2023 Volume 71 Issue 11 Pages 832-837
2023 Volume 71 Issue 11 Pages 832-837
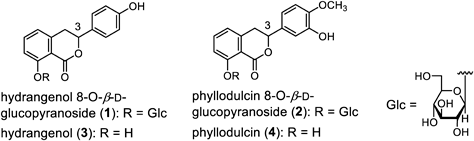
Dihydroisocoumarins, hydrangenol 8-O-β-D-glucopyranoside (1), phyllodulcin 8-O-β-D-glucopyranoside (2), hydrangenol (3), and phyllodulcin (4), are well-known as the major secondary metabolites in the leaves of Hydrangea macrophylla var. thunbergii. Dihydroisocoumarins are pharmaceutical compounds with diverse bioactivity. Although dihydroisocoumarins are commonly isolated from Hydrangea plants or via organic chemical synthesis, their production via callus induction is considered a promising alternative. In the present study, callus induction and proliferation of H. macrophylla var. thunbergii, and constituents 1–4 were quantified in calluses cultured in 17 different media. We found that the combination of the phytohormones 2,4-dichlorophenoxyacetic acid (2,4-D) and 6-benzylaminopurine (BA) was useful for callus proliferation in H. macrophylla var. thunbergii. The balance and concentrations of indole-3-acetic acid (IAA) and BA greatly affected the contents of 1–4. Particularly, 1 (2.03–3.46% yield from the dry callus) was successfully produced from the callus induced by IAA (0.5 mg/L) and BA (1.0 mg/L) at yields comparable to isolated yields from plants. To the best of our knowledge, this is the first study to show that the calluses of H. macrophylla var. thunbergii contained 1. These findings may be useful for producing bioactive dihydroisocoumarins.
Hydrangea macrophylla Seringe var. thunbergii Makino (Hydrangea serrata [Thunb.] Ser. var. thunbergii [Siebold] H. Ohba) belongs to the family Saxifragaceae (APG: Hydrangeaceae) and is native to Japan. Currently, the processed leaves and branch tips of this plant are used as crude drugs with a sweet taste for patients with diabetes and as oral refrigerants. This crude drug is listed as Sweet Hydrangea Leaf (Hydrangea Dulcis Folium) in the 18th edition of the Japanese Pharmacopoeia. Hydrangenol 8-O-β-D-glucopyranoside (1), phyllodulcin 8-O-β-D-glucopyranoside (2), hydrangenol (3), and phyllodulcin (4) are the major secondary metabolites found in the leaves1–4) (Fig. 1). The structural features of these compounds include a dihydroisocoumarin skeleton. Recently, dihydroisocoumarins 3 and 4 were reported to inhibit the binding of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein to angiotensin-converting enzyme 2 (ACE2).5) In addition, an in vitro lipid peroxidation assay revealed that 4 exhibited an antioxidative effect on reduced nicotinamide adenine dinucleotide phosphate (NADPH)-induced microsomal lipid peroxidation and Fenton-type reactions.6) The authors concluded that dihydroisocoumarins are useful pharmaceutical compounds with diverse bioactive properties. Although dihydroisocoumarins are commonly isolated from Hydrangea plants or by organic chemical synthesis,7) dihydroisocoumarin production via callus induction is also an attractive alternative. In plant science, the callus refers to an irregularly shaped cell mass formed at the site of injury. Cultured cells derived from the calluses of medicinal plants are used to produce plant secondary metabolites. However, to the best of our knowledge, there has been only one report on callus induction in H. macrophylla var. thunbergii. Compound 3 was isolated from callus induced with H. macrophylla var. thunbergii by Suzuki et al.,3) but 3 was not quantified, and there have been no reports on the isolation of 1, 2, or 4. We investigated the effects of phytohormones on callus induction in H. macrophylla var. thunbergii. Additionally, we investigated the presence of dihydroisocoumarins 1–4 in the callus and quantified 1–4 in calluses cultured in 17 different media.
