2024 Volume 72 Issue 3 Pages 258-265
2024 Volume 72 Issue 3 Pages 258-265
Glycated albumin (GA) is one of the proteins that replaces several sugar moieties and can be used as an indicator of diabetes mellitus. We developed a sensing system that uses GA in the early detection of diabetes mellitus. In this study, H6Y4C acetylated (Ac-) at the N-terminals of the peptide was combined with wheat germ agglutinin (WGA) to recognize glucose moieties. The Ac-H6Y4C-WGA was constructed as a GA-sensing probe. The tyrosine residues of Y4C exhibited an oxidation peak, and His-tag moieties were introduced to separate Ac-H6Y4C-WGA in the synthesis of the probe. The Ac-H6Y4C-WGA probe binds with the 1–2 molecules of Ac-H6Y4C per WGA using matrix assisted laser desorption/ionization-time of flight (MALDI-TOF)-MS. Next, the functions of Ac-H6Y4C-WGA were evaluated using voltammetry. The number of electron-transfers was calculated based on the relationship between the peak potential and logarithm of scan rate and was 3.03. In the electrochemical measurements with mannose and bovine serum albumin, the peak currents were similar to that of GA alone. By contrast, a decrease in the peak current was suppressed when glucose was added to the solution containing the probe. As a result, Ac-H6Y4C-WGA was selectively bound to the glucose moieties of GA. The calibration curve via differential pulse voltammetry was proportional to the concentrations of GA and ranged from 1.0 × 10−12 to 2.0 × 10−11 M with a detection limit of 3.3 × 10−13 M.
Diabetes mellitus is a disease in which the beta cells of the pancreas no longer secrete insulin due to a reduction in the insulin secretory capacity that is the result of either a relative insulin deficiency or resistance of the insulin receptors to insulin. The International Diabetes Federation reports that there will be 537 million people with diabetes worldwide in 2021,1) and diabetes ranks ninth in the WHO’s 2019 ranking of causes of death.2) If the current situation is not improved, the number of diabetes patients is estimated to reach 643 million by 2030, which contributes to several problems. One of the problems is that diabetes induces other diseases with attendant complications. The mechanisms of these diseases are speculated to be as follows. When the hyperglycemic state caused by diabetes continues, glucose binds to proteins and peptides in a non-enzymatic manner to form Amadori transfer products (AGEs) in the lood.3) Next, AGEs accumulate in vascular endothelial cells and smooth muscle cells in the liver and kidneys. As a result, diabetic complications such as kidney damage, atherosclerosis, and myocardial infarction develop.
Therefore, monitoring AGEs in the blood is significant for the diagnosis of diabetes. AGEs that are indicators of diabetes include 1,5-anhydro-D-glucitol (1,5-AG), glycated hemoglobin (HbA1c), and glycated albumin (GA).4) 1,5-AG can evaluate blood glucose levels for days to weeks.5) When urinary glucose excretion is increased due to hyperglycemia, 1,5-AG is not reabsorbed in the tubules and the blood levels decrease. These values also decrease in chronic renal failure and in pregnant women with or without diabetes.6) Glycated hemoglobin (HbA1c), which is considered the average blood glucose level over the past 2–3 months, is used as a common glycemic control marker.7) However, HbA1c levels are affected by dialysis and are not suitable for short-term blood glucose measurements because red blood cells have a life span of about 120 d. On the other hand, GA is suitable for monitoring blood glucose levels in diabetes because it reflects average blood glucose levels over 2–4 weeks and is relatively uninfluenced by other diseases.8) Glycosylation readily binds 3–5 molecules of carbohydrate per molecule of GA and occurs at Lys-52, Lys-199, Lys-281, Lys-439, and Lys-525.9)
A colorimetric method for detecting GA is due to keto-amine oxidase.10) The principle of the assay was based on the following steps. First, endogenous glycosylated amino acids are lost using ketoamine oxidase, and GA is converted to glycosylated amino acids due to protease. After the reaction with keto-amine oxidase, N,N-bis(4-sulfobutyl)3-methyl-aniline, disodium salt and 4-aminoantipyrine are condensed quantitatively. The GA concentration can be determined via the absorption of the blue-violet chromophore. Ko’s group has fabricated boronic acid-derived agarose beads on paper-based devices and attempted determination of GA.11) Shin et al. reported a quantitation of GA using isotope dilution LC-mass spectrometry, and this has also been reported using the conjugation of a peptide and an enzyme.12) In addition, monitoring of GA using electrochemical techniques is rapid, highly sensitive and cost-effective when applied to the sensing of target proteins.13,14)
Tyrosine, tryptophan, and cysteine are known as electroactive amino acids with electrode responses that are based on phenolic hydroxyl groups, indole moieties, and thiol moieties, respectively.15) Peptides that combine those amino acid residues exhibit diverse electrode responses and serve as probes for sensing targets such as cells, proteins, viruses and so on.16) The probes are considered biocompatible and to cause low levels of environmental impact upon disposal. Shinohara et al. reported a peptide containing four tyrosine and cysteine residues (Y4C) as an electron-transfer peptide.17) In a previous study, we sensed soybean agglutinin using H6Y4C and Y4CH6, in which six histidine residues were introduced into Y4C, H6W4C, and W4CH6, which also are electron-transfer peptides.18) The method involves modifying asialofetuin with an electron-transfer peptide and binding it to soybean agglutinin (SBA) to enable detection of SBA. This method has high levels of detection sensitivity and selectivity, which makes it a sensing tool appropriate for medical and other applications.
