2023 Volume 71 Issue 12 Pages 852-858
2023 Volume 71 Issue 12 Pages 852-858
Porcine acellular dermal matrix (pADM) is known to accelerate wound healing. However, the underlying molecular mechanism remains unclear. This study aimed to investigate the effects of pADM on wound healing and its underlying mechanisms. HaCaT cells were treated with hydrogen peroxide (H2O2) or pADM, and the appropriate treatment concentration was determined using the cell counting kit-8 and flow cytometry. Cell migration was assessed using a Transwell assay and scratch test. Inflammation was evaluated using enzyme-linked immunosorbent assay. Western blotting was performed to measure the levels of protein kinase B (AKT) pathway-related proteins. The results showed that H2O2 inhibited cell viability and induced apoptosis in a dose-dependent manner. pADM promoted cell migration and decreased the levels of interleukin (IL)-6, IL-8, and tumor necrosis factor-α (TNF-α) in H2O2-treated HaCaT cells. Moreover, pADM rescued the downregulation of phosphorylated (p)-AKT and p-mechanistic target of rapamycin (mTOR) induced by H2O2. LY294002, a phosphatidylinositol 3-kinase (PI3K) inhibitor, abrogated migration and anti-inflammatory response caused by pADM. In conclusion, pADM promotes cell migration and inhibits inflammation by activating the AKT pathway under oxidative stress. These findings support the use of pADM for post-traumatic therapy and reveal a novel underlying mechanism of action.
Skin injuries threaten human health; therefore, it is particularly important to promote wound healing. Wound healing is a complex dynamic process that includes hemostasis, inflammatory response, proliferation, and matrix remodeling.1) Clinically, chronic non-healing wounds, accompanied by high incidence and recurrence rates, pose a huge economic burden.2) Various cells are involved in wound closure. Keratinocytes, the most dominant cells in skin tissues, play a crucial role in wound healing.3) Thus, elucidating the pathological mechanism of keratinocytes in chronic non-healing wounds may provide a new way to develop strategies to treat wounds.
Humans, porcine, and bovine acellular dermal matrix (ADM) has the potential to accelerate acute and chronic wound repair.4) Porcine ADM (pADM) is a collagen matrix that consists of sterilized pure type I and III collagen and elastin. It can be obtained in large quantities from pig dermis.5) Several studies have revealed that pADM is widely used in clinical applications, such as burns, breast reconstructions, abdominal wall herniorrhaphy, and gingival recession.4,6–8) It has been reported that pADM not only accelerates the speed, but also improves the quality of wound healing in full-thickness skin injuries.9) However, till date, the molecular mechanism of the involvement of pADM in wound closure remains unclear.
The phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT)/mechanistic target of rapamycin (mTOR) pathway plays a critical role in cellular functions such as proliferation, survival, transcription, and translation in physiological processes and pathological disorders.10,11) This pathway has been widely studied in numerous diseases, such as tumors, degenerative diseases, inflammation, and strokes.12–14) AKT is the downstream effector of PI3K. PI3K phosphorylates PIP2 and converts it to PIP3, which then docks to AKT. Phosphorylated AKT activates the downstream effector mTOR, promoting cell growth and survival.15,16) Accumulating evidence has shown that the PI3K/AKT/mTOR pathway is involved in wound closure and tissue repair.17) However, whether pADM regulates wound healing via this pathway needs to be further studied.
In this study, we explored the effects of pADM on keratinocyte migration and inflammation. Oxidative stress is a major cause of skin injury that damages human keratinocytes.18,19) Therefore, to induce injury, we used H2O2 to treat keratinocytes. We also evaluated the regulation of pADM via the AKT pathway. These findings provide a theoretical basis for the application of pADM in skin wound healing.
HaCaT cells were purchased from American Type Culture Collection (ATCC, Manassas, VA, U.S.A.). The cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; HyClone, South Logan, UT, U.S.A.) supplemented with 10% fetal bovine serum (HyClone) at 37 °C in a humidified incubator under 5% CO2.