The callus growth rate induced by H. macrophylla var. thunbergii leaves was examined (Figs. 2–4). The apical buds and leaves just after the unfolding of H. macrophylla var. thunbergii were sterilized and placed in the callus induction medium. The induction medium consists of 4.3 g/L Murashige and Skoog (MS) medium8) with 30 g/L sucrose, 0.5 mL/L Plant Preservative Mixture™ (PPM™),9) and 3 g/L gellan gum, supplemented with 0.5 mg/L 2,4-dichlorophenoxy acetic acid (2,4-D) and 0.5 mg/L 6-furfuryl aminopurine (KIN).10) Calluses were identified approximately 2 weeks (wk) later. Approximately 25% of the apical buds and 45% of the leaves just after unfolding were callus induced. Successfully induced calluses (Callus S in Fig. 2) were isolated from the explants, transferred to induction medium, and cultured. One callus (Callus S′ in Fig. 2) was selected and cut into multiple pieces before being transferred to the growth medium with 5.0 mg/L 2,4-D and 0.5 mg/L KIN and cultured for 8 or 29 wk. The calluses obtained from Callus S′ were named Callus S″ (8 wk) or Callus S″ (29 wk), and the contents of dihydroisocoumarins 1–4 in a portion [10.8 mg for Callus S″ (8 wk), 20.0 mg for Callus S″ (29 wk)] were investigated using LC-MS analysis. Callus S″ (8 wk) and Callus S″ (29 wk) did not contain 1, 2, or 4 and the content of 3 was less than 0.01%. Next, Callus S″ (8 wk) was cut to approximately equal size (maximum diameter: 5–15 mm), transferred to A-H media,11) and cultured for 8 wk (Table 1, Fig. 2). For callus proliferation, the bottom area of callus was collected at weeks 0, 4, and 8. The Petri dish was inverted, the bottom of the callus was traced onto a piece of tracing paper, and the area was calculated using ImageJ software developed by the National Institutes of Health (NIH) (Fig. 3). When comparing calluses A–D cultured in media A–D supplemented with 2,4-D (auxin) and 6-benzylaminopurine (BA) (cytokinin) to calluses E–H cultured in media E–H supplemented with indole-3-acetic acid (IAA) (auxin) and BA, calluses A–D exhibited a slightly higher growth rate, with callus D exhibiting the highest growth rate (Table 1, Fig. 4). Phytohormones are plant growth regulators, and their types and concentrations are known to be involved in callus growth and the formation of secondary metabolites.12–15) Callus induction, growth, and plant regeneration are regulated by the balance between auxins and cytokinins. However, the types, balance, and concentrations of auxins and cytokinins are not universal and vary among plant species. Therefore, they must be examined in each plant. In the present study, the highest proliferation rates were observed in calluses induced by H. macrophylla var. thunbergii leaves when 2.0 mg/L 2,4-D and 1.0 mg/L BA were added to the basic medium. Additionally, considering that the production of secondary metabolites using callus, stem, and root redifferentiation was not necessary, shoot and root redifferentiation was not observed with this phytohormone type and concentration.


aThe bottom area of the callus was obtained at weeks 0, 4, and 8. The bottom of the callus was copied onto a piece of tracing paper from an inverted Petri dish, and the area was calculated using ImageJ software developed by the National Institutes of Health (NIH).

Proliferation rate = area at 8 wk/area at 0 wk. All values are expressed as mean ± standard deviation (S.D.) of three calluses grown in each medium. Tukey’s multiple test showed no significant differences at the 5% level in the treatment intervals. This test was performed by cutting callus (Callus S″ in Fig. 2) obtained from one leaf into eight pieces and culturing them on media A–H. The procedure was repeated three times for the callus obtained from the other two leaves of the same tree.
| Media (callus)b) | Phytohormone (mg/L)a) | ||||
|---|---|---|---|---|---|
| Auxin | Cytokinin | Auxin : Cytokinin | |||
| 2,4-D | IAA | BA | |||
| A | 0.20 | — | 0.20 | 1 : 1 | |
| B | 0.50 | — | 0.10 | 5 : 1 | |
| C | 1.00 | — | 0.10 | 10 : 1 | |
| D | 2.00 | — | 1.00 | 2 : 1 | |
| E | — | 0.50 | 2.00 | 1 : 4 | |
| F | — | 0.05 | 0.10 | 1 : 2 | |
| G | — | 0.05 | 0.20 | 1 : 4 | |
| H | — | 0.10 | 0.20 | 1 : 2 | |
| I | — | 0.25 | 0.50 | 1 : 2 | |
| J | — | 0.20 | 0.40 | 1 : 2 | |
| K | — | 0.50 | 1.00 | 1 : 2 | |
| L | — | 0.05 | 0.25 | 1 : 5 | |
| M | — | 0.05 | 0.40 | 1 : 8 | |
| N | — | 0.05 | 0.50 | 1 : 10 | |
| O | — | 0.20 | 0.20 | 1 : 1 | |
| P | — | 1.00 | 0.20 | 5 : 1 | |
| Q | — | 2.00 | 0.20 | 10 : 1 | |
a) 2,4-D: 2,4-dichlorophenoxyacetic acid; IAA: indole-3-acetic acid, BA: benzyl adenine. b) Callus cultured on media A–Q are referred to as “callus A–Q,” respectively.
