. Eur. does not.
As described above, 10 General Notices showed differences in the contents. By confirming all requirements of any of the three pharmacopoeias regarding JP GN 5 (Conformance to standards); by testing at the common temperature among the three pharmacopoeias regarding JP GN 16 (Expression of temperatures for tests or storage), JP GN 26 (Temperature for compendial tests), and JP GN 30 (Solubility) resulting from differences in temperature range due to differences such as the climate of each country/region; by testing with water conforming to the USP and Ph. Eur. monographs regarding JP GN 21 (Water in compendial tests); by using the most stringent condition regarding JP GN 37 (Constant mass); and by using the most stringent container regarding JP GN 44 (Tight containers), it is possible to ensure conformance with the three pharmacopoeias simultaneously in some cases. Thus, it is considered that a pharmacopoeial user can simplify the confirmation of compliance with the three pharmacopoeias by controlling conditions such as temperature (Supplementary Table 1). However, regarding JP GN 8 (Atomic masses), JP GN 47 (Labeling requirements on contents or potency), and JP GN 48 (Labeling requirements on origin, numerical value or physical properties), it is necessary to calculate molecular weight and confirm labelling items according to each pharmacopoeia; nevertheless, the concept of specifying the items to ensure the quality is the same.
Analysis of Items Present in the JP General Notices and USP General Notices, but Not in the Ph. Eur. General Notices (Class B)Class B consisted of 8 out of 49 JP General Notices (16%) (Fig. 1). Four out of the total eight Class B JP General Notices (JP GN 10: Potency of drugs, JP GN 23: Expression of solutions and liquid mixtures, JP GN 34: Control of elemental impurities, and JP GN 35: Control of residual solvents) were in the Ph. Eur. (Methods of Analysis, General Texts, General Monographs), while the rest of four JP General Notices (JP GN 18: In vacuum, JP GN 27: “Immediately” in compendial procedures, JP GN 29: Judgement of odor, and JP GN 43: Well-closed containers) were not in the entire Ph. Eur. (Fig. 2a, Supplementary Table 1).

Investigation results of (a) items present in both JP General Notices and USP General Notices in the entire Ph. Eur., (b) items present in both JP General Notices and Ph. Eur. General Notices in the entire USP, and (c) items only present in the JP General Notices in the entire USP and the entire Ph. Eur. are shown. The diagonal lines in the Venn diagram indicate the corresponding parts of each pie chart. The headings after the JP General Notices number are summary of the contents by the authors, and these headings are not present in the JP General Notices. GN: General Notices, JP GN: the Japanese Pharmacopoeia General Notice Number.
Investigation of the contents revealed that four out of the eight JP General Notices in Class B (JP GN 23: Expression of solutions and liquid mixtures, JP GN 34: Control of elemental impurities, JP GN 35: Control of residual solvents, and JP GN 43: Well-closed containers) were similar between the JP and USP or between the three pharmacopoeias when items were written in the entire Ph. Eur. For example, for JP GN 34 (Control of elemental impurities) and JP GN 35 (Control of residual solvents), all three pharmacopoeias control these according to the requirements specified in General Tests/General Chapters (Supplementary Table 1). It should be noted that the term “well-closed container” is used in the three pharmacopoeias, and the definition is similar between the JP and USP; however, users must be careful with this term due to the difference in the definitions in the Ph. Eur., because the definition of a well-closed container in the Ph. Eur. is similar to the definition of a tight container in the JP and USP. For the Ph. Eur., JP GN 43 (Well-closed containers) is shown as Not Listed, and JP GN 44 (Tight containers) is shown as Listed (Supplementary Table 1).
Of the four items with differences, values to be considered as decompression were different between the JP and USP in JP GN 18 (In vacuum); and the JP specifies the number of seconds until starting the next procedure but the USP does not in JP GN 27 (“Immediately” in compendial procedures) (Supplementary Table 1). Although caution is required in pharmacopoeial testing, it is considered that it is possible to confirm conformance to the three pharmacopoeias simultaneously by using the most stringent condition in some cases. Units for potency were different between the three pharmacopoeias in JP GN 10 (Potency of drugs), and the operating procedure for determining odor differed between the JP and USP in JP GN 29 (Judgement of odor) (Supplementary Table 1). These were due to differences in the operation method used since old times in each country. The concept of specifying the items to ensure the quality was the same.