Cell TreatmentHaCaT cells were seeded into 96-well plates containing culture medium (2000 cells/well). Subsequently, the cells were exposed to different concentrations of H2O2 (0, 0.25, 0.5, 1, and 2 mM; Sigma-Aldrich, St. Louis, MO, U.S.A.) at 37 °C for 12 h. Next, the cells were stimulated with 0, 0.25, 0.5, 1, and 2 mg/mL pADM (2 × 2 cm; Rickbeal, Beijing, China) at 37 °C for 24 h. To block the activation of the AKT pathway, the cells were treated with 50 µM LY294002 (a PI3K inhibitor; MedChemExpress, Monmouth Junction, NJ, U.S.A.) for 1 h before H2O2 treatment (total 13 h).20)
Cell Counting Kit-8 (CCK-8) AssayAfter treating the cells as described above, 10 µL CCK-8 (MedChemExpress, Monmouth Junction, NJ, U.S.A.) reagent was added to each well to determine cell viability. After incubation for 2 h, the absorbance was measured at 450 nm using a microplate reader (Bio-Rad, Hercules, CA, U.S.A.).
Flow CytometryAn Annexin V-PE/7-AAD apoptosis detection kit (Solarbio, Beijing, China) was used to assess cell apoptosis. HaCaT cells were washed with phosphate buffered saline (PBS) and then suspended in 250 µL binding buffer to adjust cell density to 106 cells/mL. Next, the cell suspension (100 µL) was incubated with 5 µL Annex V/PE and 10 µL 7-AAD for 15 min in the dark. After adding 400 µL PBS, apoptosis was detected by a flow cytometer (Moflo XDP, Beckman Coulter, Miami, FL, U.S.A.).
Transwell AssayTranswell chambers (8 µm, Corning, Corning, NY, U.S.A.) without Matrigel were used to detect cell migration. The harvested cells were suspended in 200 µL serum-free medium and added to the upper chambers. The lower chambers were filled with normal growth medium. After 24 h of culture, the migrated cells were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. The stained cells were imaged using five random fields under a microscope.
Scratch AssayHaCaT cells were seeded in 6-well plates at a density of 5 × 105 cells/well and cultured for 24 h. When the cells reached above 90% confluence, uniform scratch wounds were created using a sterile pipette tip. Each well was then washed with PBS to remove cell debris. Images were captured under a microscope at 0 and 24 h after scratching .
Enzyme Linked Immunosorbent Assay (ELISA)The levels of interleukin (IL)-6, IL-8, and tumor necrosis factor-α (TNF-α) were assessed using ELISA kits (Jiancheng, Nanjing, China) according to the manufacturer’s protocol.
Quantitative Real-Time PCR (qPCR)The TRIzol reagent (Invitrogen, Carlsbad, CA, U.S.A.) was used to extract total RNA from HaCaT cells. Then, the RNA was reverse transcribed to synthesize cDNA using the PrimeScript RT reagent kit (TaKaRa, Tokyo, Japan). qPCR amplification was determined using the TB Green Fast qPCR Mix (TaKaRa, Tokyo, Japan) on the LightCycler 480 real-time PCR system (Roche. Basel, Switzerland). mRNA expression was calculated using the 2−ΔΔCT method. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as the internal control.
Western Blot AssayHaCaT cells were lysed with radio immunoprecipitation assay (RIPA) buffer (Beyotime, Shanghai, China) for 30 min. Next, the samples were boiled in a loading buffer for 10 min to denature the proteins. After determining the protein concentration using a bicinchoninic acid (BCA) kit (Beyotime), the proteins were separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride (PVDF) membranes (Invitrogen, Carlsbad, CA, U.S.A.). Later, the membranes were incubated with primary antibodies (Abcam, Cambridge, MA, U.S.A.) at 4 °C overnight. The membranes were incubated with secondary antibody (Abcam) at room temperature for 1 h. Protein bands were visualized using the enhanced chemiluminescence (ECL) reagent (Beyotime). Protein levels were quantified using the ImageJ software.
Ethics ApprovalNo ethics approval was required for this study as it involved no human participants or animals.
Statistical AnalysisThe experiments were independently conducted at least three times. All data are expressed as mean ± standard deviation (S.D.), and the differences between two groups or multiple groups were analyzed using Student’s t-test or one-way ANOVA followed by Tukey’s post hoc test, respectively. Statistical significance was set at p < 0.05.
First, we established a cell injury model by treating HaCaT cells with different concentrations of H2O2 (0, 0.25, 0.5, 1, and 2 mM). The results showed that H2O2 decreased cell viability in a dose-dependent manner (Fig. 1A). In contrast, H2O2 facilitated cell apoptosis in a dose-dependent manner (Figs. 1B, C). These results demonstrated that the cell model was successfully established. Therefore, 1 mM H2O2 (about 50% cell viability) was used in the following experiment.