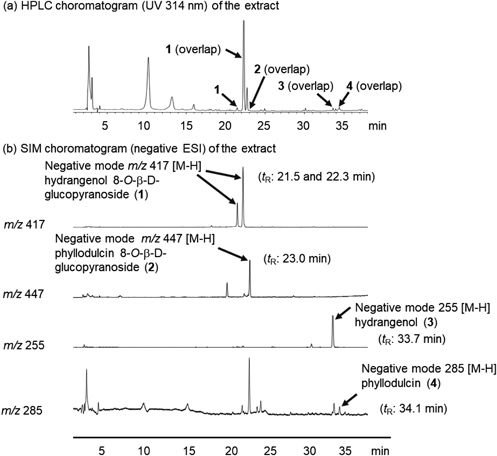
The leaves of H. macrophylla var. thunbergii have also been reported to contain hydrangenol 8-O-β-D-glucopyranoside (1), phyllodulcin 8-O-β-D-glucopyranoside (2), hydrangenol (3), and phyllodulcin (4) as principal constituents.1,2,4) Dihydroisocoumarins 1–4 were isolated according to previously reported methods and identified by comparing their spectral data with reference data.4,7) These constituents were used as standard samples. On the HPLC chromatograms for standard samples with UV detection at a wavelength of 314 nm, peaks were observed at the following retention times (tR): 1, 21.5 and 22.3 min (3R and 3S mixture); 2, 23.0 min; 3, 33.7 min; and 4, 34.1 min. However, the HPLC chromatograms of the callus methanol extracts derived from the leaves of H. macrophylla var. thunbergii overlapped with those of unidentified compounds. Therefore, we quantified constituents 1–4 in the callus using LC-MS analysis. LC-MS chromatograms for callus methanol extracts under MS detection by electrospray ionization (ESI) MS in the negative mode demonstrated good baseline separation for all peaks. Each peak was observed as a quasi-molecular ion peak ([M–H]−) [1: m/z 417; 2: m/z 447; 3: m/z 255; 4: m/z 285] (Fig. 5). These peaks were unambiguously assigned by comparing their retention times with those of authentic samples. The linear regression equations for the calibration curves of each sample are listed in Table 2. As previously reported, the most efficient extraction method for dihydroisocoumarin and its glycosides is sonication with methanol.7) Therefore, analytical samples were prepared twice by ultrasonic extraction with methanol at room temperature (25–30 °C) for 15 min.
YMC-Triart C18 column (5 µM particle size, 4.6 mm i.d. × 250 mm, YMC Co., Ltd., Japan) operated at 40 °C under the following conditions: mobile phase A (acetonitrile) and B (H2O containing 1.0% acetic acid); gradient program: 0–10 min (A : B 10 : 90, v/v, hold)→35 min (A : B 60 : 40, v/v)→40 min (A : B 100 : 0, v/v). The flow rate was 1.0 mL/min.
| Analyte | Regression equationa) | Correlation coefficient (R2) |
|---|---|---|
| Hydrangenol 8-O-β-D-glucopyranoside (1) [1]b) | y = 445806x + 1192669 | 0.9994 |
| Hydrangenol 8-O-β-D-glucopyranoside (1) [2]b) | y = 940281x + 12789203 | 0.9990 |
| Phyllodulcin 8-O-β-D-glucopyranoside (2) | y = 2740224x + 472997 | 0.9994 |
| Hydrangenol (3) | y = 13300758x + 10167590 | 0.9997 |
| Phyllodulcin (4) | y = 1272442x − 155715 | 0.9996 |
a) In the regression equation, x is the analyte solution concentration (µg/mL) and y is the analyte peak area. b) Compound 1 (3R and 3S mixtures) showed two peaks. These peaks are observed at retention times (tR) of [1] 21.5 min and [2] 22.3 min.
