2024 Volume 72 Issue 6 Pages 566-569
2024 Volume 72 Issue 6 Pages 566-569
Dihydrobenzofuran is an important skeleton for bioactive compounds and natural products. Hydroquinones can be easily modified into substituted hydroquinones, which effectively undergo oxidation to produce the corresponding benzoquinone derivatives. Benzoquinones are reactive electrophiles that are frequently utilized in coupling with olefins to dihydrobenzofurans. Herein, we report the one-pot oxidative coupling of hydroquinones bearing an electron-withdrawing group at the C2 position with olefins to dihydrobenzofurans in the presence of the Lewis acidic FeCl3 and 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ) oxidant. Furthermore, this method was applied to the oxidative coupling of N-electron-withdrawing group-substituted 4-aminophenol.
2,3-Dihydrobenzofurans (DHBs) are important skeletons for bioactive compounds and natural products, and various synthetic approaches have been developed.1–4) Among them, benzoquinones (BQs) have been widely utilized as substrates in [3 + 2] cycloaddition with olefins (e.g., styrenes,5–8) enol ethers,9–11) allyl silane12)) to afford versatile DHBs (Chart 1A). BQs are prepared by the oxidation of hydroquinones (HQs), which can be easily modified by Friedel-Crafts type reactions to the corresponding C2-functionalized hydroquinone (such as compounds bearing an electron-withdrawing group (EWG) at the C2 position, 1, Chart 1C). Therefore, the one-pot oxidative functionalization of HQs is a powerful and straightforward tool to synthesize versatile DHBs and aromatic products. Masson have reported the asymmetric and oxidative one-pot reactions of HQs with enamines to construct dihydrobenzofuran derivatives13) (Chart 1B). Additionally, we have also developed the biaryl synthesis from hydroquinones (1) and arene nuclephiles in the presence of catalytic FeCl3 as Lewis acid and 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ) oxidant.14–16) DDQ oxidation of 1 gave benzoquinone reaction intermediate (2), then FeCl3-catalyzed nucleophilic attack of indole or arene proceeded at electrophilic C3 position, arising from electron-withdrawing group at C2 position of 2, to afford the corresponding biaryl products (3 and 4). Herein, we report the application of this method to the one-pot synthesis of DHBs (6) via oxidation of 1 and [3 + 2] cycloaddition with olefins (styrenes, enol ethers, and allyl silane). Furthermore, N-electron-withdrawing group-substituted 4-aminophenols (5) underwent oxidative coupling with styrenes to give the corresponding DHBs (7).17,18) As related reactions, iron-catalyzed radical couplings of 4-methoxyphenols with olefins in the presence of DDQ were reported19) (Chart 1E). Our method realized the construction of the different type’s dihydrobenzofurans, bearing phenolic hydroxy group, electron-withdrawing groups, amino groups and so on via ionic reaction mechanism.