Analysis of Items Present in the JP General Notices and Ph. Eur. General Notices but Not in the USP General Notices (Class C)Class C consisted of 6 out of 49 JP General Notices (12%) (Fig. 1). Two out of the 6 JP General Notices (JP GN 28: Color examination, and JP GN 31: Dissolve/miscible) were in the USP (General Tests & Assays, Reference Tables), while the rest of 4 JP General Notices (JP GN 7: Expression of monograph titles, JP GN 12: Manufacture/production, JP GN 36: Potential adulteration, and JP GN 40: Content upper limit) were not in the entire USP. (Fig. 2b, Supplementary Table 1).
Investigation of the contents revealed that four out of the six JP General Notices in Class C (JP GN 7: Expression of monograph titles, JP GN 12: Manufacture/production, JP GN 31: Dissolve/miscible, and JP GN 36: Potential adulteration) were similar between the JP and Ph. Eur. or between the three pharmacopoeias when the items were written in the entire USP. For example, for JP GN 12 (Manufacture/production) and JP GN 36 (Potential adulteration), the contents in the JP and Ph. Eur. are equivalent (Supplementary Table 1). Regarding JP GN 31 (Dissolve/miscible), all three pharmacopoeias explained the meaning of “miscible.” In addition, the JP mentions that “insoluble materials other than the drug including fibers should not be detected or practically invisible, if any,”24) and the USP also has a similar description (Supplementary Table 1).
Of the two items with differences, for JP GN 28 (Color examination), the detailed examination procedures differed between the three pharmacopoeias (Supplementary Table 1). Although this was due to differences in the equipment and operation method used since old times in each country/region, the concept of specifying this item to ensure the quality was the same. JP GN 40 (Content upper limit) specifies that the content upper limit is 101.0% in case it is not indicated. Although this is a unique specification for the JP, it is considered possible to confirm conformance to the three pharmacopoeias simultaneously by using the most stringent criterion among them in some cases.
Analysis of Items Present in the JP General Notices but Not in the USP General Notices or Ph. Eur. General Notices (Class D)Class D consisted of 8 out of 49 JP General Notices (16%) (Fig. 1). Four out of the eight JP General Notices (JP GN 6: Principle on animal-derived materials, JP GN 19: Acidity or alkalinity of a solution, JP GN 20: Classification of crude drug cuttings and powder fineness, and JP GN 41: Sterility/sterilization/aseptic technique) were in the USP (General Chapters) and the Ph. Eur. (Methods of Analysis, General Texts) (Fig. 2c, Supplementary Table 1).
Investigation of the contents showed that three out of the four JP General Notices (JP GN 6: Principle on animal-derived materials, JP GN 19: Acidity or alkalinity of a solution, and JP GN 41: Sterility/sterilization/aseptic technique) were similar between the three pharmacopoeias. For example, regarding JP GN 6 (Principle on animal-derived materials), both USP and Ph. Eur. have a section on viral safety, and all three pharmacopoeias mention that healthy animals are to be used for manufacturing pharmaceuticals or their source materials.
Regarding JP GN 20 (Classification of crude drug cuttings and powder fineness) with differences, although all three pharmacopoeias specify the classification of powder fineness, the classification in the JP was different from that in the USP and Ph. Eur. (Supplementary Table 1). One of the attributes is that the JP and USP used their own standard sieves in Japan and the US, respectively.25) However, the concept of specifying this item to ensure the quality was the same between the two.