In the present study, we synthesized a protein probe that selectively binds to GA for a rapid and low-cost electrochemical measurement of GA. The concept of the assay was based on an interaction between the glucose moieties present on the GA surface and wheat germ-derived aggregate (WGA). The monomer of WGA is one that binds two molecules of N-acetylglucosamine per molecule. Bains et al. reported its association constant to be 0.4 mM−1.19) Glucose also binds to WGA based on a similar interaction, and the protein probe will interact with GA due to the binding between glucose moieties of GA and WGA. Therefore, a protein probe of electroactive acetylated (Ac-) H6Y4C combined with WGA was prepared to electrochemically sense GA. To synthesize the protein probe, C-terminal carboxy groups of Ac-H6Y4C were bound to lysine residues or to the N-terminal amino group in WGA. Acetylation of the N-terminus of peptide inhibited the bonding between the N-terminal amino group of the peptide and the C-terminal carboxy group. The electrochemical measurement principle of GA was as follows (Fig. 1). The Ac-H6Y4C-WGA probe was added to a solution as a simple sensing system for GA. The electrode responses of the probe could be observed due to oxidation of the tyrosine residue. With the addition of GA, the peak currents decreased as the concentration of GA increased. The reason happened because the Ac-H6Y4C moieties of the probe are buried inside the complex between the probe and GA. As a result, the adsorption of Ac-H6Y4C moieties on an electrode surface is suppressed based on the complex formation between the Ac-H6Y4C-WGA probe and GA. To examine the properties of the protein probe, recovery experiments of GA were performed in samples of human serum.

An ALS electrochemical analyzer model 822D (BAS Inc., Japan) was used for voltammetric measurements. The working electrode was a glassy carbon electrode (GCE: geometric area, 0.070 cm2) (No. 002016, BAS Corporation). The reference electrode was an aqueous reference electrode (RE-1B) (Ag/AgCl) (No. 012167, BAS Corporation), and a platinum wire served as the counter electrode. The measurement cell was a VC-4 cell (No. 011224, BAS Corporation). Glassy carbon electrodes were polished using polishing cloth and 1.0, 0.3, and 0.05 µm alumina (BAIKOWSKI) in that order. Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF/TOF) mass spectra were recorded in a positive mode, which was controlled using the KOPACT 2.3.5 software package for AXIMA-CFR.
ReagentsThe reagents used are as follows. Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysulfosuccinimide sodium salt (Sulfo-NHS) were supplied from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Concanavalin A (Con A), GA, human serum (H3667 SLCB9407), and WGA were purchased from Sigma-Aldrich Co. LLC (MO, U.S.A.). Bovine serum albumin (BSA) and 2-morpholinoethanesulfonic acid monohydrate (MES) were obtained from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan).
Synthesis of the Protein ProbesAn electron-transfer peptide acetylated (Ac-) on the N-terminal side (Ac-H6Y4C) was synthesized via GenScript. To prepare the Ac-H6Y4C-WGA probe, peptide bonds are formed between lysine residues or N-terminal amino groups in WGA and the C-terminal carboxy groups of H6Y4C. Acetylation of the N-terminus of the peptide inhibits the bonding between the N-terminal amino group of the peptide and the C-terminal carboxy group. The peptides were analyzed via HPLC (Pump A: 0.065% trifluoroacetic in 100% water (v/v); Pump B: 0.05% trifluoroacetic in 100% acetonitrile (v/v); Total Flow;1 mL/min; Wavelength; 220 nm; Column: Inertsil ODS-SP 4.6 × 250 mm); and, measurement was conducted using electrospray ionization (ESI)-MS (Supplementary Fig. S1).
