2023 Volume 71 Issue 7 Pages 502-507
2023 Volume 71 Issue 7 Pages 502-507
Two new diterpenes named trichoterpene I (1) and trichoterpene II (2) were isolated from the extract from the leaves of Isodon trichocarpus together with 19 known diterpenes. Their chemical structures were elucidated on the basis of chemical and physicochemical properties. Among them, oridonin (3), effusanin A (4), and lasiokaurin (9) with the α,β-unsaturated carbonyl moiety showed antiproliferative activities against breast cancer MDA-MB-231 and human astrocytoma U-251 MG cells [i.e., non-cancer stem cells (non-CSCs)] and their cancer stem cells (CSCs) isolated by sphere formation. In particular, compound 4 (IC50 = 0.51 µM) showed a higher antiproliferative activity against MDA-MB-231 CSCs than against MDA-MB-231 non-CSCs. The antiproliferative activity toward CSCs of compound 4 was equal to adriamycin (positive control, IC50 = 0.60 µM).
Isodon plants (Labiatae) such as Isodon japonicus (Burm. f.) H. Hara and Isodon trichocarpus (Maxim.) Kudô [=Rabdosia trichocarpa (Maxim.) H. Hara] are mainly distributed in Japan and China. They are popular folk medicinal plants for the treatment of gastrointestinal disorders and tumors. Previously, we reported the isolation and structural elucidation of two ent-kaurane diterpenes from the aerial parts of I. japonicus.1,2) Some of the isolated ent-kaurane-type diterpenes were found to show antimutagenic activities. In addition, there are several reports on the isolation of ent-kaurane-type diterpenes such as oridonin from the leaves of I. trichocarpus.3–6) Diterpene constituents exhibit several biological activities such as anticancer7,8) and antibacterial9) activities. In particular, oridonin is known to be important as an anticancer agent. For example, oridonin was reported to have a variety of anticancer activities, including cervical cancer, leukemia, breast cancer, and gastric cancer. On the other hand, the presence of cancer stem cells (CSCs) has recently been suggested as a major cause of drug resistance in cancer cells. CSCs are present in small numbers in various cancer cells. CSCs have self-renewal and tumorigenic potential, are resistant to current anticancer drugs and radiation, and play an important role in metastasis.10–13) However, there are currently no anticancer drugs targeting CSCs in clinical use. Therefore, there is a pressing need to develop a therapeutic medicine that targets CSCs. As part of our studies on the anticancer constituents including compounds with antiproliferative activities against CSCs from traditional medicines and medicinal foodstuffs,14–21) we examined the constituents of the leaves of I. trichocarpus. In addition, we investigated the antiproliferative activities of selected diterpenes against breast cancer MDA-MB-231 and human astrocytoma U-251 MG cells [i.e., non-cancer stem cells (non-CSCs)] and their cancer stem cells (CSCs) isolated by sphere formation. In this report, we discuss the isolation of new diterpenes, trichoterpene I (1) and trichoterpene II (2), and the antiproliferative activities of diterpene constituents against MDA-MB-231 and U-251 MG CSCs.
