2024 Volume 72 Issue 7 Pages 669-675
2024 Volume 72 Issue 7 Pages 669-675
Tendon injury is a prevalent orthopedic disease that currently lacks effective treatment. Galangin (GLN) is a vital flavonoid found abundantly in galangal and is known for its natural activity. This study aimed to investigate the GLN-mediated molecular mechanism of tendon-derived stem cells (TDSCs) in tendon repair. The TDSCs were characterized using alkaline phosphatase staining, alizarin red S staining, oil red O staining, and flow cytometry. The effect of GLN treatment on collagen deposition was evaluated using Sirius red staining and quantitative (q)PCR, while a Western bot was used to assess protein levels and analyze pathways. Results showed that GLN treatment not only increased the collagen deposition but also elevated the mRNA expression and protein levels of multiple tendon markers like collagen type I alpha 1 (COL1A1), decorin (DCN) and tenomodulin (TNMD) in TDSCs. Moreover, GLN was also found to upregulate the protein levels of transforming growth factor β1 (TGF-β1) and p-Smad3 to activate the TGF-β1/Smad3 signaling pathway, while GLN mediated collagen deposition in TDSCs was reversed by LY3200882, a TGF-β receptor inhibitor. The study concluded that GLN-mediated TDSCs enhanced tendon repair by activating the TGF-β1/Smad3 signaling pathway, suggesting a novel therapeutic option in treating tendon repair.
The tendon serves as a connecting bridge between muscle and bone, transmitting the force generated by muscle to bone, inflicting joint movement. Tendon injury is a prevalent orthopedic condition that significantly impacts patients’ health and QOL.1) Tendon injury can be either acute or chronic, including tendon rupture or tendinitis, and can result from intrinsic factors or external shock.2–5) Mild tendon injuries can self-heal and may not require surgical intervention, but healing is prolonged and may result in scar tissue formation. Similarly, severe tendon injuries are treated with conventional methods, like surgery, but their efficacy is limited.6) Moreover, some novel reported therapies, like cellular and injection therapy, still have limitations on treatment.7,8) Therefore, it is of pivotal importance to explore novel and effective tendon repair treatment strategies.
Tendon-derived stem cells (TDSCs) are a type of stem cell obtained from human and mouse tendons, displaying stem cell traits like multipotency, clonogenicity, and self-renewal ability,9) and have already been used in tendon injury healing.10) Moreover, TDSCs have recently been found to significantly promote the repair and regeneration of injured tendons.11–13)
Galangin (GLN) is an important flavonoid with natural activity extracted from the root of Alpinia officinarum Hance.14) It has recently been reported to hold healing potential in multiple diseases, including cancers, renal damage, skin and cardiovascular diseases.15–19) It has also been found to enhance expression of osteoblast differentiation markers and attenuated human osteosarcoma cell proliferation.20) However, studies on the use of GLN in treating tendon repair have not yet been reported.
Therefore, this study aimed to investigate the use of GLN-treated TDSCs to identify the regulating effect on tendon repair and elucidate its molecular mechanism, which is envisaged to provide a novel treatment strategy for tendon injury.
TDSCs were isolated as previously described.21) Patellar tendons were obtained from 6–8 weeks old female Sprague Dawley rats. The tendons were cut into small pieces and digested by 3 mg/mL collagenase type I (Merck, Darmstadt, Germany) and 4 mg/mL dispase (Roche, Basel, Swiss Confederation) in phosphate buffer saline (PBS, Thermo Scientific, Waltham, MA, U.S.A.) for 1 h at 37 °C. Cell suspensions were cultivated in Dulbecco’s Modified Eagle Medium (DMEM, Gibco, Grand Island, NY, U.S.A.) with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 µg/mL streptomycin (all bought from Gibco) at 37 °C and 5% CO2 for 8–10 d. Then, TDSCs were treated with different concentrations of GLN (0, 10, 20, 30 and 40 µM) (purity: ≥95%; Merck) for 24 h. To inhibit the transforming growth factor β1 (TGF-β1)/Smad3 signaling pathway, 25 µM LY3200882 (a TGF-β receptor inhibitor) was used to stimulate TDSCs for 24 h.
Induction of Osteogenic and Adipogenic Differentiation of TDSCsTDSCs were used for induction of osteogenic differentiation and adipogenic differentiation for subsequent experiments on the identification of TDSCs. For osteogenic differentiation, cells were cultured for 21 d in DMEM containing 100 nM dexamethasone, 10 mM β-glycerophosphate (Beyotime, Shanghai, China) and 50 mM ascorbic acid (Aladdin, Shanghai, China). For adipogenic differentiation, cells were cultured for 21 d in DMEM containing 1 µM dexamethasone, 100 µM indomethacin (Merck), 10 µg/mL insulin (Beyotime) and 500 µM 3-isobutyl-1-methylxanthine (IBMX; Merck).