To synthesize the (Ac-H6Y4C-WGA) probe, 0.3 mg of Ac-H6Y4C was dissolved in 150 µL of 0.1 M MES (containing 0.5 M NaCl) at pH 6.0; and, EDC (40 µL of 6.0 × 10−2 M), and Sulfo-NHS (40 µL of 4.6 × 10−2 M) were mixed at 28 °C for 15 min. After the addition of NaHCO3 (40 µL of 0.5 M) and WGA (60 µL of 10−3 M), the solution was incubated for 3 h. Next, the solution was loaded into a His-Tag column with His-Affinity gel (Nickel-charged agarose (30% by volume), Zymo research His-Spin Protein Miniprep Kit 2002). The unreacted WGA was removed from Ac-H6Y4C-WGA and Ac-H6Y4C using the column. When Ac-H6Y4C-WGA and Ac-H6Y4C were accumulated on the gel, the column was washed twice using 250 µL of HCl His-Wash buffer. To elute Ac-H6Y4C-WGA and Ac-H6Y4C, elution buffer was added to the column and the sample was collected to new tubes using centrifugation. A 3K spin column (Nanosep Omega Life Sciences) was used for the separation of Ac-H6Y4C-WGA from Ac-H6Y4C. First, the spin column was washed twice with 400 µL of 0.1 M NaOH and equilibrated twice with 400 µL of 0.1 M phosphate buffered saline (PBS) (pH 7.0). To purify the Ac-H6Y4C-WGA probe from Ac-H6Y4C, the samples obtained by His-tag columns were added to a spin column with a molecular weight of 3 K and centrifuged (15 °C, 17000 G); the Ac-H6Y4C-WGA on the columns was washed using 200 µL of 0.1 M PBS (pH 7.0) with centrifugation.
Structure Determination of Ac-H6Y4C-WGAFor structure determination of Ac-H6Y4C-WGA, a ZipTip (ZTC18S096, Merck) was used to purify the Ac-H6Y4C-WGA probe from the eluate mentioned above. The tip was washed 5 times with 50% ACN and equilibrated 5 times with 0.1% trifluoroacetic acid (TFA) (10 µL). Next, the eluent was placed in the tip and pushed out of the tip 10 times. The chip was equilibrated with 0.1% TFA, and the Ac-H6Y4C-WGA probe was eluted using 50% ACN. The purified Ac-H6Y4C-WGA probe was subjected to MALDI-TOF-MS. The measurement by MALDI-TOF-MS was performed by the Center for Scientific Instrument Renovation and Manufacturing Support, Osaka University, Japan. MALDI-TOF-MS measurement was performed as follows. External calibration of the MALDI-TOF-MS mass spectrum was performed using the average mass peak of the mixture. Standard materials were Ubiquitin (m/z = 8565.8), cytochrome c (m/z = 12361), myoglobin (m/z = 16952), and BSA (m/z = 66431). The prepared Native WGA and Ac-H6Y4C-WGA were measured using a Shimadzu AXIMA-PERFORMANCE (MALDI-TOF/TOF). Silver trifluoroacetate was dissolved in pure water as an ionizing agent (4.5 µM). As the matrix, sinapic acid was dissolved in methanol (1 mg/mL). The silver trifluoroacetate solution (2 µL) was applied to the sample plate and allowed to dry. Next, the matrix solution (2 µL) was spotted onto the sample plate, followed by the addition of the sample solution (2 µL), and the solution was then allowed to dry at room temperature. The sample plate was then submitted to measurement.
Estimation of the Concentration of Ac-H6Y4C-WGATo determine the concentration of Ac-H6Y4C-WGA, the spectra of 5 × 10−6 M of Ac-H6Y4C and 5 × 10−6 M of WGA and Ac-H6Y4C-WGA were measured using a NanoDrop™ 2000 ultra trace UV-visible spectrophotometer (Thermo Scientific, MA, U.S.A.). The spectra of Ac-H6Y4C and WGA obtained from the measurements were compared with that of Ac-H6Y4C-WGA to determine the concentration of Ac-H6Y4C-WGA.