The MeOH extract (8.71%) from the dried leaves of I. trichocarpus was partitioned in an EtOAc–H2O (1 : 1, v/v) mixture to give EtOAc-soluble fraction (3.28%) and an aqueous phase (5.43%). The EtOAc-soluble fraction was subjected to ordinary- and reversed-phase silica gel column chromatography and finally HPLC to furnish two new diterpenes, trichoterpenes I (1, 0.0013%) and II (2, 0.00026%), together with 17 known ent-kaurane-type diterpenes, oridonin (3, 0.35%),22) effusanin A (4, 0.0026%),23) effusanin B (5, 0.0030%),24) rabdoternin C (6, 0.0078%),25) 7,20-epoxykaur-16-ene-6β,7α,14R,15β-tetrol (7, 0.0020%),26) longikaurin A (8, 0.0014%),23,26) lasiokaurin (9, 0.0097%),26) longikaurin B (10, 0.0014%),23,26) enmenin (11, trichokaurin, 0.0013%),27) nervosanin A 1-acetate (12, 0.00089%),28) isodonal (14, 0.0017%),29) nodosin (15, 0.00043%),30) isodocarpin (16, 0.0022%),3,4,31) sculponeatin F (18, 0.0016%),32) sculponin M (19, 0.00072%),33) dihydroenmein (20, 0.00091%),5) and dihydroisodocarpin (21, 0.0022%)3,34) (Fig. 1). The aqueous phase was subjected to Diaion HP-20 column chromatography to give H2O-eluted fraction and MeOH-eluted fraction. The MeOH-eluted fraction was subjected to ordinary- and reversed-phase silica gel column chromatography and finally HPLC to furnish 4 known diterpenes, oridonin (3, 0.019%), 1α-O-β-D-glucopyranosylenmenol (13, 0.0041%),35) enmein (17, 0.00074%),30) sculponeatin F (18, 0.0014%).32)

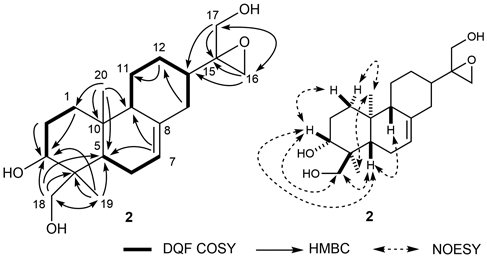
Trichoterpene I (1) was isolated as a white amorphous powder with positive specific rotation ([α]26D +87.6° in MeOH). Its IR spectrum showed absorption bands at 3400 and 1093 cm−1 due to hydroxy and ether functions. Electron ionization (EI)MS revealed a molecular ion [M]+ at m/z 364, from which the molecular formula C20H28O6 was determined via high resolution (HR)MS and 13C-NMR data. The 1H (pyridine-d5) and 13C-NMR (Table 1) spectra of 1, which were assigned by various NMR experiments, showed signals due to two methyls [δ 1.16, 1.19 (3H each, both s, 19, 18-CH3)], five methines bearing an oxygen function [δ 3.24 (1H, dd, J = 4.8, 4.8, H-12), 4.13 (1H, m, H-11), 4.28 (1H, d, J = 3.5, H-6), 4.36 (1H, m, H-1), 5.24 (1H, s, H-15)], a methylene bearing an oxygen function [δ 4.15, 4.72 (1H each, both d, J = 8.9, H2-20)], and two olefinic protons [δ 5.24, 5.58 (1H each, both s like, H2-17)]. As shown in Fig. 2, double quantum filter correlation spectroscopy (DQF COSY) experiments on 1 indicated the presence of partial structures (shown in bold). Long-range correlations were observed between the following protons and carbons in the heteronuclear multiple bond connectivity spectroscopy (HMBC) experiment: H-1 and C-3, 5, 10; H-6 and C-5, 7, 8; H-9 and C-8, 10, 11; H-11 and C-8; H-12 and C-13; H-13 and C-8, 11; H-14 and C-16; H-15 and C-8, 16, 17; H-17 and C-13, 16; H-18 and C-3, 4, 5, 19; H-19 and C-3, 4, 5, 18; H-20 and C-5, 9, 10. Next, the relative configuration of 1 was characterized by nuclear Overhauser enhancement spectroscopy (NOESY), which showed NOE correlations between H-1 and H-20a; H-5 and H3-18, H-9; H-6 and H3-19; H-9 and H-11; H-11 and H-12; H-12 and H-17; H-13 and H-14b, 15; H-14a and H-20a; H-14b and H-15; H3-19 and H-20b. The proton and carbon signals in the 1H- and 13C-NMR data of 1 were superimposable on those of known 7,20-epoxy-ent-kaurane diterpene, sculponin W,36) except for the signals around the 1-position. Therefore, the relative configuration of 1 was confirmed. In addition, the configuration at 1-position of 1 was also confirmed by the comparison of the 13C-NMR data. The 13C-NMR signal at 1-position of ent-kaurane diterpene with β-configuration such as enmelol (δ 65.8) was shifted upfield relative to that with α-configuration such as eriocalyxin D (δ 73.3).37) The 13C-NMR signal at 1-position of 1 was observed at δ 66.4, so that the configuration of 1 was suggested. On the basis of all this evidence, the chemical structure of trichoterpene I (1) was characterized as shown in Fig. 2.