The following four JP General Notices in Class D, which were not described in the entire USP or Ph. Eur., were unique to the JP (Supplementary Table 1). JP GN 4 (Monograph placement of crude drugs and related drugs) mentioned that crude drugs and their related products are placed together in the posterior part of the monographs. JP GN 11 (Being specified separately) mentioned some specifications/acceptance criteria and test methods were not included in the JP and should be established for each drug at the time of approval since there are process-related impurities or drug product tests for which a unified test method cannot be established due to differences in manufacturing methods, or the contents should be protected as part of intellectual property.26,27) JP GN 15 (Considerations on applying biological test methods) was a provision that the details of the biological test methods may be changed insofar as they do not affect the essential qualities of the test; and JP GN 17 (Measurement of drops) explained an instrument to measure the number of drops.
The four JP General Notices unique to the JP are discussed below. JP GN 4 (Monograph placement of crude drugs and related drugs) does not affect the confirmation of conformance to the JP because it explains editing manner. JP GN 11 (Being specified separately) shows a reasonable scheme under the Japanese law. The JP is basically used in combination with the marketing authorization dossier from the perspective of having various process-related impurities due to different manufacturing processes and protecting the intellectual property rights of manufacturing method developers. JP GN 15 (Considerations on applying biological test methods) clarifies that “the details of the biological test methods may be changed insofar as they do not affect the essential qualities of the test,” and it makes it possible to perform tests flexibly within a range that does not affect the nature of the test. It is considered that JP GN 17 (Measurement of drops) is not a massive burden for JP users because it is not necessary to confirm each test operation by using an instrument that meets the requirements once.
Based on the above, 27 out of the 49 JP General Notices were in both USP and Ph. Eur. General Notices (55%) (Fig. 1). When the scope of confirmation was not limited to the USP and Ph. Eur. General Notices, a total of 37 (76%), that is 27 in Class A, 4 in Class B, 2 in Class C and 4 in Class D, were in the three pharmacopoeias (Fig. 1 and Fig. 2). Additionally, it was revealed that General Notices unique to the JP, described in neither the USP nor Ph. Eur., are only four (8%) (Fig. 2c). The contents of more than half of the JP General Notices in Class A to D, except for the four JP General Notices unique to the JP, were similar between the three pharmacopoeias or between two pharmacopoeias when the information existed only in two pharmacopoeias. However, there were some differences in descriptions due to differences in the culture and climate of the country/region where the pharmacopoeias were prepared. For example, in the case of JP GN 16 (Expression of temperatures for tests or storage), JP GN 26 (Temperature for compendial tests) and JP GN 30 (Solubility), it is considered that it is possible to confirm conformity to the three pharmacopoeias simultaneously by using a common test temperature and condition in some cases. As for JP GN 5 (Conformance to standards), JP GN 18 (In vacuum), JP GN 21 (Water in compendial tests), JP GN 27 (“Immediately” in compendial procedures), JP GN 40 (Content upper limit), and JP GN 44 (Tight containers), using the most stringent condition or criterion among the three pharmacopoeias is considered acceptable in some cases. It is necessary to calculate molecular weight and confirm labelling items according to each pharmacopoeia for JP GN 8 (Atomic masses), JP GN 47 (Labeling requirements on contents or potency), and JP GN 48 (Labeling requirements on origin, numerical value or physical properties). Additionally, there are differences in the contents due to differences in equipment and test methods used for a long time in each country/region for JP GN 10 (Potency of drugs), JP GN 20 (Classification of crude drug cuttings and powder fineness), JP GN 28 (Color examination), and JP GN 29 (Judgement of odor). However, the concept of specifying these items to ensure the quality was the same. These were not differences that caused major issues when the pharmacopoeias were used after thoroughly understanding the General Notices and the related sections. However, it was clarified that attention should be paid to JP GN 43 (Well-closed containers) because a well-closed container in the Ph. Eur. corresponds to a tight container in the JP and USP, and a term corresponding to a well-closed container in the JP and USP is not defined in the Ph. Eur.