Voltammetric Measurements of GA Based on Ac-H6Y4C-WGATo voltammetrically measure GA using the Ac-H6Y4C-WGA probe, GA and the probe were incubated in 1 mL of 0.1 M phosphate buffer (pH 7.0) with stirring for 15 min. Next, the potential was scanned (scan rate: 50 mV/s) following application to a glassy carbon electrode for 5 min with stirring. For GA-sensing, the influences of coexisting substances, recovery experiments in human serum, and differential pulse voltammetry were based on the following conditions: Amplitude, 0.025 V; Pulse width, 0.01 s; Sample width, 0.005 s; Pulse period, 0.02 s; and Quiescent time, 15 s.
WGA has a monomeric molecular weight of approximately 17000, and the monomer is arranged with four similar disulfide-rich domains containing 43 amino residues, which were dimerized via head-to-tail bonding.20) MALDI-TOF-MS was used to determine the number of peptide molecules bound to the protein probe (Fig. 2). In native WGA, signals of [WGA + H+]1+ and [2WGA + H+]1+ were observed at m/z = 17214.44 and 34715.49 (Fig. 2A). For Ac-H6Y4C-WGA, the peaks due to [WGA + H+]1+ and [2WGA + H+]1+ appeared at m/z = 17272.38 and 34547.67 (Fig. 2B). The signals caused by [Ac-H6Y4C-WGA + H+]1+ and [2Ac-H6Y4C-WGA + H+]1+ were obtained at m/z = 19300.66 and 36740.86. The number of Ac-H6Y4C moieties bound to the C-terminal or lysine residue per WGA was estimated as follows. Subtracting the value of [WGA + H+]1+ m/z: 17214.44 from [Ac-H6Y4C-WGA + H+]1+ m/z: 19300.66 was 2086.00. When Ac-H6Y4C moieties were peptide bound to WGA, the molecular weight was calculated at 1621.30 per peptide moiety. Next, the obtained m/z difference (2086.00) was divided by the molecular weight of the peptide moiety. As a result, the number of peptide molecules was 1.3 per molecule of WGA. In addition, the signals attributed to WGA were detected in Ac-H6Y4C-WGA as well as in native WGA. The bond between WGA and Ac-H6Y4C was probably cleaved by the ionization of Ac-H6Y4C-WGA because the unreacted Ac-H6Y4C and WGA were removed in purification steps. Furthermore, the molecular weight of 2Ac-H6Y4C-WGA was less than twice that of Ac-H6Y4C-WGA. The ratio of [WGA + H+]1+ and [2WGA + H+]1+ in m/z became 2.0. In contrast, the ratio of [Ac-H6Y4C-WGA + H+]1+ and [2Ac-H6Y4C-WGA + H+]1+ in m/z was 1.9. When Ac-H6Y4C-WGA formed a dimer, WGA itself, or some of the Ac-H6Y4C bound to WGA, could be more broken by ionization by comparison with the ionization of a monomer.
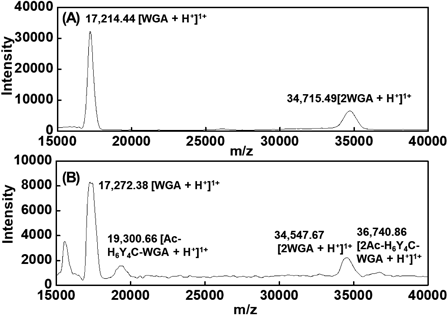
(A) Native WGA; (B) Ac-H6Y4C-WGA.
To estimate the concentration of Ac-H6Y4C-WGA, absorption spectra of 5.0 × 10−6 M Ac-H6Y4C and 5.0 × 10−6 M of WGA were recorded in 0.1 M of phosphate buffer (pH 7.0). The peaks due to amino acid residues such as tyrosine, tryptophan, and phenylalanine appear in Figs. 3(a) and (b). In addition, a synthetic spectrum of 5.0 × 10−6 M of Ac-H6Y4C and 5.0 × 10−6 M of WGA was indicated (Fig. 3(c)). In Fig. 3(d), the spectrum of the prepared Ac-H6Y4C-WGA was measured. The concentration of Ac-H6Y4C-WGA was estimated by comparing the synthetic spectrum with the spectrum of Ac-H6Y4C-WGA (Fig. 3(c)). Because the molecular number of Ac-H6Y4C mentioned above was 1.3 molecules per WGA, the concentration of the probe was determined to be 9.3 × 10−6 M.