Dihydroisocoumarin constituents 1–4 of H. macrophylla var. thunbergii have a wide range of pharmacological effects. Therefore, callus cultures may be a source of dihydroisocoumarin. As a preliminary experiment, we conducted a qualitative investigation of 1–4 in callus cultured with several phytohormones. The results showed that callus cultured with a combination of IAA and BA contained 1 and 2 (Tables 1, 3, calluses G and H, respectively). To the best of our knowledge, this is the first time compounds 1 and 2 have been identified in callus. Therefore, we attempted to proliferate calluses containing the highest concentrations of dihydroisocoumarins 1–4 by varying the concentrations and ratios of the phytohormones IAA and BA. To examine the contents of 1–4, 27 calluses (calluses I-1–Q-3) were prepared. Callus I-1–Q-3 were obtained by cutting Callus S″ (29 wk) into approximately the same size (maximum diameter: 5–15 mm), transferring them to I–Q medium, and culturing for 8 wk (Fig. 2). The concentrations of compounds 1–4 were evaluated using LC-MS analysis. Compound 1 was present at a high concentration when the ratio of IAA to BA was 1 : 2 (Tables 1, 3; calluses I, J, and K). Particularly, 2.03–3.46% yield was produced for compound 1 from callus K with IAA (0.5 mg/L) and BA (1.0 mg/L). The content of 1 in the plant [leaves of H. macrophylla var. thunbergii] has been reported as 2.15–3.58%.4) Therefore, callus K contained the same amount of compound 1 as the plant. Given that the separation and purification of 1 from plants is more difficult than that from calluses, callus K, which has a high content of 1 was considered useful as a material for supplying 1. Compounds 2 and 3 were the most abundant in callus K, similar to compound 1. However, the product yield was low. Compound 4 was rarely present in the calluses prepared in this study.
| Callus | Yield (% from dry weight) in callusa) | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| G-1 | 0.15 | <0.01 | <0.01 | <0.01 |
| H-1 | 0.54 | 0.012 | <0.01 | <0.01 |
| I-1 | 0.81 | 0.014 | 0.022 | <0.01 |
| I-2 | 1.31 | 0.024 | <0.01 | <0.01 |
| I-3 | 1.09 | 0.011 | <0.01 | <0.01 |
| J-1 | 1.03 | 0.015 | <0.01 | NDb) |
| J-2 | 2.44 | 0.042 | 0.024 | <0.01 |
| J-3 | 2.47 | 0.027 | 0.017 | <0.01 |
| K-1 | 3.46 | 0.044 | 0.015 | <0.01 |
| K-2 | 2.03 | 0.036 | 0.022 | <0.01 |
| K-3 | 2.14 | 0.037 | 0.076 | <0.01 |
| L-1 | 1.17 | <0.01 | <0.01 | NDb) |
| L-2 | 1.29 | 0.010 | <0.01 | NDb) |
| L-3 | 0.65 | <0.01 | <0.01 | NDb) |
| M-1 | 0.82 | <0.01 | <0.01 | <0.01 |
| M-2 | 1.08 | 0.017 | <0.01 | <0.01 |
| M-3 | 2.26 | 0.021 | 0.032 | <0.01 |
| N-1 | 0.70 | 0.014 | <0.01 | <0.01 |
| N-2 | 1.22 | 0.018 | <0.01 | <0.01 |
| N-3 | 2.04 | 0.032 | 0.019 | <0.01 |
| O-1 | <0.01 | <0.01 | <0.01 | <0.01 |
| O-2 | <0.01 | <0.01 | <0.01 | <0.01 |
| O-3 | <0.01 | <0.01 | <0.01 | <0.01 |
| P-1 | 0.23 | <0.01 | <0.01 | <0.01 |
| P-2 | <0.01 | <0.01 | <0.01 | <0.01 |
| P-3 | 0.50 | <0.01 | <0.01 | <0.01 |
| Q-1 | 0.32 | <0.01 | <0.01 | NDb) |
| Q-2 | 0.05 | <0.01 | <0.01 | NDb) |
| Q-3 | <0.01 | <0.01 | <0.01 | NDb) |
a) Callus S″ (8 wk) and Callus S″ (29 wk) did not contain 1, 2, or 4 and the content of 3 was less than 0.01%. b) ND: Not detected.