Investigation of Items Not in the JP General Notices but in the USP General Notices and/or Ph. Eur. General NoticesFigure 3 and Supplementary Table 2 show the results of classifying items that existed in the USP General Notices and/or Ph. Eur. General Notices but not in the JP General Notices. Supplementary Table 2 also provides the location of each item (in case of multiple locations, a representative example is provided) and the contents of the USP and Ph. Eur. Furthermore, regarding items which are not present in the entire USP or Ph. Eur., although we also investigated the “Submission Guideline for Chemical Medicines,”21) “European pharmacopoeia style guide (2017),”22) and “Technical guide for the elaboration of monographs on medicinal products containing chemically defined active substances (2020),”23) which correspond to the JP drafting guidelines for the USP and Ph. Eur. for Class E to G, there were no items that were present only in these documents.
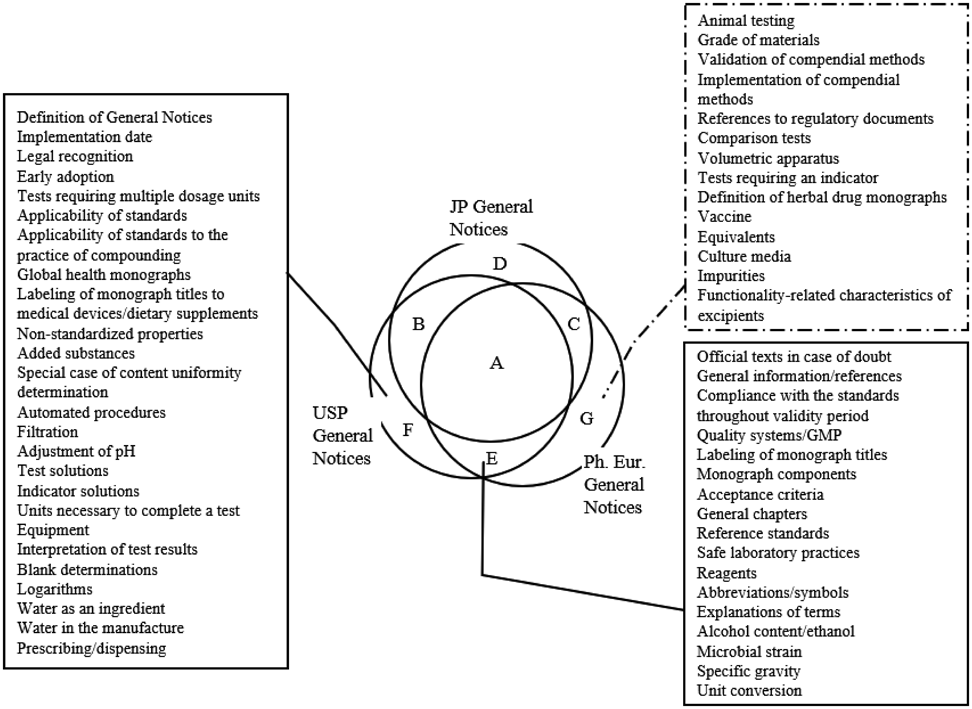
The solid line indicates the items counted based on the USP General Notices, and the dashed line indicates those counted based on the Ph. Eur. General Notices. GMP: Good Manufacturing Practice.
Sixteen out of 17 items (94%) in Class E except for 1 item (Unit conversion) were in the JP other than the General Notices (Preface, General Tests, Monographs, General Information, Laws, and Notifications) (Fig. 4a, Supplementary Table 2).

Investigation results of (a) items present in both USP General Notices and Ph. Eur. General Notices in the entire JP (including the laws and notifications), (b) items only present in the USP General Notices in the entire JP (including the laws and notifications) and the entire Ph. Eur., and (c) items only present in the Ph. Eur. General Notices in the entire JP (including the laws and notifications) and the entire USP are shown. The diagonal lines in the Venn diagram indicate the corresponding parts of each pie chart. The solid line indicates the items counted based on the numbers of the USP General Notices, and the dashed line indicates the items counted based on the numbers of the Ph. Eur. General Notices. Underlined items are those present in the laws and notifications for the JP. GN: General Notices, GMP: Good Manufacturing Practice.