(a) 5.0 × 10−6 M of Ac-H6Y4C; (b) 5.0 × 10−6 M of WGA; (c) 5.0 × 10−6 M of Ac-H6Y4C + 5.0 × 10−6 M of WGA; and, (d) 9.3 × 10−6 M of Ac-H6Y4C-WGA. The spectra were recorded using 0.1 M of phosphate buffer (pH 7.0). To determine the concentration of the Ac-H6Y4C-WGA, the absorbance was measured at 275 nm.
First, cyclic voltammograms of 1.5 × 10−7 M of Ac-H6Y4C were measured at −0.20 V for 5 min using a glassy carbon electrode to evaluate the performance of Ac-H6Y4C-WGA (Fig. 4(A)). The reason that the potential at −0.2 V was applied to the electrode for 5 min was to improve the sensitivity of the peak current due to AcH6Y4C moieties (Supplementary Fig. S3). When the potential was scanned between −0.2 and 0.9 V using cyclic voltammetry, a pair of peaks based on the redox of the phenolic hydroxyl group of tyrosine were observed at 0.65 and 0.15 V, respectively. Because the electrode reaction indicated irreversible properties, the oxidation peak that was greater than the reduction peak was used to evaluate the behavior of the probe. Next, the peak current was recorded after 1.5 × 10−7 M of Ac-H6Y4C-WGA and 1.0 × 10−11 M of GA were incubated with stirring. As a result, the peak current of the probe was decreased compared with that of the probe alone (Fig. 4(B)). By contrast, no decrease in the peak current was observed by the mixing of 1.5 × 10−7 M of Ac-H6Y4C and 1.0 × 10−11 M of GA ((c) in Fig. 4 (A)). WGA has four sugar recognition sites that bind to N-acetylglucosamine, glucosamine, and glucose.5) The electrode responses of the peptide moieties in the probe were suppressed based on the binding between the glucose moieties of GA and the recognition sites of WGA to glucose moieties. Accordingly, it was clear that GA was detected using the Ac-H6Y4C-WGA probe.
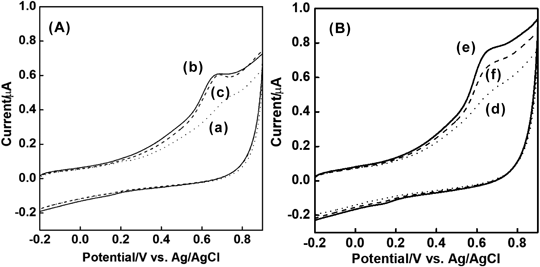
(A) (a) Blank; (b) 1.5 × 10−7 M of Ac-H6Y4C; (c) 1.5 × 10−7 M of Ac-H6Y4C + 1.0 × 10−11 M GA; (B) (d), Blank; and, (e) 1.5 × 10−7 M of Ac-H6Y4C-WGA; and, (f) 1.5 × 10−7 M of Ac-H6Y4C-WGA + 1.0 × 10−11 M of GA. After 1.5 × 10−7 M of Ac-H6Y4C-WGA and 1.0 × 10−11 M of GA were incubated in 0.1 M phosphate buffer (pH 7.0) for 30 min, a potential of −0.2 V was applied to the electrode for 5 min. Then, the potential was scanned in a positive direction (Sample interval, 0.001 V; scan rate, 50 mV/s).
To confirm whether the electrode response of Ac-H6Y4C-WGA was based on diffusion or a surface-controlled electrochemical process, we measured the relationship between the oxidation peak of the probe and the scan rate (Supplementary Fig. S2). The electrode reaction was due to a surface-controlled electrochemical process because the peak currents were proportional to the scan rates. The Ac-H6Y4C-WGA probe was not immobilized on the electrode. The assay for GA was carried out based on the formation of a complex between the probe and GA. The electrode response sites of the probe were Ac-H6Y4C, and the probe was added to a solution. When the probe was incubated with GA in the solution, the target was detected by the changes in the peak currents caused by the formation of a complex.
The relationship between the oxidation current value of 1.5 × 10−7 M of Ac-H6Y4C and the scan rate was recorded in a range of from 5–150 mV/s (Supplementary Fig. S2). The peak currents were proportional to the scan rates. This phenomenon suggested that the probe was accumulated on the electrode based on the adsorption of peptide moieties. In addition, we evaluated the relationship between the time of application potential (accumulation time) to the electrode and the oxidation peaks. The electrode responses of the Ac-H6Y4C-WGA probe increased as the accumulation time increased up to 5 min (Supplementary Fig. S3). Accordingly, the decrease in the peak current was not due to a decrease in the diffusion constant of the complex.