In the present study, the combination of the phytohormones 2,4-D and BA was useful for callus proliferation. On the other hand, the balance and concentration of IAA and BA significantly affected the contents of dihydroisocoumarins in callus of H. macrophylla var. thunbergii. Particularly, 2.03–3.46% yield was produced for compound 1 from callus K with IAA (0.5 mg/L) and BA (1.0 mg/L). Therefore, callus cultures can be used for mass production of bioactive dihydroisocoumarins. In addition, only a high content of 1 was observed in the calluses. The reason and culture conditions for calluses with high contents of 2–4 will be investigated in the future.
Apical buds and leaves were collected from Hydrangea macrophylla Seringe var. thunbergii Makino was grown at the Medicinal Botanical Garden of Kyoto Pharmaceutical University between September and November 2022. The plant materials were identified by one of the authors (J. T.). A voucher specimen of the medicinal plant (KPU-20041) was deposited in the garden. Immediately after unfolding, the apical buds and leaves were used as explants to induce callus formation. Explants were soaked in 70% ethanol for 1 min, followed by 1% Sodium Hypochlorite for 15 min, and rinsed with distilled water three times. Then the explants were immersed in a 5% Plant Preservative Mixture™ (PPM™) solution and sterilized by agitation for 4 h.
Chemicals and ReagentsThe reagents for callus induction, multiplication, and HPLC analysis were purchased from FUJIFILM Wako Pure Chemical Corporation, Japan; NACALAI TESQUE, Inc., Japan; and KENIS LIMITED, Japan.
Media and Culture ConditionsMurashige and Skoog (MS) medium8) containing vitamins was used in all experiments. The MS medium supplemented with 30 g/L sucrose, 0.5 mL/L PPM™ and different combinations of phytohormones. The pH of the medium was adjusted to 5.8 using either 0.1 M HCl or 0.1 M NaOH before adding 3 g/L gellan gum and autoclaving at 121 °C for 20 min. Petri dishes (9 cm diameter × 2 cm height) were used to culture the callus. All the cultures were maintained under dark conditions in a growth chamber (Cool Incubator CN-25C) at 25 ± 2 °C.
Callus Induction and SubcultureFor callus induction, the MS medium containing vitamins (30 g/L sucrose, 0.5 mL/L PPM™, and 3 g/L gellan gum) was supplemented with two kinds of phytohormones: 2,4-dichlorophenoxy acetic acid (2,4-D) 0.5 mg/L and 6-furfuryl aminopurine (KIN) 0.5 mg/L. Sterilized explants were inoculated on the media and cultured under dark conditions in a growth chamber at 25 ± 2 °C for approximately 4 wk. After callus formation was confirmed, the successfully induced callus was isolated from the explant, transferred to the medium conditioned for callus induction and cultured under dark conditions in a growth chamber at 25 ± 2 °C. One callus (Callus S′ in Fig. 2) was selected and cut into several pieces and transferred to the growth medium, and cultured for 8 wk (Callus S″ in Fig. 2). In the growth medium, MS medium including vitamins (with 30 g/L sucrose, 0.5 mL/L PPM™, and 3 g/L gellan gum) was supplemented with two kinds of phytohormones: 2,4-D 5.0 mg/L and KIN 0.5 mg/L.9) Calluses were passed on at approximately 8 wk intervals using the growth medium to take over the callus.
Determination of Callus MultiplicationTo determine the effects of phytohormones on callus multiplication, callus (Callus S″ in Fig. 2) were cut into several pieces and transferred to their respective media (Table 1). Callus proliferation was evaluated by approximating the bottom area of the callus on a solid medium at 4 and 8 wk after callus placement.16) Tracing paper was placed in close contact with the bottom of the Petri dish in which the callus was to be cultured, and the edges of the callus were copied. Callus area was determined using ImageJ software (National Institutes of Health).
