2024 Volume 72 Issue 3 Pages 313-318
2024 Volume 72 Issue 3 Pages 313-318
Generating reliable data on functional group compatibility and chemoselectivity is essential for evaluating the practicality of chemical reactions and predicting retrosynthetic routes. In this context, we performed systematic studies using a functional group evaluation kit including 26 kinds of additives to assess the functional group tolerance of carbene-mediated reactions. Our findings revealed that some intermolecular heteroatom–hydrogen insertion reactions proceed faster than intramolecular cyclopropanation reactions. Lewis basic functionalities inhibited rhodium-catalyzed C–H functionalization of indoles. While performing these studies, we observed an unexpected C–H functionalization of a 1-naphthol variant used as an additive.
A variety of functional groups exist in medicinal molecules, natural products, and pharmaceutical active ingredients. In their synthetic processes, it is common to mask the more reactive functionalities with protecting groups when less reactive functionalities must be converted. This may increase the number of steps required, however, and decrease the overall synthetic efficiency. In addition, it is not always clear which functional groups are compatible with a particular reaction or which functional groups react first in complex molecular syntheses. Therefore, it is essential to obtain comprehensive information on the chemoselectivity in various molecular transformations. Typically, the compatibility of functional groups is assessed by examining the substrate generality. Given the time required to synthesize reactants with multiple functionalities, this conventional method may not be suitable for a comprehensive evaluation. Accordingly, applying an exhaustive set of external additives to comprehensively investigate the effects of functional groups will be a useful approach.1,2)
Some research groups evaluate functional group tolerances by adding compounds with specific functional groups as additives.3,4) In 2009, the Fox group reported Rh-catalyzed multi-component reactions using α-alkyl-α-diazoesters, aldehydes, and dipolarophiles, and evaluated the functional group compatibility with 11 different external additives5) (eq 1, Chart 1). An efficient “robustness screen” was established by the Glorius group in 2013 (eq 2).1,6) The functional group tolerance was assessed with the change in the product yield and the remaining additives carrying specific functional groups. In 2014, the Mukherjee7) and Malik8) groups also independently reported enantioselective nucleophilic addition and allylic C–H acetoxylation, respectively, and assessed chemoselectivity or its functional group tolerance (eqs 3, 5). With the addition of biomolecules into the reaction system, the Chen group demonstrated that the developed deboronative alkynylation reaction could be compatible with nucleic acids, cell lysates, nucleosides, and proteins (eq 4).9) Very recently, a powerful method was established by the Ohshima group that takes into account two essential aspects - statistical evaluation and identification of side products (eq 6).10) This methodology provides valuable information to better understand specific reactions11–13) and to build an original database14–16) for machine learning.17–22)

As part of our ongoing research program to synthesize biologically important molecules,23) we focused on developing carbene chemistry using metal catalysis.24) Metal carbenes25,26) are reactive transient species that enable insertion reactions into C–H bonds, cyclopropanation reactions of olefins, and so on.27–29) Transition metals, mainly in group 8–11 elements, are commonly used as catalytic core elements to generate carbene species from the corresponding diazo compounds or their chemical equivalents.30–32) In this study, we evaluated chemoselectivity using a functional group evaluation (FGE) kit in rhodium(II)-catalyzed intramolecular cyclopropanation and rhodium(III)-catalyzed intermolecular C–H functionalization reactions based on the Ohshima method (eq 7). To the best of our knowledge, there are no reports of comprehensive additive screening in these catalytic transformations. Herein we report the results of a detailed examination using the FGE kit.