| Compound Position | 1 (pyridine-d5) | 2 (CD3OD) | 2 (pyridine-d5) | |||
|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1α | 66.4 | 4.36 (m) | 38.8 | 1.82 (m) | 37.9 | 1.80 (m) |
| 1β | 1.14 (m) | 1.22 (m) | ||||
| 2 | 27.8 | 1.85, 1.92 (both m) | 27.7 | 1.64 (m) | 27.7 | 1.91 (m) |
| 3 | 34.6 | 1.28 (m) | 74.2 | 3.60 (m) | 73.4 | 4.23 (m) |
| 2.27 (dd, 3.6, 13.5) | ||||||
| 4 | 34.0 | 43.5 | 42.8 | |||
| 5 | 55.2 | 2.35 (d, 3.5) | 43.3 | 1.49 (dd, 4.4, 11.7) | 42.9 | 1.91 (m) |
| 6 | 74.0 | 4.28 (d, 3.5) | 23.8 | 1.93 (m) | 23.3 | 2.05 (m) |
| 7 | 98.3 | 122.7 | 5.36 (s like) | 120.5 | 5.42 (s like) | |
| 8 | 51.7 | 137.4 | 137.5 | |||
| 9 | 37.9 | 4.36 (m) | 53.8 | 1.64 (m) | 52.7 | 1.73 (br d like, 13.3) |
| 10 | 42.3 | 36.1 | 35.1 | |||
| 11 | 51.8 | 4.13 (m) | 26.6 | 1.06, 1.79 (both m) | 25.7 | 1.14 (m) |
| 1.79 (br d like, 13.3) | ||||||
| 12 | 53.4 | 3.24 (dd like, 4.8, 4.8) | 26.7 | 1.16, 1.83 (both m) | 26.1 | 1.54, 2.17 (both br d like, 12.4) |
| 13 | 38.7 | 3.04 (br dd like, 4.8, 4.8) | 42.8 | 1.55 (m) | 42.0 | 2.02 (m) |
| 14a | 26.2 | 2.74 (br d like, 12.2) | 36.0 | 1.93 (m) | 35.5 | 2.44 (dd like, 14.4, 14.4) |
| 14b | 2.22 (dd, 4.8, 12.2) | 2.24 (br d like, 13.6) | 2.66 (br d like, 14.4) | |||
| 15 | 75.3 | 5.24 (s) | 76.1 | 75.4 | ||
| 16 | 154.0 | 48.2 | 3.60 (d like, 11.3) | 48.8 | 4.13 (d like, 11.5) | |
| 3.70 (d, 11.3) | 4.29 (d, 11.5) | |||||
| 17 | 108.5 | 5.24 5.58 (both br s like) | 63.9 | 3.53, 3.58 (both d, 11.2) | 63.6 | 4.15, 4.23 (both d, 10.4) |
| 18 | 33.0 | 1.19 (s) | 67.3 | 3.22, 3.46 (d, 10.9) | 67.3 | 3.66 (d, 10.4) |
| 4.13 (d like, 10.4) | ||||||
| 19 | 22.3 | 1.16 (s) | 12.7 | 0.77 (s) | 13.0 | 1.14 (s) |
| 20a | 67.6 | 4.72 (d, 8.9) | 16.1 | 0.83 (s) | 15.8 | 0.90 (s) |
| 20b | 4.15 (d, 8.9) | |||||

Trichoterpene II (2) was isolated a white amorphous powder. Its IR spectrum showed absorption bands due to hydroxy and ether functions. EIMS revealed a molecular ion [M]+ at m/z 336, from which the molecular formula C20H32O4 was determined via HRMS and 13C-NMR data. The 1H-NMR (CD3OD and pyridine-d5) and 13C-NMR (Table 1) data of 2, assigned via various NMR experiments, showed resonances assignable to a diterpene with three hydroxy groups and an epoxy moiety. The linkages of the three hydroxy groups and an epoxy moiety as well as the structure of the diterpene moiety were confirmed based on DQF COSY and HMBC experiments. HMBC correlations were observed between the following proton and carbon pairs: H-1 and C-3; H-2 and C-3; H-7 and C-5, 9; H-12 and C-11; H-14 and C-12; H-16 and C-13, 15, 17; H-17 and C-13, 15, 16; H-18 and C-3, 4, 5, 19; H-19 and C-3, 4, 5, 18; H-20 and C-1, 5, 9, 10. Next, the relative configuration of 2 was characterized by NOESY experiment, which showed NOE correlations between H-1β and H-3; H-1α and H3-20; H-3 and H-5, H2-18; H-5 and H-9, H2-18; H3-19 and H3-20. The proton and carbon signals in the 1H- and 13C-NMR spectra of 2 were superimposable on those of known ent-abietane diterpene, hebeiabinin B,38) except for the signals around the 15-postion of 2. On the basis of all this evidence, the chemical structure of trichoterpene II (2) was characterized as shown in Fig. 3.