Preparation of Callus for Quantification of Compounds 1–4To determine the effects of phytohormones on the production of compounds 1–4 in the callus, callus (Callus S″ in Fig. 2) were cut into several pieces and transferred to their respective media, I–Q, as shown in Table 1, from the growth medium. Preliminary component analysis of calluses cultured in media A–H revealed peaks of compounds 1–4 in calluses cultured with media G and H. Quantitative analysis of calluses cultured in media I–Q (Table 3) was performed based on these results. Calluses cultured on the media were freeze-dried in a lyophilizer (Eyela FDU-1200). The lyophilized calluses were crushed and sonicated in MeOH (800 µL) for 15 min at room temperature. The extracts were centrifuged at 3000 rpm for 5 min, and the supernatant was transferred into a 2 mL volumetric flask. The extraction process was repeated twice. The combined solution was made up to the volume with MeOH. The solution was filtered through a syringe filter (0.45 µm), and subjected to LC-MS analysis.
Preparation of Standard SolutionIsolation and structure determination of the reference samples of compounds 1–4 were performed using previously described methods.4) Accurately weighed 10.00 mg of each reference sample 1–4 was introduced into a 20 mL volumetric flask and made up to the volume with methanol (MeOH). The resulting solution was used as a standard stock solution (0.50 mg/mL for 1). Aliquots of 2 or 4 mL of the stock standard solution (0.50 mg/mL) were transferred into 20 mL volumetric flasks, and the volume was made up of MeOH. The resulting solution was used as a standard stock solution (0.05 mg/mL for 2 and 0.10 mg/mL for 1). Subsequently, 2 and 4 mL aliquots of each stock standard solution (0.05 mg/mL) were transferred to a 10 mL volumetric flask, and the volume was made up with MeOH. The obtained solution was used as a standard stock solution (0.01 mg/mL for 1–4 and 0.02 mg/mL for 1). Aliquots of 2 mL of the stock standard solution (0.02 mg/mL) were transferred into a 10 mL volumetric flask, and MeOH was added in sufficient quantity. The resulting solution was used as the standard stock solution (0.004 mg/mL for 1–4). Aliquots of 2 mL of the stock standard solution (0.004 mg/mL) were transferred into a 20 mL volumetric flask, and the volume was made up with MeOH. The obtained solution was used as a standard stock solution (0.0004 mg/mL for 1–4). This solution was used to prepare working solutions (0.4, 4.0, 10, 20, 100, and 500 µg/mL for 1; 0.4, 4.0, 10, and 50 µg/mL for 2; and 0.4, 4.0, and 10 µg/mL for 3 and 4) to construct the calibration curves. For calibration, an aliquot (4 mL or 20 µL) of each solution was injected into the LC–MS system. Each peak was observed at the following retention times: (tR): (tR): 1, 21.5 and 22.3 min (3R and 3S mixture, respectively); 2, 23.0 min, 3, 33.7 min; and 4, 34.1 min. The linearity of compounds 1–4 was plotted using a linear regression of the peak area versus concentration. The coefficient of correlation (R2) was used to determine the linearity.
HPLC Instrument and ConditionsCompounds 1–4 were analyzed using an HPLC system and an LCMS-8040 tandem quadrupole mass spectrometer (Shimadzu Corporation, Kyoto, Japan) equipped with an ESI interface. The instruments were operated using LabSolutions LCMS Ver. 5.6. The HPLC system was equipped with a system controller (CBM-20A), a PDA detector (SPD-M20A), two binary pumps (LC-20AD), an autosampler (SIL-20AC), a column heater (CTO-20AC), and a degasser (DGU-20A). The chromatographic separation was performed on a YMC-Triart C18 column (5 µM particle size, 4.6 mm i.d. × 250 mm, YMC Co., Ltd., Japan) operated at 40 °C under the following conditions: mobile phase A (acetonitrile) and B (H2O containing 1.0% acetic acid); gradient program: 0–10 min (A : B 10 : 90, v/v, hold)→35 min (A : B 60 : 40, v/v)→40 min (A : B 100 : 0, v/v). The flow rate was 1.0 mL/min and the injection volume was 20 µL. Detection was performed at 314 nm (UV) under selected-ion monitoring (SIM) using negative-mode electrospray ionization mass spectrometry. The operating parameters for MS detection were as follows: nebulizer gas flow, 3 L/min; drying gas flow, 15 L/min; desolvation line temperature, 250 °C; heat block temperature, 400 °C; and interface voltage, 4.5 kV.
This research was funded by the JSPS KAKENHI (Grant number 23H02642).
The authors declare no conflict of interest.
This article contains supplementary materials.