2023 Volume 71 Issue 10 Pages 782-786
2023 Volume 71 Issue 10 Pages 782-786
Catechols possessing electron-withdrawing groups at the C3 position effectively underwent oxidative functionalization at the C4 position in the presence of phenyliodine(III) diacetate (PIDA) and heteroarene nucleophiles (e.g., indole, indazole, and benzotriazole) to produce the corresponding biaryl products. The PIDA-mediated oxidation of catechol derivatives afforded the ortho-benzoquinone intermediate, which subsequently underwent regioselective nucleophilic addition to the α,β-unsaturated carbonyl moiety of ortho-benzoquinone using indole, indazole, and benzotriazole to give 4-substituted catechol derivatives in a one-pot manner. Notably, the nucleophilic substitution positions of indazole and benzotriazole were perfectly controlled. Additionally, the reaction using N-methylaniline as the nucleophile afforded a tertiary amine product.
ortho-Benzoquinones (1,2-benzoquinones) are readily prepared by the oxidation of catechols or phenols and are utilized as reactive reaction intermediates to form complex benzene skeletons (Chart 1A). ortho-Benzoquinones, which possess two carbonyl groups and two olefin moieties, can function as enones, dienones, carbodienes, heterodienophiles, heterodienes, and dienophiles in various organic reactions.1–3) Therefore, controlling the reaction position is challenging. Nucleophilic additions to the enone (α,β-unsaturated carbonyl) moiety of ortho-benzoquinones using alcohols,4) phenols,4–6) secondary-amines,2) and thiols4) have been recently accomplished. We have also recently reported the regioselective nucleophilic addition of indoles to ortho-benzoquinone intermediate (I) derived from 3-methoxycarobonylcatechol (1a) to afford arylindoles, biaryl products, in the presence of phenyliodine(III) diacetate (PIDA) with or without BF3·Et2O as a Lewis acid7) (Chart 1B). While a C4-adduct (2) was selectively obtained in the absence of BF3·Et2O, the use of a catalytic amount of BF3·Et2O gave a C5-adduct (3). To expand our synthetic strategy, we performed the regioselective and oxidative functionalization of catechols substituted with an electron-withdrawing group (EWG) at the 3-position using indole, indazole, benzotriazole, and N-methylaniline (Chart 1C).

Indoles are pharmaceutically useful skeletons8–13) and behave as electron-rich arene nucleophiles. The electron-rich arenes can be useful nucleophiles, because the preliminary functionalization, which causes the production of wastes, is not required.14,15) In our previous work7) (Chart 1B), BF3·Et2O played a key role in switching the regioselectivity of the nucleophilic position on 3-methoxycarbonyl ortho-benzoquinone (I) using an indole nucleophile. Acetic acid (AcOH) was produced during the PIDA-mediated oxidation of 1a. AcOH effectively activated the C2-carbonyl moiety on A, which facilitated the 1,4-addition of indole at the C4-position to give 2. On the other hand, catalytic BF3·Et2O preferentially coordinated to the C1-carbonyl group of I to produce 3 via the 1,4-addition of indole at the C5-position. When 3-nitrocatechol (1b) was used as a substrate instead of 1a to react with an indole nucleophile, a 4-adduct (4) was selectively obtained with or without BF3·Et2O (Chart 2). After the oxidation of 1b using PIDA in CH2Cl2 or tetrahydrofuran (THF), the addition of the indole afforded 4-adduct 4a as the main product in 71 and 70% yields, respectively (entry 1, left). BF3·Et2O (5 mol%) had no effect on the regioselectivity, affording 4a as the main product in 55% yield and 5a in 11% yield (entry 1, right). A similar behavior was observed when 5-fluoroindole and 2-methoxycarbonylindole were used as nucleophiles to produce other 4-adducts (4b and 4c; entries 2 and 3). While the reaction using 2-phenylindole gave 4-adduct 4d as the sole product in the absence of BF3·Et2O, the reaction using BF3·Et2O selectively afforded a 5-adduct (5d).16) Although density functional theory (DFT) calculations have been conducted to elucidate these selectivities, the reason remains unclear.