In this study, the antiproliferative activities of eight isolated compounds against breast cancer MDA-MB-231 and human astrocytoma U-251 MG cells and their CSCs were evaluated. As compounds for the evaluation, compound 3, the major secondary metabolite of Isodon plants, and compounds 4 and 9 with an α,β-unsaturated carbonyl structure similar to 3 were selected. In order to compare their activities, compounds 1, 6, and 11 without an α,β-unsaturated carbonyl moiety and 6,7-seco-ent-kauranes 20 and 21 were also selected. Adriamycin (ADR) was used as the positive control and CSCs were prepared by sphere formation, which was confirmed by assessing cell morphology. The upregulated expression of stem cell markers Nanog and Nestin was confirmed by immunofluorescence analysis as described previously.39) Cell viability was assayed using CellTiter-Glo® 3D (Promega Corp., Madison, WI, U.S.A.). As a result, oridonin (3), effusanin A (4), and lasiokaurin (9) with the α,β-unsaturated carbonyl moiety showed significant antiproliferative activities against both MDA-MB-231 CSCs and MDA-MB-231 non-CSCs (Tables 2, 3, respectively). On the other hand, trichoterpene I (1), rabdoternin C (6), enmenin (11), dihydroenmein (20), and dihydroisodocarpin (21) did not show such activities. These results suggest that the α,β-unsaturated carbonyl moiety plays an important role in these activities. Interestingly, compound 4 (IC50 = 0.51 µM) exhibited higher antiproliferative activity against MDA-MB-231 CSCs than against MDA-MB-231 non-CSCs (IC50 > 3 µM). The antiproliferative activity toward CSCs of compound 4 was equal to ADR (IC50 = 0.60 µM). Therefore, compound 4 shows potential as an anticancer drug that selectively acts on CSCs. Furthermore, compounds 3, 4, and 9 also showed antiproliferative activities against both U-251 MG CSCs and U-251 MG non-CSCs (Tables 4, 5, respectively).
| Compound | Inhibition (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Conc. (µM) | 0 | 0.39 | 0.78 | 1.56 | 3.13 | 6.25 | 12.5 | 25 | 50 | IC50 (µM) |
| 1 | 0.0 ± 17.7 | — | — | — | — | 10.6 ± 20.5 | −1.5 ± 19.7 | 3.9 ± 20.2 | 23.9 ± 13.2** | — |
| 3 | 0.0 ± 2.3 | — | — | — | — | −27.9 ± 12.1 | 27.8 ± 1.0** | 99.1 ± 0.2** | 100 ± 0.1** | ca.17 |
| 4 | 0.0 ± 19.1 | 27.2 ± 6.7 | 63.6 ± 10.9** | 92.5 ± 2.0** | 98.0 ± 0.5** | — | — | — | — | 0.51 |
| 9 | 0.0 ± 5.6 | — | — | — | 12.0 ± 3.4** | 83.0 ± 5.4** | 97.5 ± 0.2** | 100 ± 0.0** | 100 ± 0.0** | 4.8 |
Each value represents the mean ± S.E.M. (N = 3). The statistical significance of difference was analyzed using Dunnett’s test (* p < 0.05, ** p < 0.01 compared with control group). Cells were incubated with test samples for 6 d. Compounds 6, 11, 20, and 21 did not show the antiproliferative activities (The inhibitory effects at 50 µM were less than 15%). The IC50 value of adriamycin (positive control) was 0.60 µM.