The desired one-pot oxidative coupling of 2-methoxycarbonyl hydroquinone (1a) with styrene (1.5 equivalent (equiv.)) proceeded smoothly in the presence of FeCl3 (10 mol%) and DDQ (1.0 equiv.) in CH2Cl2 at 0 °C to room temperature (r.t.) for 24 h to give the dihydrobenzofuran derivative (6a) in 95% NMR yield (Table 1, entry 1). FeCl2 was also effective to produce 6a in 87% yield (entry 2), whereas no catalyst or the use of other Lewis acids (FeBr3, Fe(acac)3, AuCl3, ZnCl2, or BF3·Et2O) resulted in no reaction or low yields (entries 3–8). Reactions in other solvents, such as (CH2Cl)2, CHCl3, toluene, CH3CN, tetrahydrofuran (THF), and CH3OH, instead of CH2Cl2 using FeCl3 and DDQ, gave lower yields (entries 1 vs. 9–14). DDQ was found to be an adequate oxidant in comparison to other oxidants (PhI(OAc)2, cerium(IV) diammonium nitrate (CAN), OXONE, and NaClO·5H2O) (entries 1 vs. 15–19). A reduction in the catalytic amount of FeCl3 (10 to 5 mol%) maintained a high yield of 6a (entries 1 vs. 20), and further reduction to 1 mol% resulted in a lower yield (entry 21). The reaction at a constant temperature (r.t. or 0 °C) led to a lower yield of 6a (entries 1 vs. 22 and 23). The use of FeCl3 as inexpensive and abundant metal species is useful in organic reactions.20,21)
 | ||||
|---|---|---|---|---|
| Entry | Lewis acid | Oxidant | Solvent | Yield (%) |
| 1 | FeCl3 | DDQ | CH2Cl2 | 95 (90)b) |
| 2 | FeCl2 | DDQ | CH2Cl2 | 87 |
| 3 | — | DDQ | CH2Cl2 | 0 |
| 4 | FeBr3 | DDQ | CH2Cl2 | 53 |
| 5 | Fe(acac)3 | DDQ | CH2Cl2 | 15 |
| 6 | AuCl3 | DDQ | CH2Cl2 | 61 |
| 7 | ZnCl2 | DDQ | CH2Cl2 | 23 |
| 8 | BF3·Et2O | DDQ | CH2Cl2 | 15 |
| 9 | FeCl3 | DDQ | (CH2Cl)2 | 68 |
| 10 | FeCl3 | DDQ | CHCl3 | 28 |
| 11 | FeCl3 | DDQ | Toluene | 76 |
| 12 | FeCl3 | DDQ | CH3CN | 27 |
| 13 | FeCl3 | DDQ | THF | 2 |
| 14 | FeCl3 | DDQ | CH3OH | 0 |
| 15 | FeCl3 | Chloranil | CH2Cl2 | 0 |
| 16 | FeCl3 | PhI(OAc)2 | CH2Cl2 | 8 |
| 17 | FeCl3 | CAN | CH2Cl2 | 10 |
| 18 | FeCl3 | OXONE | CH2Cl2 | 0 |
| 19 | FeCl3 | NaClO·5H2O | CH2Cl2 | Trace |
| 20c) | FeCl3 | DDQ | CH2Cl2 | 90 |
| 21d) | FeCl3 | DDQ | CH2Cl2 | 46 |
| 22e) | FeCl3 | DDQ | CH2Cl2 | 55 |
| 23f) | FeCl3 | DDQ | CH2Cl2 | 85 |
a) Yield was determined by 1H-NMR using 1,1,2,2-tetrachloroethne as an internal standard. b) Isolated yield. c) 5 mol% of FeCl3 was used. d) 1 mol% of FeCl3 was used. e) At r.t. f) At 0 °C.; THF; tetrahydrofuran, acac; acetylacetonate, CAN; cerium(IV) diammonium nitrate.
The reaction using 2-methoxycarbonyl benzoquinone (2a), the oxidized form of 1a, could be used as a substrate under FeCl3-catalyzed reaction conditions to give 6a in 59% yield (Chart 2A), clearly indicating that 2a was a reaction intermediate in the one-pot oxidative coupling of 1a to 6a. 1a undergoes oxidation by DDQ to give 2a; then, FeCl3 activates the carbonyl groups at C1 position of 2a and the addition of styrene to the electrophilic C3 position of 2a produces the benzylic cation intermediate A. Subsequent intramolecular cyclization afforded dihydrobenzofuran 6a. The radical coupling of phenol derivatives bearing electron-donating methoxy groups and styrenes in the presence of catalytic FeCl3 and DDQ has also been reported.19) Therefore, a reaction mechanism involving radical intermediates cannot be completely ruled out.

The effect of the substituent on the C2 position of hydroquinone was investigated (Chart 3). 2-Methylcarbonyl, 2-formyl, and 2-tert-butoxycarbonyl-subsituted hydroquinones underwent oxidative coupling with styrene to afford the corresponding benzofurans (6b–6d) in high yields. In contrast, the reaction of 2-nitro substance afforded a low yield, probably because of its low solubility in CH2Cl2. Additionally, no desired dihydrobenzofuran products (6f–6h) were obtained using non-substituted, 2-chloro- and 2-methoxy-hydroquinones as substrates. The electrophilicity at the C3 position, enhanced by the electron-withdrawing group at the C2 position of benzoquinone reaction intermediate 2, was presumed to be important for facilitating the addition of styrene in the second step. When yields were lower in the reactions, there are many byproducts. Meanwhile, 2-methoxycarobonyl-1,4-dihydroxynaphthalene was effectively transformed into the corresponding three-membered dihydronaphthalene product (6i) in 70% yield.