Following treatment of HaCaT cells with 0, 0.25, 0.5, 1, and 2 mM H2O2 for 12 h, (A) cell viability was assessed by CCK-8; (B) Representative plot of Annex V/PE (x-axis) and 7-AAD (y-axis) double staining by flow cytometry; (C) Late cell apoptosis rate (Annexin V+ and PI+ cells in the upper right quadrant) was quantified from the results of flow cytometry.
To determine the appropriate concentration of pADM to be used for subsequent tests, HaCaT cells were stimulated with 0, 0.25, 0.5, 1, or 2 mg/mL of pADM to assess cell viability and apoptosis. As shown in Fig. 2A, compared with 0 mg/mL pADM, 0.25, 0.5, and 1 mg/mL pADM did not affect cell viability, whereas 2 mg/mL pADM inhibited cell viability (Fig. 2A). In addition, 2 mg/mL pADM induced apoptosis, whereas 0, 0.25, 0.5, and 1 mg/mL pADM did not (Figs. 2B, C). Thus, 1 mg/mL pADM was used in subsequent experiments.

Following treatment of HaCaT cells with 0, 0.25, 0.5, 1, and 2 mg/mL pADM, (A) cell viability was assessed by CCK-8; (B) Representative plot of Annex V/PE (x-axis) and 7-AAD (y-axis) double staining by flow cytometry; and (C) Late cell apoptosis rate (Annexin V+ and PI+ cells in the upper right quadrant) evaluated using flow cytometry was quantified.
To investigate the biological functions of pADM in wound healing, in vitro experiments were performed. Results of the Transwell assay showed that H2O2 inhibited cell migration, whereas pADM increased the number of migrated cells (Fig. 3A). The scratch test results also showed that pADM promoted wound healing in H2O2 treated cells (Fig. 3B). However, pADM did not affect HaCaT cell migration (Supplementary Figs. 1A and B). Moreover, the levels of IL-6, IL-8, and TNF-α were increased by H2O2 treatment, whereas pADM reduced their levels, which were evaluated using ELISA and qPCR (Figs. 3C–H). Nevertheless, pADM did not affect the expression of IL-6, IL-8, and TNF-α in HaCaT cells (Supplementary Figs. 1C–H). These results demonstrate that pADM facilitates cell migration and inhibits inflammation in H2O2-induced HaCaT cells rather than HaCaT cells.
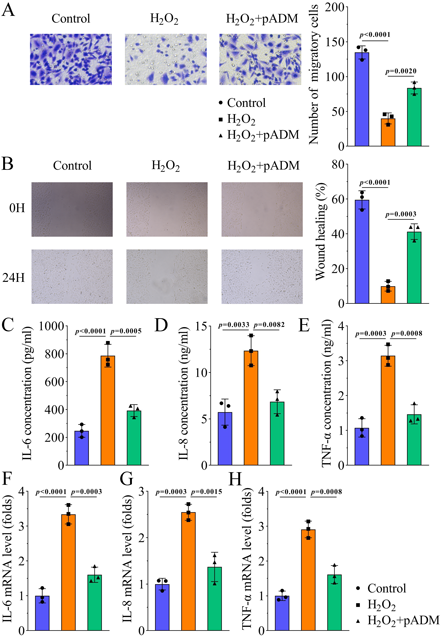
(A) Cell migration was analyzed using Transwell assay, and the number of migratory cells was counted. (B) Scratch test was used to assess cell migration, and the percentage of wound healing was quantified. The levels of (C) IL-6, (D) IL-8, and (E) TNF-α were measured using ELISA. The mRNA expression of (F) IL-6, (G) IL-8, and (H) TNF-α was examined using qPCR.
Since the PI3K/AKT/mTOR pathway is related to wound healing, we investigated whether pADM is involved in wound healing by regulating this pathway. To explore the potential mechanism of action, we measured the levels of the AKT pathway-related proteins. As shown in Fig. 4A, H2O2 downregulated the p-AKT and p-mTOR levels in a dose-dependent manner. However, H2O2 did not affect AKT or mTOR levels. Thus, the ratios of p-AKT/AKT and p-mTOR/mTOR were reduced by H2O2 (Fig. 4A). In addition, pADM reversed the downregulation of p-AKT and p-mTOR levels, p-AKT/AKT, and p-mTOR/mTOR induced by H2O2 (Fig. 4B). However, the levels of p-AKT, AKT, p-mTOR, and mTOR were all not regulated by pADM in HaCaT cells (Supplementary Fig. 1I). Cell apoptosis induced by H2O2 was suppressed by pADM (Fig. 4C).