| Compound | Inhibition (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Conc. (µM) | 0 | 0.78 | 1.56 | 3.13 | 6.25 | 12.5 | 25 | 50 | IC50 (µM) |
| 1 | 0.0 ± 10.9 | −7.8 ± 8.8 | −10.1 ± 16.8 | 3.1 ± 14.5 | 17.0 ± 17.4* | 33.3 ± 9.8** | — | ||
| 3 | 0.0 ± 8.2 | 8.1 ± 17.3 | −2.5 ± 17.4 | 43.9 ± 0.8** | 100.2 ± 0.0** | 100.2 ± 0.1** | 13.6 | ||
| 4 | 0.0 ± 11.4 | −6.7 ± 10.2 | −14.1 ± 8.6* | 27.8 ± 8.9** | 95.6 ± 0.77** | 100.0 ± 0.01** | — | — | — |
| 9 | 0.0 ± 3.7 | −5.5 ± 9.1 | −12.2 ± 5.0 | −16.3 ± 5.0 | −5.7 ± 4.5 | 53.5 ± 9.5** | — | — | — |
Each value represents the mean ± S.E.M. (N = 3). The statistical significance of difference was analyzed using Dunnett’s test (* p < 0.05, ** p < 0.01 compared with control group). Cells were incubated with test samples for 72 h. Compounds 6, 11, 20, and 21 did not show the antiproliferative activities (The inhibitory effects at 50 µM were less than 15%). The IC50 value of adriamycin (positive control) was 0.21 µM.
| Compound | Inhibition (%) | ||||||
|---|---|---|---|---|---|---|---|
| Conc. (µM) | 0 | 3.13 | 6.25 | 12.5 | 25 | 50 | IC50 (µM) |
| 3 | 0.0 ± 7.0 | −1.4 ± 5.5 | 3.1 ± 21.6 | 45.5 ±5.7** | 66.9 ± 5.2** | 99.2 ± 2.3** | 16.3 |
| 4 | 0.0 ± 8.0 | 23.6 ± 17.7** | 78.1 ± 1.8** | 93.2 ± 2.0** | 83.5 ± 5.5** | 85.2 ± 2.7** | 4.1 |
| 9 | 0.0 ± 3.0 | −6.4 ± 3.6 | −8.0 ± 4.5 | 5.4 ± 4.4 | 36.5 ± 16.5** | 95.5 ± 4.0** | 30.0 |
| 20 | 0.0 ± 4.6 | 4 ± 7.7 | −18 ± 14.7 | −18.7 ± 10.4 | −7.4 ± 8.3 | 54.5 ± 4.1** | — |
Each value represents the mean ± S.E.M. (N = 3). The statistical significance of difference was analyzed using Dunnett’s test (* p < 0.05, ** p < 0.01 compared with control group). Cells were incubated with test samples for 6 d. Compounds 1, 6, 11, 20, and 21 did not show the antiproliferative activities (The inhibitory effects at 50 µM were less than 15%). The IC50 value of adriamycin (positive control) was 0.051 µM.
| Compound | Inhibition (%) | ||||||
|---|---|---|---|---|---|---|---|
| Conc. (µM) | 0 | 3.13 | 6.25 | 12.5 | 25 | 50 | IC50 (µM) |
| 3 | 0.0 ± 15.1 | 11.5 ± 10.3 | 45.5 ± 5.9** | 84.9 ± 3.4** | 100.4 ± 0.0** | 100.4 ± 0.0** | 6.6 |
| 4 | 0.0 ± 2.2 | 8.7 ± 3.2 | 39.1 ± 9.5** | 81.2 ± 2.1** | 99.6 ± 0.2** | 100 ± 0.0** | 7.4 |
| 9 | 0.0 ± 0.9 | 19.5 ± 3.0** | 69.1 ± 2.4** | 78.4 ± 4.1** | 99.8 ± 0.2** | 99.9 ± 0.2** | 5.2 |
| 20 | 0.0 ± 7.6 | 0.4 ± 4.8 | 1.4 ± 10.3 | 8.3 ± 4.7 | 19.8 ± 5.1 | 56.5 ± 6.1** | 44.9 |
Each value represents the mean ± S.E.M. (N = 3). The statistical significance of difference was analyzed using Dunnett’s test (* p < 0.05, ** p < 0.01 compared with control group). Cells were incubated with test samples for 72 h. Compounds 1, 6, 11, and 21 did not show the antiproliferative activities (The inhibitory effects at 50 µM were less than 15%). The IC50 value of adriamycin (positive control) was 0.041 µM.
Two new diperpenes, trichoterpenes I (1) and II (2), were isolated from the leaves of I. trichocarpus together with 19 known diterpenes. In particular, effusanin A (4) showed higher antiproliferative activity against MDA-MB-231 CSCs and than against MDA-MB-231 non-CSCs. We conclude that diterpenes with the α,β-unsaturated carbonyl moiety, such as effusanin A (4), could be useful for the treatment of cancers. The detailed mechanisms of action of 4 need to be studied further.