Alkaline Phosphatase (ALP) StainingIn order to investigate the osteogenic differentiation of TDSCs, BCIP/NBT alkaline phosphatase colour development kit (Beyotime) was used to stain cells. Briefly, cultured-cells were fixed in 4% paraformaldehyde for 20 min and washed three times with distilled water. Then, the cells were stained with the working solution for 30 min in the dark. After that, the cells were washed with distilled water and observed using an optical microscope (Olympus, Tokyo, Japan).
Alizarin Red S StainingAlizarin red S staining was measured by an alizarin red S staining kit for osteogenesis (Beyotime) to detect osteoblast mineralization nodules. The cells after washing with PBS were fixed in the fixing solution for 20 min, and stained with alizarin red S solution for 30 min. The stained cells were observed using an optical microscope (Olympus).
Oil Red O StainingThe Oil Red O staining was measured by a modified Oil Red O staining kit (Beyotime) to observe intracellular lipid. According to the guidelines, the cells were fixed in 10% formaldehyde solution for 10 min and stained with the modified Oil Red O staining solution for 10 min. Finally, the cells were observed by a microscope (Olympus).
Flow CytometryThe surface antigens of cells were detected by flow cytometry. The antibodies anti-CD34, anti-CD45 and anti-CD45 (all bought from Abcam, Cambridge, U.K.) were incubated with TDSCs for 30 min at room temperature and then washed with PBS twice. Finally, the cells were resuspended in PBS and analyzed using a FACSCalibur cytometer (BD, Franklin Lakes, NJ, U.S.A.).
Cell ViabilityThe effect of GLN on the viability of TDSCs was counted by Cell Counting Kit (CCK-8) (Yeasen, Shanghai, China). In brief, TDSCs were incubated in 96-well plates at a density of 1 × 103 cells/well along with different concentrations of GLN for 24 h. Afterwards, CCK8 solution (10 µL) was added to each well and incubated for 2 h. Finally, a microplate reader was used to measure the absorbance at a wavelength of 450 nm.
Sirius Red StainingCollagen content was detected by sirius red staining as previously described.22) After treatment, TDSCs were fixed with 4% paraformaldehyde for 10 min and washed three times with PBS. Sirius red reagent (Hyclone, Logan, UT, U.S.A.) was added to stain for 30 min. The color was eluted with a 1 mL mixed solution of NaOH and absolute methanol at an equal ratio. The absorbance was measured at a wavelength of 540 nm using a spectrophotometer.
Quantitative Real-Time PCR (qRCR)Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, U.S.A.). PrimeScript RT reagent kit (TaKaRa Bio, Shiga, Japan) was used to synthesize cDNAs. The cDNAs were subjected to qPCR analysis in an ABI-7500 PCR instrument (Applied Biosystems, Waltham, MA, U.S.A.) using the SYBR qPCR master mix kit (Invitrogen). The expression of collagen type I alpha 1 (COL1A1), decorin (DCN) and tenomodulin (TNMD) were calculated by the 2−∆∆Ct method with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the endogenous reference. The primers for qPCR were as follows: COL1A1, 5′-GGAGAGAGCATGACCGATGG-3′ (sense) and 5′-GGGACTTCTTGAGGTTGCCA-3′ (antisense); DCN, 5′-TGGCAGTCTGGCTAATGT-3′ (sense) and 5′-ACTCACGGCAGTGTAGGA-3′ (antisense); TNMD, 5′-GTGGTCCCACAAGTGAAGGT-3′ (sense) and 5′-GTCTTCCTCGCTTGCTTGTC-3′ (antisense); TGF-β1, 5′-TCAACTGTGGAGCAACACGT-3′ (sense) and 5′-CACTCAGGCGTATCAGTGGG-3′ (antisense); Smad3, 5′-CAGGAGGAGAAGTGGTGCGA-3′ (sense) and 5′-TCCAGTGACCTGGGGATGGTAA-3′ (antisense); GAPDH, 5′-ACCACAGTCCATGCCATCAC-3′ (sense) and 5′-TCCACCACCCTGTTGCTGAT-3′ (antisense).
