- J-STAGE home
- /
- Chemical and Pharmaceutical Bu ...
- /
- Volume 72 (2024) Issue 3
- /
- Article overview
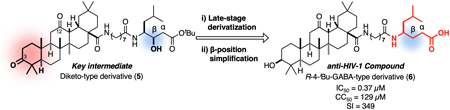
-
Reon Takeuchi
Graduate School of Science and Technology, Shizuoka University
-
Junko Fujimoto
Department of Applied Chemistry and Biochemical Engineering, Faculty of Engineering, Shizuoka University
-
Yoshinori Taguchi
Graduate School of Medical Photonics, Shizuoka University
-
Ryuji Ide
Course of Applied Chemistry and Biochemical Engineering, Department of Engineering, Graduate School of Integrated Science and Technology, Shizuoka University
-
Ryuji Kyan
Research Institute of Green Science and Technology, Shizuoka University
-
Kohei Sato
Graduate School of Science and Technology, Shizuoka University Department of Applied Chemistry and Biochemical Engineering, Faculty of Engineering, Shizuoka University Course of Applied Chemistry and Biochemical Engineering, Department of Engineering, Graduate School of Integrated Science and Technology, Shizuoka University Research Institute of Green Science and Technology, Shizuoka University
-
Nobuyuki Mase
Graduate School of Science and Technology, Shizuoka University Department of Applied Chemistry and Biochemical Engineering, Faculty of Engineering, Shizuoka University Course of Applied Chemistry and Biochemical Engineering, Department of Engineering, Graduate School of Integrated Science and Technology, Shizuoka University Research Institute of Green Science and Technology, Shizuoka University
-
Masaru Yokoyama
Pathogen Genomics Center, National Institute of Infectious Diseases
-
Shigeyoshi Harada
Corresponding author
AIDS Research Center, National Institute of Infectious Diseases
-
Tetsuo Narumi
Corresponding author
Graduate School of Science and Technology, Shizuoka University Department of Applied Chemistry and Biochemical Engineering, Faculty of Engineering, Shizuoka University Graduate School of Medical Photonics, Shizuoka University Course of Applied Chemistry and Biochemical Engineering, Department of Engineering, Graduate School of Integrated Science and Technology, Shizuoka University Research Institute of Green Science and Technology, Shizuoka University
Supplementary material
2024 Volume 72 Issue 3 Pages 330-335
- Published: March 25, 2024 Received: December 20, 2023 Released on J-STAGE: March 25, 2024 Accepted: February 14, 2024 Advance online publication: - Revised: -
(compatible with EndNote, Reference Manager, ProCite, RefWorks)
(compatible with BibDesk, LaTeX)