General experimental procedures were shown in supplementary materials.
Plant MaterialThe leaves of Isodon trichocarpus, which was cultivated in Japan, were obtained from Kurohime medical herb tea Co., Ltd. (Nagano, Japan) in 2006. The leaves were identified by one of the authors (H. M.). A vouchen specimen is on file in our laboratory (KPU IT-2006-1).
Extraction and IsolationThe leaves (6.0 kg) were extracted with MeOH three times under reflux for 3 h. Evaporation of the solvent under reduced pressure provided the MeOH extract (522.7 g, 8.71%). A part of the MeOH extract (447.8 g) was partitioned into an EtOAc–H2O (1 : 1, v/v) mixture to furnish an EtOAc-soluble fraction (168.5 g, 3.28%) and aqueous phase (279.3 g, 5.43%). A part of the EtOAc-soluble fraction (138.4 g) was subjected to normal-phase silica gel column chromatography (CC) [3.0 kg, CHCl3–MeOH–H2O (50 : 3 : 1 (lower layer)→20 : 3 : 1 (lower layer)→10 : 3 : 1 (lower layer)→7 : 3 : 1 (lower layer)→6 : 4 : 1, v/v/v)→MeOH] to give 12 fractions [fr. 1–fr. 5, fr. 6 (12.5 g), fr. 7 (14.4 g), fr. 8 (9.1 g), fr. 9 (25.4 g), fr. 10 (4.8 g), fr. 11, fr. 12]. Fraction 6 (12.5 g) was separated by reversed-phase silica gel CC [380 g, MeOH–H2O (30 : 70→40 : 60→50 : 50→60 : 40, v/v)→MeOH] to give 11 fractions [fr. 6-1, fr. 6-2, fr. 6-3 (5.40 g), fr. 6-4 (500 mg), fr. 6-5 (362 mg), fr. 6-6 (1.12 g), fr. 6-7–fr. 6-11]. Fraction 6-3 (5.40 g) was separated by reversed-phase silica gel CC [MeOH–H2O (40 : 60→50 : 50→60 : 40, v/v)→MeOH] to give 7 fractions [fr. 6-3-1 (523 mg), fr. 6-3-2 (1, 53 mg), fr. 6-3-3 (406 mg), fr. 6-3-4–fr. 6-3-6, fr. 6-3-7 (740 mg)]. Fraction 6-3-1 (523 mg) was purified by normal-phase silica gel CC [CHCl3–MeOH–H2O (30 : 3 : 1 (lower layer)→20 : 3 : 1 (lower layer)→7 : 3 : 1 (lower layer), v/v/v)] and HPLC [MeOH–H2O (55 : 45, v/v)] to give 11 (7.4 mg), 16 (49 mg), and 21 (37 mg). Fraction 6-3-3 (406 mg) was purified by HPLC [MeOH–H2O (55 : 45, v/v)] to give 16 (40 mg), 19 (30 mg), and 21 (54 mg). Fraction 6-3-7 (740 mg) was purified by HPLC [MeOH–H2O (55 : 45, v/v)] to give 11 (45 mg). Fraction 6-4 (500 mg) was separated by HPLC [MeOH–H2O (65 : 35, v/v)] to give 2 fractions [fr. 6-4-1 (103 mg), fr. 6-4-2 (114 mg)]. Fraction 6-4-1 (103 mg) was purified by HPLC [MeOH–H2O (60 : 30, v/v)] to give 12 (37 mg). Fraction 6-4-2 (114 mg) was purified by normal-phase silica gel CC [CHCl3–MeOH (45 : 1, v/v)→CHCl3–MeOH–H2O (50 : 3 : 1 (lower layer), v/v/v)] to give 5 (76 mg). Fraction 6-5 (362 mg) was purified by HPLC [MeOH–H2O (65 : 35, v/v)] to give 2 (11 mg) and 5 (51 mg), and 7 (83 mg). Fraction 6-6 (300 mg) was purified by HPLC [MeOH–H2O (70 : 30, v/v)] to give 6 (87 mg). Fraction 7 (14.4 g) was separated by reversed-phase silica gel CC [420 g, MeOH–H2O (10 : 90→20 : 80→30 : 70→40 : 60, v/v)→MeOH] to give 14 fractions [fr. 7-1-7-4, fr. 7-5 (1.32 g), fr. 7-6, fr. 7-7 (2.00 g), fr. 7-8 (3.58 g), fr. 7-9 (700 mg), fr. 7-10 (455 mg), fr. 7-11–7-14]. Fraction 