Various olefins were applicable for the oxidative coupling of 1a (Chart 4). 4-Methyl-, 4-tert-butyl-, 4-bromo-, and 4-chloro-styrenes underwent the coupling with 1a to afford the corresponding dihydrobenzofuran derivatives (6j–6m) in high yields. In contrast, the reaction of 3-chlorostyrene gave no desired product (6n), and dihydrobenzofuran derivative 6o, derived from 2-chlorostyrene, was obtained in low yield. Unfortunately, 4-methoxy and 4-trifluoromethylstyrene were not applicable to give no desired products. 1,1-Diphenylethylene, 3,4-dihydro-2H-pyran, and butyl vinyl ether were coupled with 1a to form the corresponding dihydrobenzofuran derivatives (6p–6r). Furthermore, ally trimethylsilane (TMS) was used as an olefin to give the dihydrobenzofuran derivative (6t) in 43% yield, accompanied by the generation of allylated side products (8).22,23)

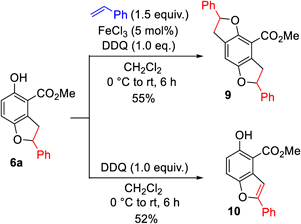
Further transformations of 6a, obtained by oxidative coupling of 1a with styrene, were performed (Chart 5). The reaction of 6a was repeated under the same reaction conditions using styrene, FeCl3, and DDQ to give the three-membered product (9) by coupling the phenol moiety of 6a with styrene (the radical reaction mechanism described in Reference 19 was presumed). Additionally, DDQ oxidation of 6a afforded an aromatized product (10).
This oxidative coupling with styrenes was applicable to N-electron-withdrawing group-substituted 4-aminophenols (5) (Chart 6). N-Acetyl-(Ac) (acetaminophen, a pharmaceutical drug) was coupled with styrene to give the corresponding dihydroxybenzofuran derivative (7a) in 59% yield. The electron-withdrawing groups on the nitrogen atom of 5 were essential, and N-mesyl-(Ms) and N-benzoyl-(Bz) substances were transformed into the desired products (7b and 7c). No dihydrobenzofuran derivatives (7d and 7e) were obtained using N-benzyl- (Bn) or N-free substances. 4-Fluoro- and 4-bromo-styrenes were also applicable in the present oxidative coupling of N-Ac-substance to give the corresponding products (7f and 7g). Furthermore, N-Ac-3-methoxycarbonyl-4-aminophenol was converted into the corresponding dihydrobenzofuran derivative (7h).

Ac; Acetyl, Ms; Mesyl, Bz, Benzoyl, Bn; Benzyl
We accomplished the oxidative coupling of hydroquinones bearing electron-withdrawing groups at the C2 position with olefins (styrenes, enol ethers, and allyl silane) to produce pharmaceutically useful dihydrobenzofuran derivatives in a one-pot manner. Additionally, N-electron-withdrawing group-substituted 4-aminophenols were applicable. This method can easily yield various dihydrobenzofurans that can contribute to drug discovery.
Typical Procedure; To a solution of hydroquinone (1; 0.20 mmol) in CH2Cl2 (1.0 mL) were added styrene (0.3 mmol), FeCl3 (0.02 mmol), and DDQ (0.20 mmol) under argon at 0 °C. The reaction temperature was gradually increased to room temperature, and the reaction mixture was stirred at room temperature for total 24 h. After quenching with sat. NaHCO3 aq., the reaction mixture was extracted with CH2Cl2 (10 mL × 2). The organic layer was dried over Na2SO4 and concentrated in vacuo. Purification using flash column chromatography on silica gel (n-hexane-ethyl acetate) gave dihydrobenzofuran derivative (6).
This study was partially supported by JSPS (MEXT grant in aid-for transformative research areas (B) Deuterium Science) KAKENHI Grant Number 20H05738 (for Y.S.), and Life Science and Drug Discovery (Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)) from AMED under Grant Number 23ama121054 (for Y.S.) and Research Foundation for the Electrotechnology of Chubu (REFEC) (for Y.S.).
The authors declare no conflict of interest.
This article contains supplementary materials.