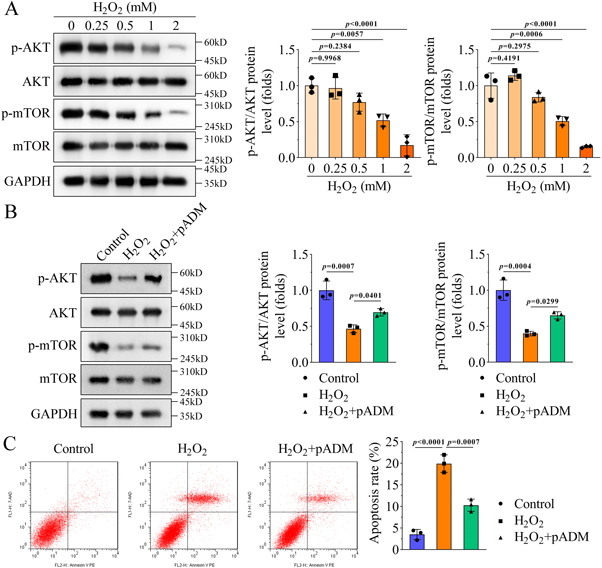
(A) Following treatment of HaCaT cells with 0, 0.25, 0.5, 1, and 2 mM H2O2, the levels of p-AKT, AKT, p-mTOR, and mTOR were measured using Western blotting, and the ratios of p-AKT/AKT and p-mTOR/mTOR were quantified. (B) Following treatment of HaCaT cells with H2O2 and pADM, Western blotting was performed to detect the levels of p-AKT, AKT, p-mTOR, and mTOR. p-AKT/AKT and p-mTOR/mTOR ratios were quantified. (C) Effect of H2O2 and pADM on HaCaT cell apoptosis was analyzed using flow cytometry. Cells were double stained with Annex V/PE (x-axis) and 7-AAD (y-axis). Late cell apoptosis rate (Annexin V+ and PI+ cells in the upper right quadrant) was quantified.
Consequently, a rescue study was performed to confirm the role of the AKT pathway. LY294002 was used to inactivate the AKT pathway in HaCaT cells because the levels of p-AKT and p-mTOR were downregulated (Fig. 5A). LY294002 treatment inhibited pADM-induced cell migration (Figs. 5B, C). Additionally, LY294002 abrogated the decreased levels of IL-6, IL-8, and TNF-α caused by pADM (Figs. 5D–I). These data suggest that pADM promotes cell migration and inhibits inflammation by activating the AKT pathway.
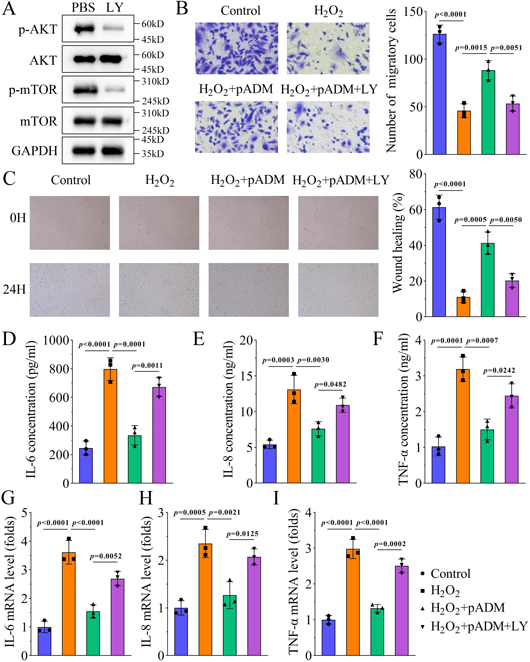
HaCaT cells were treated with H2O2, pADM, and LY294002, and (A) effects of LY294002 on the protein levels of p-AKT, AKT, p-mTOR, and mTOR were assessed using Western blotting; (B) a Transwell assay was performed to assess cell migration and the number of migratory cells was counted; (C) a scratch test was performed to analyze cell migration, and the percentage of wound healing was quantified; the levels of (D) IL-6, (E) IL-8, and (F) TNF-α were measured using ELISA; the mRNA expression of (G) IL-6, (H) IL-8, and (I) TNF-α was detected using qPCR.