7-5 (1.32 g) was purified by normal-phase silica gel CC [CHCl3–MeOH (50 : 1→30 : 1→10 : 1, v/v)] to give 15 (18 mg). Fraction 7-7 (2.00 g) was purified by normal-phase silica gel CC [CHCl3–MeOH (50 : 1, v/v)→CHCl3–MeOH–H2O (50 : 3 : 1 (lower layer)→20 : 3 : 1 (lower layer)→10 : 3 : 1 (lower layer), v/v/v)] to give 3 fractions [fr. 7-7-1 (350 mg), fr. 7-7-2 (1.36 g), fr. 7-7-3 (277 mg)]. Fraction 7-7-1 (350 mg) was purified by HPLC [MeOH–H2O (55 : 45, v/v)] to give 14 (71 mg). Fraction 7-7-2 (500 mg) was purified by HPLC [MeOH–H2O (55 : 45, v/v)] to give 4 (40 mg). Fraction 7-8 (240 mg) was purified by HPLC [MeOH–H2O (65 : 35, v/v)] to give 8 (3.9 mg). Fraction 8 (9.1 g) was separated by reversed-phase silica gel CC [300 g, MeOH–H2O (30 : 70→40 : 60→50 : 50→60 : 40→70 : 30→80 : 20→90 : 10, v/v)→MeOH] to give 15 fractions [fr. 8-1-8-3, fr. 8-4 (523 mg), fr. 8-5, fr. 8-6, fr. 8-7 (705 mg), fr. 8-8 (309 mg), fr. 8-9, fr. 8-10, fr. 8-11–8-15]. Fraction 8-4 (475 mg) was purified by normal-phase silica gel CC [CHCl3–MeOH (30 : 1→10 : 1, v/v)] and HPLC [MeOH–H2O (55 : 45, v/v)] to give 18 (61 mg), and 20 (35 mg). Fraction 8-7 (100 mg) was purified by HPLC [MeOH–H2O (70 : 30, v/v)] to give 9 (49 mg). Fraction 8-8 (309 mg) was purified by HPLC [MeOH–H2O (55 : 45, v/v)] to give 9 (64 mg) and 10 (58 mg). Fraction 9 (10.0 g) was separated by reversed-phase silica gel CC [300 g, MeOH–H2O (40 : 60→50 : 50→60 : 40→70 : 30→80 : 20→90 : 10, v/v)→MeOH] to give 7 fractions [fr. 9-1, fr. 9-2 (8.24 g), fr. 9-3 (5.03 g), fr. 9-4 (209 mg), fr. 9-5 (194 mg), fr. 9-6-9-15]. Fraction 9-2 (3.22 g) was purified by HPLC [MeOH–H2O (60 : 40 and 50 : 50, v/v)] to give 3 (1.84 g). Fraction 9-3 (200 mg) was purified by HPLC [MeOH–H2O (60 : 40, v/v)] to give 3 (46 mg). Fraction 9-4 (209 mg) was purified by HPLC [MeOH–H2O (62.5 : 37.5, v/v)] to give 4 (75 mg). A part of the aqueous phase (202.9 g) was subjected to Diaion HP-20 column chromatography (3.5 kg, H2O→MeOH) to give H2O-eluted fraction (161.3 g, 4.32%) and MeOH-eluted fraction (39.7 g, 1.06%). A part of the MeOH-eluted fraction (29.4 g) was subjected to normal-phase silica gel CC [1.0 kg, CHCl3–MeOH–H2O (20 : 3 : 1 (lower layer)→10 : 3 : 1 (lower layer)→7 : 3 : 1 (lower layer)→6 : 4 : 1, v/v/v)→MeOH] to give 6 fractions [fr. 1, fr. 2 (2.3 g), fr. 3 (6.5 g), fr. 4 (8.1 g), fr. 5, fr. 6]. Fraction 2 (2.3 g) was subjected to reversed-phase silica gel CC [MeOH–H2O (40 : 60→50 : 50→60 : 40→70 : 30, v/v)→MeOH] to afford 6 fractions [fr. 2-1, fr. 2-2 (203 mg), fr. 2-3 (1.00 g), fr. 2-4-2-6]. Fraction 2-2 (203 mg) was purified by HPLC [MeOH–H2O (40 : 60, v/v)] to give 17 (21 mg) and 18 (40 mg). Fraction 2-3 (203 mg) was purified by HPLC [MeOH–H2O (50 : 50, v/v)] to give 3 (95 mg). Fraction 3 (6.5 g) was subjected to reversed-phase silica gel CC [MeOH–H2O (30 : 70→40 : 60→50 : 50→60 : 40→70 : 30→80 : 20→90 : 10, v/v)→MeOH] to afford 4 fractions [fr. 3-1, fr. 3-2, fr. 3-3 (0.97 g), fr. 3-4]. Fraction 3-3 (0.97 g) was purified by HPLC [MeOH–H2O (50 : 50, v/v)] to give 3 (68 mg). Fraction 4 (8.1 g) was subjected to reversed-phase silica gel CC [MeOH–H2O (30 : 70→40 : 60→50 : 50→60 : 40→70 : 30→80 : 20→90 : 10, v/v)→MeOH] to afford 5 fractions [fr. 4-1, fr. 4-2 (1.43 g), fr. 4-3-4-5]. Fraction 4-2 (200 mg) was purified by HPLC [MeOH–H2O (35 : 65, v/v)] to give 13 (16 mg).