In 16 out of 17 items in Class E, except for 1 item (Alcohol content/ethanol), the content was similar between the USP and Ph. Eur. for “Unit conversion” and between the three pharmacopoeias for the rest of them. For example, regarding “Official texts in case of doubt,” all three pharmacopoeias specify the official texts: the online version for the USP, the English and French versions for the Ph. Eur., and the Japanese version for the JP. “General information/references” does not mean the judgment of conformance to standards in all three pharmacopoeias. Regarding “Compliance with the standards throughout validity period,” all three pharmacopoeias require compliance with standards in the monograph until the expiration date (Supplementary Table 2). As for “Labeling of monograph titles,” all three pharmacopoeias mention that labeling of monograph titles indicates compliance with the compendial standards (Supplementary Table 2). “Reference standards” are the official reference materials used in the tests specified in all three pharmacopoeias (Supplementary Table 2). As for “Safe laboratory practices,” the USP and Ph. Eur. state precautions for handling drugs, reagents, and test solutions, and the JP mentions precautions individually in the monographs, such as instructions like “add it carefully.” “Acceptance criteria” are set by considering factors such as analytical error, manufacturing and formulation variability, and acceptable degradation in all three pharmacopoeias (Supplementary Table 2).
Regarding “Compliance with the standards throughout validity period,” “Labeling of monograph titles,” and “Reference standards,” the method of handling these items was common between the three pharmacopoeias. However, as the USP and Ph. Eur. are clearly described in the General Notices, it makes it easier for pharmacopoeial users to understand them. Moreover, for “Safe laboratory practices,” all three pharmacopoeias describe performing procedures “with care” in individual operations and are considered useful for ensuring the safety of pharmacopoeial users. As for “Acceptance criteria,” it is important to understand acceptance criteria consider factors such as analytical error, manufacturing and formulation variability, and acceptable degradation. If added to the JP General Notices in the future revision, the understanding of the JP users will be further deepened, and General Notices between the three pharmacopoeias will become more similar.
In contrast, for “Alcohol content/ethanol,” all three pharmacopoeias have a definition of alcohol/ethanol; however, their definitions are different. Alcohol is another name for ethanol in the JP. Both alcohol and ethanol point at the monograph of alcohol in the USP, whereas ethanol means anhydrous ethanol and alcohol means ethanol (96%) in the Ph. Eur. One of the reasons underlying this difference is names used traditionally in each country/region. When pharmacopoeias are used after a thorough understanding of the General Notices or applicable sections in case the item is not present in the General Notices, it does not cause significant problems. However, these differences in terms can easily lead to confusion, and thus, should be disseminated actively.
Analysis of Items Not Present in the JP General Notices or Ph. Eur. General Notices but in the USP General Notices (Class F)Seventeen out of 25 items (68%) in Class F (Definition of General Notices, Implementation date, Legal recognition, Tests requiring multiple dosage units, Non-standardized properties, Added substances, Automated procedures, Filtration, Adjustment of pH, Test solutions, Indicator solutions, Units necessary to complete a test, Equipment, Blank determinations, Logarithms, Water as an ingredient, and Water in the manufacture) were in various sections, including the JP General Rules for Preparations, General Tests, Monographs, General Information, Laws, Notifications, and the Ph. Eur. Cover Page, Introduction, Methods of Analysis, Reagents, General Monographs, Monographs (Fig. 4b, Supplementary Table 2).
Of 10 items in Class F, other than seven items unique to the USP mentioned later, the content was similar between the USP and JP for “Applicability of standards” and between the three pharmacopoeias for the rest of the nine items. For example, regarding “Implementation date,” the USP mentions the implementation date for the revisions, and the online version shows the effective date of the changed description. Similarly, the JP and Ph. Eur. list an implementation date of the version.