The FGE kit contains 26 kinds of compounds with various functional groups (A1–A26). To evaluate the experimental results using statistical methods, a control experiment was conducted with 1-butyl-4-chlorobenzene (additive A1) based on the original Ohshima work.10) A1 is an unreactive p-disubstituted benzene and easily detectable by 1H-NMR. Reproducibility was ensured by repeating the reaction five times to confirm that the common 4-chlorophenyl unit does not have an undesired impact on the reaction outcome. The value of the average yield needs to be within standard deviation σ ≤ 5. The systematic evaluation of functional groups was mainly limited to intermolecular reactions in previous studies (Chart 1). We evaluated an intramolecular cyclopropanation reaction under rhodium (II) catalysis. Allyl 2-diazo-2-phenylacetate was selected as a model substrate. The corresponding products are synthetic intermediates of Milnacipran,33) a drug with serotonin noradrenaline reuptake inhibitor activity,34,35) and intermediates of compounds acting on orexin receptors and sigma receptors.36,37)
In the presence of A1 (1 equivalent (equiv.)) and a Rh2(OAc)4 catalyst (5 mol%), 1 was added dropwise over 2 min at 50 °C in CH2Cl2, giving 2 in 70% yield (additive remaining 93%, entry 1, Table 1). In this system, no side-products derived from the reaction of the Rh-carbene species with A1 were detected. Thus, various additives (A2–A26) were subsequently examined based on the above-mentioned assay protocol. No major changes were observed in the reactions using A13 with a siloxy group, A15 with an epoxide functionality, A16 with an unsaturated ketone structure, A17 with a methyl ketone functionality, A19 with a benzonitrile moiety, or A24 with an ester moiety. For the sake of clarity, we used the following notation:  ; a significant increase supported by statistical analysis.
; a significant increase supported by statistical analysis.  ; comparable to that of the control group.
; comparable to that of the control group.  ; a statistically significant reduction, equivalent to, or greater than half of the control group’s average.
; a statistically significant reduction, equivalent to, or greater than half of the control group’s average.  ; a statistically significant reduction, less than half of the control group’s average. Further, no major changes were observed even with A11 having a terminal olefin and A12 having a terminal alkyne, which are potentially reactive with carbene species, along with trisubstituted arene (A4, A9, A10) and arylboronic ester A21 useful for coupling reactions. On the other hand, nitrogen-containing heteroaromatics (A8, A22) as well as Lewis-basic amine or sulfide (A23, A26) inhibited the cyclopropanation reaction, recovering diazoester 1 and the additives. This is presumably because the functional groups in the additives deactivated the rhodium catalyst used.
; a statistically significant reduction, less than half of the control group’s average. Further, no major changes were observed even with A11 having a terminal olefin and A12 having a terminal alkyne, which are potentially reactive with carbene species, along with trisubstituted arene (A4, A9, A10) and arylboronic ester A21 useful for coupling reactions. On the other hand, nitrogen-containing heteroaromatics (A8, A22) as well as Lewis-basic amine or sulfide (A23, A26) inhibited the cyclopropanation reaction, recovering diazoester 1 and the additives. This is presumably because the functional groups in the additives deactivated the rhodium catalyst used.
 |
a) Ar represents p-chlorophenyl group.
The following results indicated that the intermolecular carbene insertion reaction38) into the X–H bond (X=O, N, S) proceeded preferentially over the intramolecular cyclization reaction. Use of the primary amide (A7) gave N–H insertion product 4 in a high yield (93%, eq 2, Chart 2). Likewise, reaction with a primary alcohol (A3) provided an O–H insertion product 3 (eq 1). Thiol functionality (A20) also completely prevented the cyclopropanation, giving 5, albeit in low yield (38% yield, eq 3). Surprisingly, these intermolecular X–H insertion reactions were faster than intramolecular cyclopropanation reactions under dilute conditions (0.02 M).

Next, we focused on intermolecular C–H functionalization, a characteristic reaction of carbene species. In 2020, our group reported a rhodium(III)-catalyzed C–H activation of indoles at the C4 position using an enone-directing group.39) We evaluated the functional group compatibility of the transformation using the FGE kit (Table 2). The reaction using 6, acceptor/acceptor-substituted diazo compound (7), and A1 was performed under rhodium catalysis at 35 °C for 24 h, producing 2 in 65% yield and recovering 86% of A1. The 4-chlorophenyl unit did not hinder the progress of the reaction; therefore, we investigated various additives based on the same protocol.