Trichoterpene I (1): White amorphous powder; [α]26D +87.6 (c 2.5, MeOH); IR (KBr) νmax 3400, 2938, 1093 cm−1; 1H- and 13C-NMR (pyridine-d6, 600 MHz): given in Table 1; HREIMS m/z 364.1882 (Calcd for C20H28O6 [M+], 364.1886).
Trichoterpene II (2): White amorphous powder; [α]24D +23.4 (c 0.5, MeOH); IR (KBr) νmax 3450, 2936, 1080 cm−1; 1H- and 13C-NMR (CD3OD and pyridine-d6, 600 MHz): given in Table 1; HREIMS m/z 336.2304 (Calcd for C20H32O4 [M+], 336.2300).
Cell CultureThe breast cancer cell line MDA-MB-231 and the glioblastoma cell line U-251 MG were purchased from the American Type Culture Collection (Manassas, VA, U.S.A.). MDA-MB-231 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM)/high glucose (Wako, Osaka, Japan). Each medium contained 10% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO, U.S.A.) and 1% penicillin/streptomycin (PC/SM; Wako). U-251 MG cells were cultured in (DMEM/low glucose (Wako).
Cancer Stem Cell PreparationCSCs of MDA-MB-231 and U-251 MG were prepared by the sphere formation assay as described previously.13,39)
CellTiter-Glo 3D® Cell Viability Assay for CSCs and Non-CSCsFor CSCs: The cells were seeded at a density of 3.0 × 103 cells/90 µL per well in ultra-low attachment 96-well plates and treated with test compounds (10 µL per well) at 24 h after seeding. After 6 d, the cell-containing media were transferred to a 96-well white plate (96F Nunclon TM Delta White Microwell SI; Thermo Fisher Scientific). CellTiter-Glo® 3D Reagent (Promega, Madison, WI, U.S.A.) was added at 100 µL per well, mixed by shaking for 5 min at room temperature (r.t.), and incubated for 25 min at 37 °C. Luminescence was measured with a luminometer (GloMax® Discover System; Promega). For non-CSCs: The cells were seeded at a density of 3.0 × 103 cells/90 µL per well in 96-well plates and treated with test compounds (10 µL per well) 24 h after seeding. After 3 d, cell viability was evaluated with CellTiter-Glo® 3D Reagent using a 96-well white plate.13)
Statistical AnalysisStatistical analyses were performed using GraphPad Prism 8.21 software. The statistical analysis was conducted using one-way ANOVA followed by a Dunnett’s test to analyze differences between treatment groups. The differences were considered significant when * p < 0.05 or ** p < 0.01.
This research was funded by JSPS KAKENHI Grant Number 20K07109 (S. Nakamura and S. Nakashima), 20H03397 (S. Nakamura and T.M.), and 21K06637 (S. Nakamura and S. Nakashima).
The authors declare no conflict of interest.
This article contains supplementary materials.