Furthermore, there were only seven items unique to the USP. These were “Early adoption,” which is the ability to apply changes/additions prior to the date of enforcement; “Global health monographs,” which are provided for possible use outside of the US at the discretion of individual government authorities; “Applicability of standards to the practice of compounding” and “Prescribing/dispensing,” which are provisions for compounding, prescribing and dispensing of medicines; “Labeling of monograph titles to medical devices/dietary supplements,” which are provisions for medical devices and dietary supplements; “Special case of content uniformity determination,” which explains a case in which content uniformity determinations use the same analytical methodology specified in the assay; and “Interpretation of test results,” which explains how to compare analytical results with acceptance criteria (Fig. 4b, Supplementary Table 2).
It is considered that “Early adoption” does not affect the JP and/or Ph. Eur. users because users select effective versions of the JP and Ph. Eur. whenever they use them. “Applicability of standards to the practice of compounding,” “Labeling of monograph titles to medical devices/dietary supplements,” and “Prescribing/dispensing” also do not affect the JP and/or Ph. Eur. users because the scope of the USP is different from that of the JP and Ph. Eur. by covering compounding, prescribing, and dispensing of medicines, medical devices, and dietary supplements. It is assumed that the USP lists global health monographs because the USP is utilized globally, and its mission is to improve global health. “Special case of content uniformity determination” is an advantage for the JP and/or Ph. Eur. users to omit tests under certain conditions when they use the USP (Supplementary Table 2). There is no issue with “Interpretation of test results” because the JP and/or Ph. Eur. users implement the same approaches.
Analysis of Items Not Present in the JP General Notices or USP General Notices but in the Ph. Eur. General Notices (Class G)Eleven out of 14 items in Class G (Grade of materials, Validation of compendial methods, Implementation of compendial methods, References to regulatory documents, Comparison tests, Volumetric apparatus, Definition of herbal drug monographs, Vaccine, Equivalents, Culture media, and Impurities) (79%) were in sections such as the JP General Tests, Monographs, General Information, and the USP General Chapters, Monographs, Indicators and Indicator Test Papers (Fig. 4c, Supplementary Table 2).
The content was similar between two pharmacopoeias for “Animal testing,” “Tests requiring an indicator,” and “Functionality-related characteristics of excipients,” and between the three pharmacopoeias for the rest of the items. For example, regarding “Validation of compendial methods,” all three pharmacopoeias mean test methods have been validated at the time of listing in the pharmacopoeia (Supplementary Table 2). As for “References to regulatory documents,” the Ph. Eur. mentions that European Union (EU) directives and notes for guidance in monographs and general chapters are provided for information for users. The USP lists Food and Drug Administration (FDA) guidances in some General Chapters numbered 1000 to 1999, and the JP also lists references in some General Information.
As for “Validation of compendial methods,” the handling is the same in the three pharmacopoeias. However, as it was only written in the Ph. Eur. General Notices, it was friendly for analysts of the Ph. Eur. tests. The JP and USP contain similar descriptions in the General Information and General Chapters for informational purposes, respectively. Although General Information in the JP is not within the scope of the Ministry of Health, Labour and Welfare (MHLW) Ministerial Notification and is provided as reference information, it was shown that understanding the General Information is also useful. In addition, as for “References to regulatory documents,” it is the users’ responsibility to check the latest reference information; however, this approach is beneficial because users can easily access at least the information listed in the Ph. Eur. Regarding the JP, it is difficult to cite reference information in JP General Tests and Monographs according to the legal system. However, it is considered that a list of notifications and guidelines is to be used as a reference to help deepen the understanding of the users.
Of the 42 items not in the JP General Notices but present in the USP (Class E and F), 33 items (79%) were in the three pharmacopoeias (for Japan, including the laws and notifications), and of the 14 items not present in the JP or USP General Notices but present in the Ph. Eur. (Class G), 11 items (79%) were present in the three pharmacopoeias (for Japan, including the laws and notifications) (Fig. 4). Moreover, among the 56 items (Class E to G), 9 items (16%) were present in the Japanese law or notifications. Since the JP is a Ministerial notification from the MHLW, supportive or related information for the JP is described in notifications, which is a lower position than the JP. By including them, we could confirm the contents described in the USP and Ph. Eur. General Notices.