In addition, the electron transfer number was estimated using Laviron's equation.21) When the redox of tyrosine residues was an irreversible electrode reaction, the number of electron transfers was calculated to be 3.03 (Fig. 5). The value was smaller than that of the previously reported electron-transfer number of 3.89 for Y4C.17) This was caused by the acetylation at the N-terminal and by the influence of H6 in Ac-H6Y4C.
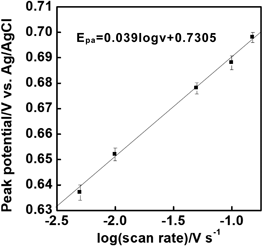
A potential of 0.3 V was applied to the electrode for 5 min in 0.1 M of phosphate buffer with 1.5 × 10−7 M of Ac-H6Y4C-WGA, and the potential was scanned in a positive direction (scan rate, 5–150 mV/s).
The calibration curve for GA sensing was constructed based on the electrode responses of the probe using differential pulse voltammetry. The peak currents were measured in the solutions containing 1.5 × 10−7 M of Ac-H6Y4C-WGA and various concentrations of GA (1.0 × 10−12 M–5.0 × 10−11 M) (Fig. 6). The peak currents decreased linearly and ranged from 1.0 × 10−12 to 2.0 × 10−11 M of GA (I(µA) = −0.0146CGA + 0.0022 (R: 0.995)). The relative standard deviation of 5.0 × 10−12 was 4.9% (n = 5). The detection limit was calculated from LOD = 3.3 × SD/m (SD, standard deviation of low concentration of GA; m, slope of the calibration curve) and was 3.3 × 10−13 M.

After 1.5 × 10−7 M of Ac-H6Y4C-WGA + GA was incubated in 0.1 M of phosphate buffer (pH 7.0) for 30 min, a potential of 0.2 V was applied to the electrode for 5 min. Then, the potential was scanned in a positive direction (pulse amplitude, 25 mV; pulse width, 0.01 s; sample width, 0.005 s; and, pulse period, 0.002 s).
To evaluate the selectivity for the sensing of GA, we examined the current change that coexisting substances would cause in a solution with 1.5 ×10−7 M of Ac-H6Y4C-WGA and 5.0 × 10−12 M of GA (Fig. 7). The oxidation peak obtained by 1.5 ×10−7 M of Ac-H6Y4C-WGA with 5.0 × 10−12 M of GA (Fig. 7 (b) was lower compared with that by 1.5 ×10−7 M of Ac-H6Y4C-WGA alone (Fig. 7(a)). Glucose was added in order to evaluate the binding between glucose and WGA. The peak current of the probe was increased by the addition of glucose (Fig. 7(c)). When the concentration of glucose was 10−6 M in the solution, the peak current approximated the 1.5 ×10−7 M that was measured for Ac-H6Y4C-WGA alone (Fig. 7(d)). This phenomenon indicated that the binding between the probe and GA was suppressed by the binding between glucose and WGA. In the certificate of analysis (Sigma-Aldrich) of the GA that was used, three molecules of carbohydrate are bound to each molecule of GA. When the carbohydrate moieties were glucose moieties, WGA combines with GA selectively. Since mannose did not interact with WGA, the change in the peak current was rarely observed compared with that of 1.5 ×10−7 M of Ac-H6Y4C-WGA and 5.0 × 10−12 M of GA (Fig. 7(e)). The value with BSA was also similar to that of Ac-H6Y4C-WGA and WGA because BSA is a simple protein without carbohydrates and did not combine with WGA (Fig. 7 (f)). Con A selectively combines with glucose and mannose and is a lectin that forms dimers and tetramers.22) When 1.5 × 10−7 M of Ac-H6Y4C-WGA, 5.0 × 10−12 M of GA, and 5.0 × 10−11 M of Con A were incubated for 30 min, the peak current was increased due to the competitive reactions between Ac-H6Y4C-WGA and Con A to GA (Fig. 7(g)). The coexistance of SBA with one of the glycoproteins did not influence the measurements of GA.