Next, we examined the oxidative functionalization of 1a using an indazole instead of an indole as the nucleophile (Table 1). Indazole is also a pharmaceutically useful skeleton,17–25) and the selectivity of the nucleophilic position (N1 or N2-atom on indazole) depends on the reaction conditions.17–25) Specifically, the reaction of 1a with indazole can possibly furnish four regioisomers [C4/N2-adduct (6a), C5/N2-adduct (7a), C4/N1-adduct (8a), and C5/N1-adduct (9a)]. After screening the reaction conditions, 6a was selectively obtained as the main product and its structure was determined by X-ray structural analysis. Compound 6a was obtained in 51% yield using BF3·Et2O (entry 1), while the reaction without BF3·Et2O gave 6a in 58% yield (entry 2). The use of other Lewis acid catalysts, such as InCl3 and FeCl3, was less effective in improving the yield, and the regioselectivity remained unchanged (entries 3 and 4). The use of THF instead of CH2Cl2 in the absence of BF3·Et2O improved the reaction efficiency, giving 6a in 68% yield (entry 5). Hexafluoroisopropanol (HFIP), CHCl3, and toluene were ineffective in this reaction (entries 6–8).
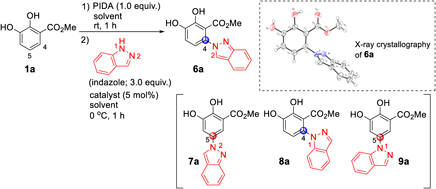 | ||||
|---|---|---|---|---|
| Entry | Catalyst | Solvent | Yield (%) | |
| 6a | 7a, 8a, or 9a | |||
| 1 | BF3·Et2O | CH2Cl2 | 51 | ND |
| 2 | — | CH2Cl2 | 58 | ND |
| 3 | InCl3 | CH2Cl2 | 58 | ND |
| 4 | FeCl3 | CH2Cl2 | 53 | ND |
| 5 | — | THF | 68 | ND |
| 6 | — | HFIP | 46 | ND |
| 7 | — | CHCl3 | 38 | ND |
| 8 | — | toluene | 23 | ND |
PIDA; phenyliodine(III) diacetate, THF; tetrahydrofuran, HFIP; hexafuluoroisopropanol, ND; not detected.
The oxidative functionalization of 1a using 5-bromoindazole and 6-methoxycarbonylindazole in CH2Cl2 also afforded the C4/N2-adducts (6b and 6c) with or without BF3·Et2O (Chart 3). The use of benzotriazole also afforded a C4-adduct (6d), and CH2Cl2 was found to be a better solvent than THF. Although the reason for the behavior of the nucleophilic positions of indazole17–25) and benzotriazole26–35) is unclear, it is noteworthy that the nucleophilic positions were perfectly controlled. N-Methylaniline was also compatible in this reaction, furnishing C4-adduct 6e. The reactivity in THF was higher than that in CH2Cl2.
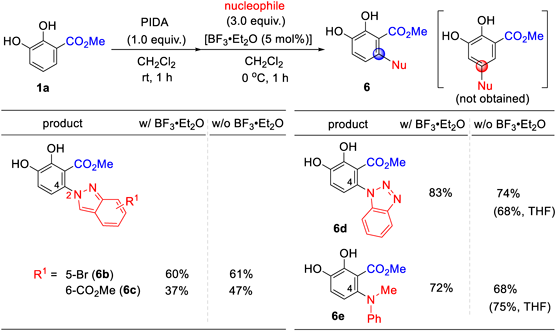
The scope and limitations of this study were investigated (Chart 4). The reactions of 1b, a 3-nitro compound, with indazole and N-methylaniline with or without BF3·Et2O resulted in a complex mixture and did not give the desired products (6f and 6g). Oxidative functionalization using benzotriazole effectively produced a C4-adduct (6h) in 68% yield. 3-Cyanocatechol 1c also underwent oxidative functionalization using benzotriazole to give C4-adduct 6l, while products (6i–6k) were not obtained from indole, indazole, and N-methylaniline, respectively, in the presence or absence of BF3·Et2O. In the latter reactions, the reactions resulted in complex mixture probably due to no regioselectivty of nucleophiles. Unfortunately, the structures of byproducts could not be identified.

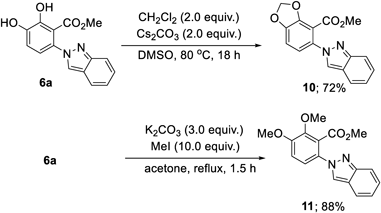
Further functionalization of the synthesized product 6a was conducted. The catechol moiety of 6a was effectively transformed into a 1,2-methylenedioxybenzene skeleton (10) in the presence of CH2Cl2 and Cs2CO3 (Chart 5, top). Additionally, the dimethylation of the two hydroxy groups in 6a was successfully accomplished to give 11.
We accomplished the oxidative coupling of catechol derivatives possessing an electron-donating group at the C3 position in the presence of a PIDA oxidant and nucleophiles. Our previous study showed that the regioselectivity of the nucleophilic positions changed in the absence or presence of BF3·Et2O when using a combination of 3-methoxycarbonylcatechol (1a) and an indole nucleophile to selectively give C4- or C5-adduct, as shown in Chart 1B.7) In contrast, this study revealed that the use of other nucleophiles toward 1a and other substrates possessing 3-nitro- and 3-cyano groups with indole as well as indazole and benzotriazole nucleophiles afforded C4-adducts as the main products with or without BF3·Et2O. The biaryl skeletons obtained are pharmaceutically useful. Additionally, the use of N-methylaniline gave a tertiary amine product. The present one-pot functionalization method of catechols is valuable for the construction of various complex benzene-linked skeletons. The elucidation of the regioselectivity based on DFT calculations is currently underway.
Typical Procedure without BF3·Et2O; To a suspension of catechol (1; 0.20 mmol) in CH2Cl2 (1.0 mL) in a dry brown test tube was added PIDA (64.4 mg, 0.2 mmol) under argon. The reaction mixture was placed on an organic reactor, Chemi Station (EYELA, Tokyo Rikakikai Co., Ltd., Tokyo, Japan), and the mixture was stirred at 25 °C for 1 h. Nucleophile (3.0 equivalents (equiv.)) and CH2Cl2 (1.0 mL) were added sequentially, and the mixture was stirred at 0 °C for 1 h. After quenching with H2O, the reaction mixture was extracted with CH2Cl2 (10 mL×3). The organic layer was dried over Na2SO4 and concentrated in vacuo. Purification using flash column chromatography on silica gel (n-hexane-ethyl acetate) gave 4-adduct.
This study was partially supported by JSPS (MEXT Grant in Aid-for transformative research areas (B) Deuterium Science) KAKENHI Grant Number 20H05738 (for Y.S.), and Life Science and Drug Discovery (Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)) from AMED under Grant Number 23ama121054 (for Y.S.) and Research Foundation for the Electrotechnology of Chubu (REFEC) (for Y.S.).
The authors declare no conflict of interest.
This article contains supplementary materials.