Except for seven items unique to the USP, the contents in most of the items classified in Class E to G were similar between the three pharmacopoeias, or two pharmacopoeias in case items described only in two of them. However, since the definitions of alcohol/ethanol were different, it is considered that users need to pay attention to them.
This study revealed that more than 70% of the 105 items listed in the General Notices of the three pharmacopoeias (Class A to G) were present in all three pharmacopoeias (for Japan, including the laws and notifications). Moreover, by analyzing the contents of the 105 items (Supplementary Tables 1, 2), we found that contents were similar for most of the items; however, differences were observed in 19 items. Among items present in two of the three pharmacopoeias, although two items (JP GN 18: In vacuum, and JP GN 27: “Immediately” in compendial procedures) had differences such as numerical values; and the contents between the JP and Ph. Eur. were different in JP GN 40 (Content upper limit), it is possible to confirm conformance to the three pharmacopoeias simultaneously by using the most stringent condition or criterion in some cases. Although seven items (JP GN 5: Conformance to standards, JP GN 16: Expression of temperatures for tests or storage, JP GN 21: Water in compendial tests, JP GN 26: Temperature for compendial tests, JP GN 30: Solubility, JP GN 37: Constant mass, and JP GN 44: Tight containers) had differences such as numerical values and test conditions, it is possible to ensure conformance to the three pharmacopoeias simultaneously by using the most stringent condition or a common temperature/condition in some cases. Moreover, seven items (JP GN 8: Atomic masses, JP GN 10: Potency of drugs, JP GN 20: Classification of crude drug cuttings and powder fineness, JP GN 28: Color examination, JP GN 29: Judgement of odor, JP GN 47: Labeling requirements on contents or potency, and JP GN 48: Labeling requirements on origin, numerical value or physical properties) showed it is necessary to confirm the items according to each pharmacopoeia due to differences such as equipment and operation methods used traditionally in each country/region. However, the concept of defining these items for quality assurance was the same, and it is expected that the pharmacopoeias will be used after a thorough understanding of the General Notices and applicable sections. However, as 2 items (JP GN 43: Well-closed containers, and “Alcohol content/ethanol”) differed in terms of their definition, the users should be aware of them.
Since the pharmacopoeias have been developed individually under the regulatory framework of each country/region, the structure of the pharmacopoeias including the General Notices is unique. For the JP, some of the contents listed in the USP and/or Ph. Eur. General Notices were described in the laws and notifications, in addition to the sections other than the General Notices in the JP. It is considered difficult for overseas users to obtain such information considering the need to read and examine content other than the JP, especially since the notifications are issued only in Japanese. It is necessary to disseminate information in English actively, and it is desirable to develop tools that facilitate access to such information. This study revealed no major differences in the descriptions in the General Notices of the three pharmacopoeias for most of the items even though users need to be careful on some items. For the pharmacopoeias, the user must understand the General Notices before referring to the monographs and test methods, and it is expected manufacturing of APIs, excipients, and drug products that conform to multiple pharmacopoeias in response to the globalization of drug distribution will be facilitated by manufacturers’ understanding of the similarities and differences highlighted in this study. Moreover, we hope that this study helps users who are already using one or two of the pharmacopoeias to understand the content in the rest of the pharmacopoeias and expand users of the three pharmacopoeias.
In this study, we compared the existence of items and the contents described in the General Notices of the JP, USP, and Ph. Eur. Investigation of the existence of items revealed that more than 70% of the 105 items in General Notices in the three pharmacopoeias were in the entire pharmacopoeias (for Japan, including Japanese laws and notifications). Furthermore, an investigation of the contents showed that approximately 20% of the 105 items have some differences, such as numerical values and test conditions; however, it is possible to simplify the confirmation of compliance with the three pharmacopoeias by controlling conditions in some cases, and it was indicated that most of the items did not have major differences.