In this study, we investigated the role of pADM in wound healing and underlying molecular mechanisms. We then assessed the effects of pADM on migration and inflammation of keratinocytes. These results showed that pADM inhibited cell migration and inflammation by regulating the AKT pathway.
Previous studies have reported the potential role of pADM in wound healing. Compared with human ADM, pADM has a lower cost and easier access, and displays similar effects.21) Therefore, pADM has a strong potential to be used in the field of surgery. pADM accelerates wound repair and enhances the quality of skin recovery.9,22) However, the underlying mechanisms are complex. For example, pADM downregulates the expression of miR-124-3p.1 and miR-139-5p in epidermal stem cells to facilitate wound healing and scar formation.22) In addition, pADM promotes growth of granulation tissue to facilitate wound healing.23) In addition, pADM activates the Notch/Jagged1 signaling pathway to promote wound healing.24) Keratinocytes play a crucial role in skin repair by restoring epidermal re-epithelialization. pADM can stimulate proliferation and differentiation of keratinocytes, thereby promoting wound repair.25) Impaired wound healing is regulated by many factors, among which oxidative stress is an important factor. It can affect wound healing by regulating cell migration and proliferation.26) In the present study, we used H2O2 to treat HaCaT cells to generate a damaged cell model. These results showed that H2O2 suppressed cell viability and induced apoptosis in a dose-dependent manner, suggesting that this model was established successfully. Cell migration is essential in maintaining tissue homeostasis and promoting wound repair.27) In addition, inflammation is one of the main phases of wound healing.28) Keratinocytes interact with immune cells or microbiota to function at each stage of wound healing.3) Under inflammatory conditions, oxidative stress promotes migration of inflammatory cell across endothelial junctions, inducing tissue injury.29) Hence, the effects of pADM on cell migration and inflammatory response were evaluated. We found that pADM promoted HaCaT cell migration and downregulated the levels of pro-inflammatory factors including IL-6, IL-8, and TNF-α. Previous studies have reported that inflammatory cytokines IL-6, IL-8, and TNF-α promotes the migration of HaCaT cells.30–32) However, we considered that pADM promoted keratinocyte cell migration by inhibiting the release of pro-inflammatory cytokines, inconsistent with previous studies mentioned above. This may be because we used an H2O2 cell model, whereas previous studies used keratinocytes. These findings suggested that pADM promotes wound healing. However, oxidative damage is not the only cause of skin wounds, so a limitation of this study is that we only used H2O2 to establish cell injury model. In future work, we will use other stimuli to establish cell model and investigate the effect of pADM.
The AKT pathway is involved in favorable outcomes related to numerous diseases, including wound healing. Importantly, this pathway is closely associated with the biological functions of keratinocytes. For example, ropivacaine suppresses proliferation and migration of HaCaT cells to delay wound closure by inactivating the PI3K/AKT/mTOR pathway.33) miR-26a inhibits HaCaT cell migration by blocking the PI3K/AKT pathway.34) In addition, deficiency of SETD2 accelerates re-epithelialization by facilitating keratinocyte migration via activation of the AKT/mTOR pathway, thus promoting cutaneous wound healing.35) These studies indicate that activation of the PI3K/AKT/mTOR pathway has the potential to promote wound healing. In the current study, we found that H2O2 decreased the protein levels of p-AKT and p-mTOR, whereas pADM abrogated the downregulation induced by H2O2, suggesting that pADM activated the AKT pathway. Moreover, results of the rescue experiments showed that blocking the AKT pathway reversed pADM-mediated cell migration and inflammation. Taken together, pADM activates the AKT pathway to promote migration and suppresses inflammation of keratinocytes.
In conclusion, the results of this study reveal that pADM promoted migration and inhibited inflammation of keratinocytes induced by oxidative stress by activating the AKT pathway. They also suggest that the pADM-regulated AKT pathway may facilitate wound healing. Thus, these findings provide novel evidence for the role of pADM in wound repair.
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by D.L. and T.C. The first draft of the manuscript was written by X.H. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
The authors declare no conflict of interest.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
This article contains supplementary materials.