Western Blot AssayTotal protein was isolated from TDSCs using radio immunoprecipitation assay (RIPA) lysis buffer (Thermo Scientific) and quantified using a BCA kit (Beyotime). Protein samples were loaded on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred to polyvinylidene fluoride (PVDF) membranes. After washing, the membrane was incubated overnight with primary antibodies, including anti-TGF beta 1 (ab215715, 1 : 1000), anti-Smad3 (ab40854, 1 : 1000), anti-p-Smad3 (ab52903, 1 : 2000), anti-PI3K (ab302958, 1 : 1000), anti-p-PI3K (ab182651, 1 : 200), anti-p-AKT (ab38449, 1 : 1000), anti-IkBα (ab32518, 1 : 1000), anti-p-IkBα (ab92700, 1 : 1000), anti-p65 (ab32536, 1 : 1000), anti-p-p65 (ab76302, 1 : 1000), anti-GAPDH (ab9485, 1 : 2500) (all bought from Abcam) and anti-AKT (#9272 1 : 1000) (Cell Signaling, Danfoss, MA, U.S.A.). After incubation with secondary antibody (Abcam, ab6721, 1 : 2000) at room temperature for 2 h, blots were visualized using the ECL reagent (Yeasen) and photographed with an optical luminescence instrument (GE, U.S.A.).
Statistical AnalysisData processing was completed with SPSS 22.0 software. All results were represented as the mean ± standard deviation from three replicated experiments. Comparisons between two or more groups were implemented with unpaired two-tailed Student’s t-test or one-way ANOVA. p < 0.05 was recognized as statistically significant.
The multipotential differentiation ability of cells isolated from the patellar tendons of rats was used to identify TDSCs. The ALP staining revealed significant ALP precipitation (Fig. 1A), while alizarin red S staining demonstrated extensive mineralized calcium deposits in most cells (Fig. 1B). Similarly, the Oil Red O staining showed adipogenic differentiation (Fig. 1C), which was followed by using flow cytometry to identify surface antigens of TDSCs, and results showed CD34 and CD45 were negatively expressed, while CD90 exhibited strong positive expression (Fig. 1D). These data verified the nature of cells extracted from rat patellar tendons being TDSCs.

The osteogenic differentiation was measured by A. ALP staining and B. alizarin red S staining. (scale bar: 50 µm) C. The intracellular lipid was measured by Oil Red O staining (scale bar: 50 µm) D. Flow cytometry analysis of the surface antigens of TDSCs.
Collagen fibers are essential components of a healthy tendon, and the deposition of collagen serves as an indicator to evaluate the effectiveness of tendon repair.23) The chemical structure of GLN is displayed in Fig. 2A. To investigate the effect of GLN treatment on collagen deposition of TDSCs, varying concentrations of GLN (0, 10, 20, 30, and 40 µM) were used to stimulate TDSCs, followed by assessing cell viability using CCK-8 assay. Results showed that GLN had no significant effect on TDSCc, exhibiting no cytotoxicity (Fig. 2B). Moreover, collagen deposition as a function of GLN treatment of TDSCs was assessed via Sirius red staining, and the result indicated that GLN significantly increased the collagen deposition at different tested GLN concentrations (20, 30, and 40 µM), particularly at 30 and 40 µM (Figs. 2C–D). Since 30 µM GLN concentration showed promising effects on collagen deposition; hence, this concentration was selected for subsequent experiments.

A. Chemical structure of GLN. B. CCK8 assay was applied to measure the cell viability of TDSCs treated with different concentrations of GLN (0, 10, 20, 30, and 40 µM). C, D. Sirius red staining for measure collagen deposition in TDSCs treated with different concentrations of GLN (0, 10, 20, 30, and 40 µM). ** p < 0.01, and *** p < 0.001 vs. the control group.
The GLN treatment effectiveness was evaluated by measuring levels of three tendon markers, i.e., COL1A1, DCN, and TNMD levels, in TDSCs. The qPCR results confirmed that the relative expression of above-stated markers in GLN-treated TDSCs was more than two folds increased compared with the control (Figs. 3A–C). Similarly, Western blot also indicated that GLN promoted the protein levels of COL1A1, DCN and TNMD (Figs. 3D, E). All these results demonstrated that GLN treatment incited an increased expression of COL1A1, DCN and TNMD in TDSCs.

A–C. The qPCR detection of COL1A1, DCN and TNMD expression in control (CON) and GLN treatment groups. D, E. The protein levels of COL1A1, DCN and TNMD were measured by Western blot in TDSCs treated with or without GLN. *** p < 0.001 vs. the control group.