(a) 1.5 × 10−7 M of Ac-H6Y4C-WGA alone; (b) 1.5 × 10−7 M of Ac-H6Y4C-WGA and 5.0 × 10−12 M of GA; (c) 1.5 × 10−7 M of Ac-H6Y4C-WGA, 5.0 × 10−12 M of GA, and 1.0 × 10−9 M of Glucose; (d) 1.5 × 10−7 M of Ac-H6Y4C-WGA, 5.0 × 10−12 M of GA, and 1.0 × 10−6 M of Glucose; (e) 1.5 × 10−7 M of Ac-H6Y4C-WGA, 5.0 × 10−12 M of GA, and 1.0 × 10−6 M of Mannose; (f) 1.5 × 10−7 M of Ac-H6Y4C-WGA, 5.0 × 10−12 M of GA, and 5.0 × 10−10 M of BSA; (g) 1.5 × 10−7 M of Ac-H6Y4C-WGA, 5.0 × 10−12 M of GA, and 5.0 × 10−11 M of Con A; and, (h) 1.5 × 10−7 M of Ac-H6Y4C-WGA, 5.0 × 10−12 M of GA, and 5.0 × 10−10 M of SBA. After 1.5 × 10−7 M of Ac-H6Y4C-WGA, 5.0 × 10−12 M of GA, and a coexisting substance were incubated in 0.1 M phosphate buffer (pH 7.0) for 30 min except for (a) and (b), a potential of 0.2 V was applied to the electrode for 5 min. Then, the potential was scanned in a positive direction (pulse amplitude, 25 mV; pulse width, 0.01 s; sample width, 0.005 s; and, pulse period, 0.002 s). The measurement of (a) was performed without incubation. In the experiment of (b), the voltammogram was recorded in the solution with 1.5 × 10−7 M of Ac-H6Y4C-WGA and 5.0 × 10−12 M of GA after the incubation.
We compared the detection range and detection limits in GA sensing with that found by other research groups (Table 1). For the detection of GA, single-strand DNA with ferrocene that recognized GA was modified via methoxy/polyethylene on a gold electrode.14) The sensor was proportional to the concentration of GA and ranged from 75 nM to 10 µM. On the other hand, a procedure was developed using a graphene oxide complex with an immobilized free GA-recognizing aptamer.23) GA was detected within a range of between 0.15 and 750 nM with a LOD of 0.13 nM. Mahobiya’s group proposed a biosensor using microscreen-printed electrodes with bi-metallic gold-platinum nanomaterial.24) The calibration curve for a bimetallic nanoparticles-modified electrode was linear and ranged from 0.1 nM to 500 mM. The detection limit of GA was 0.1 nM. Also, a disposable enzyme sensor was constructed using an interdigitated electrode.25) GA was detected by the measurement of fructosyl lysine produced from the protease digestion product of GA. The concentration of GA was linear and ranged from 50–500 µM with a LOD of 1.2 μΜ. Li et al. fabricated a glassy carbon electrode with a cuprous oxide/reduced graphene oxide (Cu2O-rGO) nanozyme for the detection of GA.13) Measurements were carried out using the interaction between methylene blue-labeled DNA tripods and GA. The electrode responses were proportional to the concentration of GA (0.33 nM–23 μΜ). The detection limit of GA was 0.11 nM. A boronate affinity sandwich assay was performed using an indium tin oxide electrode modified with nanomaterials of Prussian blue nanoparticles/3-aminophenylboronic acid. The calibration curve was linear and ranged from 76 nM to 15 μΜ with a LOD of 53 nM.26) Choi et al. synthesized urchin-like Pt nanozymes (uPtNZs) for the sensitive determination of GA using 3,3′,5,5′-tetramethylbenzidine and thionine.27) The responses were linear and ranged from 76 nM to 150 μΜ with a LOD of 58 nM. Maraming’s group detected GA using a polydopamine nanoparticle-functionalized electrochemical aptasensor.28) The measurements of GA ranged from 15 to 15000 nM with a LOD of 6.0 nM. Furthermore, a sensing system of GA was designed using an electrode with reduced graphene oxide/Au nanoparticles/anti-GA aptamer.29) The thiolated aptamer was immobilized on the electrode, and the linear range of that system was 30–150 nM with a detection limit of 1.1 nM. When using this method, WGA selectively recognized GA with multiple carbohydrate moieties, and Ac-H6Y4C moieties were buried in GA. Therefore, the electrode responses of Ac-H6Y4C moieties were decreased. In addition, the complexes such as WGA-GA-WGA-GA could be formed to further decrease the electrode response. The effects could cause changes in the peak currents on the order of 10−13 M, resulting in a highly sensitive sensing system.