The JP 18th Edition (JP18, including the English version of JP17),6) USP and the National Formulary 2021 (USP-NF 2021),8) and Ph. Eur. 10th edition (Ph. Eur.10.0)10) were analyzed and compared in this study. In addition, as guidelines for the preparation of pharmacopoeial drafts that describe how to describe the draft pharmacopoeia, “the 18th Revision of the JP Drafting Guidelines,”27) “Submission Guideline for Chemical Medicines,”21) “European pharmacopoeia style guide (2017),”22) and “Technical guide for the elaboration of monographs on medicinal products containing chemically defined active substances (2020)”23) were also used in this study. They were the latest versions of each pharmacopoeia as of July 1, 2021.
Investigation of Descriptions in the USP and Ph. Eur. Corresponding to the JP General NoticesFor 49 JP General Notices, we investigated whether the same items existed in the USP General Notices and/or Ph. Eur. General Notices. For each JP General Notice, items present in both USP General Notices and Ph. Eur. General Notices were classified as Class A, items present in the USP General Notices but not in the Ph. Eur. General Notices were classified as Class B, items not present in the USP General Notices but present in the Ph. Eur. General Notices were classified as Class C, and items not present in the USP General Notices or Ph. Eur. General Notices were classified as Class D. Moreover, we investigated whether the same items were present in the entire pharmacopoeia, “Submission Guideline for Chemical Medicines,”21) “European pharmacopoeia style guide (2017),”22) and “Technical guide for the elaboration of monographs on medicinal products containing chemically defined active substances (2020)”23) for Class B to Class D. Subsequently, we investigated similarities and differences between the contents for each item in Class A to Class D. The number of items was counted based on the number of the JP General Notices. When the USP or Ph. Eur. General Notices mentioned referring to other sections for details, the items were also counted as items present in the General Notices.
Investigation of Items Not in the JP General Notices but in the USP General Notices and/or Ph. Eur. General NoticesItems not present in the JP General Notices but present in the USP General Notices and/or Ph. Eur. General Notices were classified as whether they existed in both USP General Notices and Ph. Eur. General Notices or one of them. Items present in both USP General Notices and Ph. Eur. General Notices were classified as Class E, items present in the USP General Notices but not in the Ph. Eur. General Notices were classified as Class F, and items not present in the USP General Notices but present in the Ph. Eur. General Notices were classified as Class G. Next, for Class E, we investigated whether items existed in the entire JP or “the 18th Revision of the JP Drafting Guidelines.”27) For Class F and Class G, we also investigated whether the items existed in the pharmacopoeia sections other than General Notices, “the 18th Revision of the JP Drafting Guidelines,” “Submission Guideline for Chemical Medicines,”21) “European pharmacopoeia style guide (2017),”22) and “Technical guide for the elaboration of monographs on medicinal products containing chemically defined active substances (2020).”23) In addition to the items specified in the PMD Act, various notifications and notices from the MHLW provide much supplementary information and related information on points to consider when interpreting and using the information in the JP.11) Therefore, we also investigated whether the same items existed in Japanese laws (the PMD Act, Enforcement Ordinance of the PMD Act, Enforcement Regulations of the PMD Act) and notifications for the items in Class E to Class G. Subsequently, we investigated similarities and differences in the contents for each item in Class E to Class G. Since the Ph. Eur. General Notices are only roughly classified into items, the number of items is mainly counted based on the USP General Notices and items not existing in USP General Notices but present in Ph. Eur. General Notices (Class G) were counted based on the number of items shown in Fig. 3 and Supplementary Table 2. For the JP, items present in the JP sections other than the MHLW Ministerial notification such as Preface and General Information, the Japanese laws, and notifications including “the 18th Revision of the JP Drafting Guidelines”27) were counted as those in the entire JP (including the laws and notifications).
Koko Tanaka is an employee of Amgen K.K. Rieko Saito and Maki Matsuhama are employees of the Pharmaceuticals and Medical Devices Agency. Seiko Miyazaki is an executive director of Pharmaceutical and Medical Device Regulatory Science Society of Japan. The views expressed in this manuscript are those of the authors and do not necessarily reflect the views of the authors’ organizations.
This article contains supplementary materials.