The mechanism of tendon repair mediated by TDSCs following GLN treatment was investigated by examining the effects of GLN on intracellular signaling molecules since three signaling pathways, namely TGF-β1/Smad3, PI3K/AKT and NF-κB, have been reported to be involved in tendon injury.24,25) Western blot results following GLN treatment indicated that protein levels of TGF-β1 and p-Smad3 were increased, cementing the activation of the TGF-β1/Smad3 signaling pathway by GLN (Figs. 4A, B), while no changes in other protein levels were detected with or without GLN application (Figs. 4C–F). Moreover, the expression of TGF-β1 and Smad3 was also increased by GLN (Figs. 4G, H). All these results suggested that GLN might activate the TGF-β1/Smad3 signaling pathway in TDSCs.

The protein levels of A and B. TGF-β1/Smad3 pathway, C and D. PI3K/AKT pathway and E and F. NF-κB pathway with or without GLN treatment were measured using Western blot. G, H. The expression of TGF-β1 and Smad3 was measured by qPCR.
The GLN-mediated regulation of tendon repair by activating the TGF-β1/Smad3 signaling pathway. LY3200882, a TGF-β receptor inhibitor, was used for pathway inhibition. The Sirius red staining results indicated reduced collagen deposition in GLN-treated TDSCs post LY3200882 application (Figs. 5A, B), in addition to significantly reduced mRNA and protein levels of COL1A1, DCN, and TNMD, compared with the GLN treatment group (Figs. 5C–G). These data demonstrated that LY3200882 inhibited the repair effect in GLN-treated TDSCs, confirming that GLN promoted tendon repair by activating TGF-β1/Smad3 signaling pathway in TDSCs.
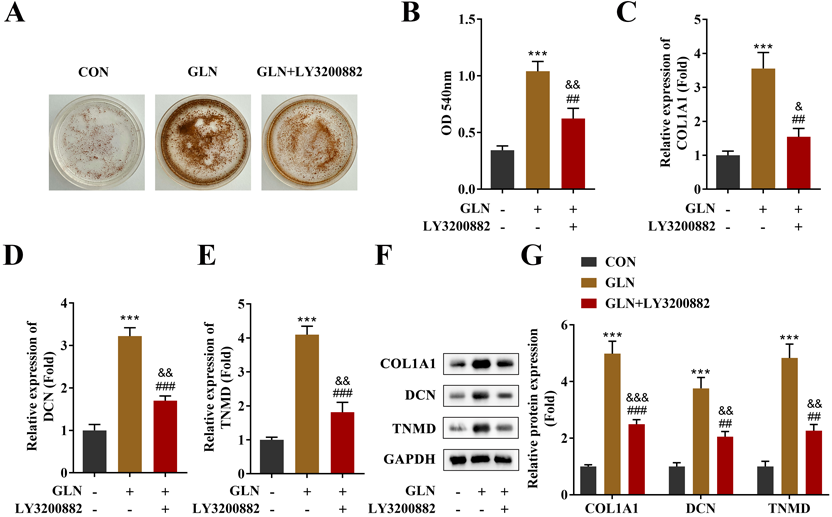
A, B. The collagen deposition in TDSCs was detected by Sirius red staining in control, GLN and GLN + LY3200882 treatment groups. C–E. The mRNA expression of COL1A1, DCN and TNMD in control, GLN, and GLN + LY3200882 groups was measured by qPCR detection. F, G. The protein levels of COL1A1, DCN, and TNMD were measured by Western blot. ##p < 0.01 vs. the GLN group. ###p < 0.001 vs. the GLN group. ***p < 0.001 vs. the control group. & p < 0.05 vs. the control group. && p < 0.01 vs. the control group. &&& p < 0.001 vs. the control group.
This study is the first to show the effect of GLN on TDSCs, where data indicated that GLN accelerated the collagen deposition and elevated the expression of tendon markers COL1A1, DCN, and TNMD, cementing the fact that GLN activated TGF-β1/Smad3 signaling pathway in TDSCs was involved in promoting tendon repair.
Research on utilizing Chinese medicine ingredients for treating diseases has been on the rise in recent years. GLN, a natural flavonoid, has been demonstrated to be beneficial in promoting the healing of multiple types of injuries.26–28) However, the effect of GLN on tendon repair has not been previously reported. TDSCs are believed to possess the capacity to promote tendon injury healing and are suitable for application in cell therapy.29) Therefore, this study investigated the effects of GLN on TDSCs to evaluate its potential for tendon repair.