| Procedure | Linear range | Detection limit | Reference |
|---|---|---|---|
| Flexible gold electrode modified with single strand DNA | 75 nM–10 µM | 25 nM | 14 |
| Voltammetry based on oxidation of [Fe(CN)6]3- using a graphene oxide-aptamer complex | 0.15–750 nM | 0.13 nM | 23 |
| Microscreen printed electrodes based on a bi-metallic gold-platinum nanomaterial | 0.1 nM–500 mM | 0.1 nM | 24 |
| Interdigitated electrode-based disposable enzyme sensor | 50–500 µM | 1.2 μM | 25 |
| Electrode with cuprous oxide/reduced graphene oxide nanocomposites | 0.33 nM–23 μM | 0.11 nM | 13 |
| Electrochemical detection using a Prussian blue nanozyme-based on boronate affinity assay | 76 nM–15 µM | 53 nM | 26 |
| Electrochemical sensing based on a GA antibody/urchin-like Pt nanozyme/GA/boronic acid complex | 76 nM–150 μM | 58 nM | 27 |
| Sensor modified with polydopamine nanoparticles/DNA | 15–15000 nM | 6.0 nM | 28 |
| Electrode modified with a reduced-graphene oxide/Au nanoparticles/anti-GA aptamer | 30–150 nM | 1.1 nM | 29 |
| Voltammetric method using an electron- transfer peptide modified with a wheat germ agglutinin probe | 1.0–20 pM | 0.33 pM | This work |
To clarify whether the proposed method could be applied to the detection of GA, GA was monitored in human serum. The serum was diluted 5000000-fold using 0.1 M of phosphate buffer (pH 7.0). The electrode response of the probe was comparable to that obtained without human serum, indicating that the serum component does not decrease the electrode response. Concentrations of GA ranging from 1.0 × 10−12 to 2.0 × 10−11 M were added to the human samples as recovery experiments (Table 2). The recoveries of GA were 98–101%, which is sufficient for the electrochemical detection of GA. The value of GA initially contained in human serum obtained by the standard addition method was 5 × 10−6 M (R.S.D. = 5.1%, n = 5). The concentration of GA in the diluted sample was 1.0 × 10−12 M. Although this concentration was lower than those commonly reported,30,31) the value was in agreement with the value of 0.6–3% for GA.32) As a result, this procedure could be a form of sensing for GA in serum samples.
| Sample | GA added (10−12 M) | Found (10−12 M) | Recovery (%) | R.S.D. (%) |
|---|---|---|---|---|
| 1 | 1.0 | 0.98 | 98 | 5.1 |
| 2 | 5.0 | 4.8 | 98 | 4.7 |
| 3 | 10.0 | 10.1 | 101 | 5.0 |
| 4 | 20.0 | 19.8 | 99 | 4.8 |
1 : 5000000 dilution of human serum (0.0000002%) using 0.1 M phosphate buffer (pH 7.0). Number of determinations (n = 5).
In the present study, we developed a voltammetric system capable of sensing GA for the early detection of diabetes mellitus. First, a protein probe wherein the electron transfer peptides were bound to WGA recognized glucose. When cyclic voltammograms were recorded using a glassy carbon electrode, an oxidation peak of phenolic hydroxyl groups was observed. In the presence of GA, the oxidation peak was decreased due to the binding between Ac-H6Y4C-WGA and GA. The electrode reaction was based on the control of adsorption because the peak currents were proportional to the scan rates. Furthermore, the number of electron transfers was estimated from the peak potential and the logarithm of scan rate and found to be 3.03. The calibration curve of GA using differential pulse voltammetry was linear and ranged from 10−13 to 10−11 M. In addition, measurements with coexisting substances were performed via differential pulse voltammetry. The GA measurements with mannose and BSA were similar to that of GA alone. Following the recognition of glucose, the GA and protein probe was incubated in the solution, and the decrease in the peak current was suppressed since the glucose combined with the protein probe. This phenomenon indicated that Ac-H6Y4C-WGA selectively binds to glucose and to the glucose moieties of glycoproteins. To examine whether this method would be applicable to the detection of GA in human serum, recovery experiments of GA were performed in the serum. These experiments resulted in recoveries that ranged from 98–102% with GA concentrations ranging from 10−12 to 10−11 M. Therefore, the Ac-H6Y4C-WGA-based sensing system could be a sensitive and rapid method to detect GA.
This work was supported by JSPS KAKENHI Grant Number JP 22K05155.
The authors declare no conflict of interest.
This article contains supplementary materials.