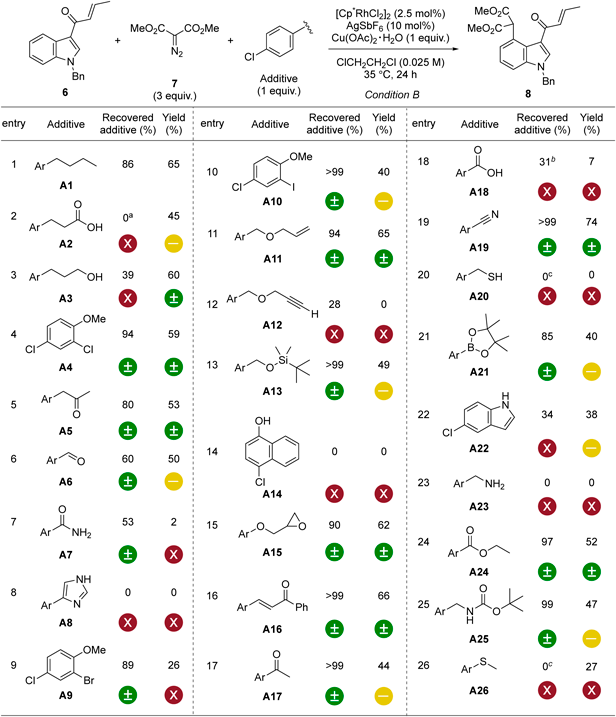 |
a) Ar represents p-chlorophenyl group. b) Reaction was quenched with aqueous NaHCO3 solution. c) Reaction was quenched with aqueous NaClO solution.
Likewise, in intramolecular cyclopropanation, no statistically significant changes were observed in C–H functionalization with additives A5 with an arylpropan-2-one structure, A15, A16, and A24. On the other hand, in the reactions using A6 with a formyl group, and A10, A13, A17, A21, and A25 with a carbamate group, each additive used was recovered well, while the product yield was slightly lower. Although a terminal olefin was tolerated under the reaction conditions (A11), indole 6 remained intact in the reaction using A12 with a terminal alkyne. In this respect, different trends were observed for intramolecular and intermolecular reactions under rhodium catalysis. Interestingly, although the reaction using A3 with free alcohol did not decrease the yield of 6, the recovery yield of A3 was 39%. Aliphatic carboxylic acid (A2) and aromatic carboxylic acid (A18) were incompatible, like rhodium (II) catalysis. In the reaction using primary amide (A7), 6 disappeared, and no detectable side product was obtained. In general, functional groups that could act as ligands for Rh(III) inhibited the progress of the reaction. Although the addition of A19 slightly improved the yield of 8, it was statistically comparable to the control group.
Of particular note, the reaction with 4-chloronaphthalen-1-ol (A14) provided C–H functionalized product 9 (29% yield) and O–H insertion product 10 (11% yield) without any production of 8 (eq 1, Chart 3). In a related reaction, the Swamy group reported a Rh(III)-catalyzed C–H activation of 1-naphthol derivative with an acceptor/acceptor-substituted diazo compound using pyrimidine as the directing group in 2017.40) The Zhao group achieved a Rh(III)-catalyzed functionalization of C(sp2)–H bond in 1-naphthol variant directed by a thiocarbamate group in 2019.41) The yields, however, were not satisfactory in either case (3440) and 40%,41) respectively). We were interested in the result of eq 1 because there have been no reports of reactions with 1-naphthol lacking directing groups. Preliminary studies were performed using A14 as a starting material. Control experiments revealed that 9 was obtained in an isolated yield of 44% under silver catalysis without using a rhodium catalyst (eq 2).42,43) When 9-phenanthrol was used instead of A14, an O–H insertion reaction proceeded to give 11 (eq 3), indicating substrate-dependent chemoselectivity.44) Such acceleration of serendipitous discoveries is part of the appeal of systematic studies utilizing the FGE kit.

We investigated functional group compatibility and chemoselectivity in rhodium-carbene-mediated transformations. Systematic studies using the FGE kit developed by Ohshima’s group revealed that Lewis basic functionalities inhibited the reaction progress. On the other hand, protic functional groups may cause chemoselective X–H insertion reactions, even in intramolecular reactions. In addition, the presence of terminal alkynes did not affect the reaction results of the intramolecular cyclopropanation, but significantly reduced the yield of the intermolecular C–H functionalization. The comprehensive additive screening serendipitously found a directing group-free C–H functionalization of 1-naphthol variants. We believe that gathering dependable information incorporating even negative experimental findings will play a pivotal role in advancing organic synthesis.
This work was supported by the Grant-in-Aid for Transformative Research Areas (A) 22H05337 Digitalization-driven Transformative Organic Synthesis (Digi-TOS) from the Ministry of Education, Culture, Sports, Science and Technology, Japan. It was also supported by JSPS KAKENHI (Grant Numbers: 22H02741 and 21K06471). We thank Ohshima Group for providing us with the FGE kit.
The authors declare no conflict of interest.
This article contains supplementary materials.