Collagen deposition is a key step in wound healing,30) and also plays an important role in tendon repair. It has been reported that HUMSC-Exos accelerated the tendon healing process and elicited higher collagen deposition in TDSCs in vitro.22) Moreover, the absence of estrogen receptor β in a mouse model was found to decrease the collagen type I deposition and disturbed tendon repair.31) Some studies have revealed the GLN potential to inhibit collagen degradation, e.g., treatment of HS68 human dermal fibroblasts with varying GLN concentrations (10, 20, and 30 µM) demonstrated that the latter two concentrations could alleviate the H2O2 and UVB mediated reduced cell viability, where 30 µM was found to be more effective and not cytotoxic; moreover, 30 µM GLN treatment could enhance collagen synthesis and reduce dermal senescence of HS68 cells.32) Similarly, 30 µM GLN was found to decrease matrix metalloproteinase-9 expression in human fibrosarcoma HT-1080 cells, thereby inhibiting type IV collagen degradation in the basement membrane.33) All these studies advocated the effectiveness of 30 µM GLN concentration in promoting collagen synthesis. Our data also demonstrated excellent effects on stimulating collagen deposition in TDSCs with 30 µM GLN application without cytotoxicity, suggesting that GLN treatment may enhance the TDSCs’ repair ability. In addition, GLN systemic administration has defects such as low bioavailability, high clearance and off-target, which need to be further confirmed by more studies.34)
The tendon repair process had been previously correlated with tendon markers, where COL1A1 was found to be the major component of type I collagen.35) It has been shown that aspirin treatment increased the COL1A1 content and promoted tendon injury healing.36) Similarly, the absence of DCN has been found to inhibit late-stage healing of tendons in mice,37) while TNMD is the best-known mature marker for tendon and ligament lineage cells, and has been reported to be involved in early tendon healing.38) In this study, the expressions of COL1A1, DCN, and TNDM were evaluated, and results showed that GLN treatment increased their mRNA expression and protein levels in TDSCs, indicating that GLN promoted TDSCs mediated tendon repair.
Moreover, our results also showed that GLN treatment activated the TGF-β1/Smad3 pathway in TDSCs. TGF-β is a ubiquitous and potent cytokine with three different isoforms, TGF-β1, TGF-β2, and TGF-β3 that play an important role in tendon development.39–41) During the initial period after Achilles tendon rupture, TGF-β1 expression levels are elevated, stimulating the synthesis of collagen type I and type III through the TGF-β/Smad signaling pathway. It has been reported that benzyl alcohol accelerates recovery from Achilles tendon injury, potentially via TGF-β1/Smad2/3 pathway.42) Similarly, GLN has been reported to relieve dermal aging by activating the TGF-β/Smad signaling pathway.32) However, the mechanism of GLN-mediated TDSCs in regulating tendon repair remains unclear.
Furthermore, the protein levels of p-PI3K and p-AKT in TDSCs were also detected, and results showed no GLN-mediated effect on their protein expression, suggesting that the PI3K/AKT signaling pathway was not activated by GLN in TDSCs. However, it has been reported that TGF-β activated the PI3K/AKT signaling pathway through the TRAF6-mediated ubiquitylation of p85α, not by the Smad pathway,43) which was different from our results. Hence, it is speculated that GLN may also activate PI3K by triggering TGF-β1, but our results did not show the strong activating effect of TGF-β1 on PI3K, only activating the TGF-β1/Smad3 pathway; hence, this specific mechanism will be further analyzed in our subsequent studies.
It was further observed that LY3200882 reversed the promotion of collagen deposition in TDSCs induced by GLN but did not eliminate the effect. It is hence hypothesized that collagen deposition by TDSCs is not solely regulated by the TGF-β1/Smad3 pathway, which is why LY3200882 could not completely offset the promotion of GLN-mediated collagen deposition. For instance, Yao et al.22) confirmed that human umbilical cord mesenchymal stem cell-derived exosomes increase collagen deposition in TDSCs through mTOR signaling and promote tendon regeneration. Similarly, anti-inflammatory cytokine interleukin-10 has been reported to inhibit the expression of collagen-related genes in TDSCs through the JAK/Stat3 signaling pathway.44) These studies’ data revealed that collagen synthesis in TDSCs was not only mediated by TGF-β1/Smad3 pathway, which could be the reason despite treatment of TDSCs with LY3200882, the promotion of collagen deposition stimulated by GLN was not completely diminished.
Nevertheless, this study is still constrained by certain limitations. We only utilized a cell model to identify the repair function of GLN in TDSCs, which might be validated through animal experiments in our future research.
In conclusion, it was demonstrated that GLN promoted tendon repairs mediated by TDSCs through activating the TGF-β1/Smad3 signaling pathway, which is envisaged to provide a theoretical basis for the clinical application of GLN in the treatment of tendon repair.
The work was supported by the Foundation of Jiangxi Provincial Science and Technology Department under Grant Number: 20224BAB206103.
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Q. L., H. Y., H. H. and S. F. The first draft of the manuscript was written by X. D. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
The authors declare no conflict of interest.
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.