2019 Volume 66 Issue 6 Pages 515-522
2019 Volume 66 Issue 6 Pages 515-522
Cushing’s disease is almost always caused by hypersecretion of adrenocorticotropic hormone (ACTH) from a pituitary adenoma. A mutation in the deubiquitinase gene USP8 has been found in human ACTH-producing pituitary adenoma cells. This mutational hotspot hyperactivates USP8, rescuing epidermal growth factor receptor (EGFR) from lysosomal degradation and ensuring its sustained signaling in Cushing’s disease. An EGFR inhibitor would be an effective anti-tumor agent in EGFR-related tumors. We investigated the effect of a potent dual tyrosine kinase inhibitor, lapatinib, on ACTH production and cell proliferation in AtT-20 mouse corticotroph tumor cells. Lapatinib decreased proopiomelanocortin (Pomc) mRNA levels and ACTH levels in AtT-20 cells and also inhibited cell proliferation, induced apoptosis, and decreased pituitary tumor-transforming gene 1 (Pttg1), a hallmark of pituitary tumors, mRNA levels. KSN/Slc nude mice were subcutaneously inoculated with AtT-20 cells. After 1 week, the mice were randomized either to control or lapatinib groups. The inhibitor decreased the tumor weight of AtT-20 allografts in vivo versus control mice. Lapatinib also significantly decreased Pomc and Pttg1 mRNA levels in the tumor and plasma ACTH and corticosterone levels in vivo. Thus, lapatinib decreases the ACTH production and proliferation of corticotroph tumor cells. An EGFR-targeting therapy could be an important treatment for Cushing’s disease.
CUSHING’S DISEASE is a clinical syndrome in which hypercortisolemia results from excessive production of adrenocorticotropic hormone (ACTH) by pituitary corticotroph adenomas [1, 2]. This leads to osteoporosis, infections, hyperglycemia, hypertension, and atherosclerosis [3]. Surgical excision of the adenoma from the pituitary is the primary treatment for Cushing’s disease, but curative surgery is challenging. To treat the resultant hypercortisolism, additional therapy is required [4-6]. Although some drugs have been identified to suppress ACTH production, a more effective medical treatment that targets pituitary ACTH-producing adenomas is required.
A mutation in the deubiquitinase gene USP8 has been found in human ACTH-producing pituitary adenomas [7, 8]. This mutational hotspot hyperactivates USP8, rescuing epidermal growth factor receptor (EGFR) from lysosomal degradation and ensuring its sustained signaling in Cushing’s disease. Accordingly, the phosphorylated EGFR expression has been found in most Cushing’s disease [9], and an EGFR inhibitor would be an effective anti-tumor agent in EGFR-related tumors. Indeed, the EGFR tyrosine kinase inhibitor gefitinib suppresses ACTH production and tumor growth in an experimental mouse model of Cushing’s disease [10].
Lapatinib is an orally active and specific receptor tyrosine kinase inhibitor of both EGFR and p185her2/neu (HER2), and it shows broad spectrum of anti-tumor activity [11]. Clinical trials revealed that it is effective in breast cancer patients and salivary gland carcinoma patients [12, 13]. Recently, lapatinib was found to be effective in female transgenic mice with HER2-driven prolactinomas [14]. Accordingly, here we studied the effect of the potent dual tyrosine kinase inhibitor lapatinib on ACTH production and cell proliferation in corticotroph tumor cells. We also examine the possible effects of lapatinib in vivo to determine the effects of lapatinib on tumor weight, tumor proopiomelanocortin (Pomc) pituitary tumor-transforming gene 1 (Pttg1) mRNA levels, and plasma ACTH and corticosterone levels, in mice with AtT-20 allografts.
Lapatinib was purchased from Selleckchem (Houston, TX, USA).
Cell cultureMurine corticotroph tumor AtT-20 cells were obtained from ATCC (Manassas, VA, USA). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 100 μg/mL streptomycin, 100 U/mL penicillin, and 10% fetal bovine serum at 37°C with 5% CO2. One day before each experiment, the DMEM medium was changed to DMEM with 0.2% bovine serum albumin.
RNA isolationAtT-20 cells grown to 40% confluence were incubated either with or without lapatinib at appropriate concentrations (0.1–10 μM) for the indicated (0–24 h) times. Total cellular RNA was isolated using an RNeasy Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. Total RNA (0.5 μg) was used as a template to synthesize cDNA with random hexamer primers using the SuperScript First-Strand Synthesis System for Reverse Transcription-Polymerase Chain Reaction (RT-PCR; Invitrogen Corp., Carlsbad, CA, USA) as described previously [15, 16].
Quantitative real-time RT-PCRThe cDNAs were subjected to quantitative real-time RT-PCR. Specific primer and probe sets (Assays-on-Demand Gene Expression Products; Applied Biosystems, Foster City, CA, USA) were used to measure the expression of mouse Pomc (NM_008895.3) and Pttg1 (NM_001131054.1) mRNA. To standardize expression levels, β2-microglobulin (B2mg) was measured as a reference gene. The total volume (25 μL) of real-time PCR solution included gene expression products (Mm00435874_m1 for mouse Pomc, Mm00479224_m1 for mouse Pttg1, and Mm00437762_m1 for mouse B2mg), TaqMan universal PCR master mix (Applied Biosystems), and 500 ng cDNA. The samples were amplified with the following cycle parameters: 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, and 60°C for 1 min, using an ABI PRISM 7000 Sequence Detection System (Applied Biosystems). All data are expressed as a function of the threshold cycle (CT) for quantitative analyses using ABI PRISM 7000 SDS software (Applied Biosystems).
Cell proliferation assayAtT-20 cells were treated with the appropriate concentrations (0.1–10 μM) of lapatinib for 48 h. Viable cells were determined by a Cell Counting Kit-8 (Dojin, Kumamoto, Japan).
Cell death detection assayAtT-20 cells were treated with the appropriate concentrations (0.1–10 μM) of lapatinib for 24 h. DNA fragmentation was measured by a Cell Death Detection ELISA Kit (Roche, Penzberg, Germany) and each enrichment factor was calculated according to the manufacturer’s instructions.
Western blot analysisCells were treated with 10 μM lapatinib for the indicated (0–24 h) times. After each treatment, cells were washed with cold phosphate-buffered saline (PBS), and were lysed with Laemmli sample buffer for protein extraction. The lysates were centrifuged, and the supernatant was recovered. Western blot analysis was performed as described previously [17]. In brief, whole cell lysates were heated, and proteins were separated on a 2–15% gradient polyacrylamide gel and transferred to a polyvinylidene fluoride membrane (Daiichi Kagaku, Tokyo, Japan). Membranes blocked with Detector Block® buffer (Kirkegaard & Perry Laboratories, Gaithersburg, MD, USA) were incubated for 1 h with an anti-EGFR antibody (1:1,000 dilution) (Boster Biological Technology, Pleasanton, CA, USA) and anti-β-actin antibody (1:10,000 dilution) (ab8227, Abcam, Cambridge, MA). After washes with PBS containing 0.05% Tween 20, the membrane was incubated with horseradish peroxidase-labeled anti-rabbit immunoglobulin G (Daiichi Kagaku). Hybridization signals were detected by the chemiluminescent substrate SuperSignal West Pico (Pierce Chemical Co., Rockford, IL, USA), and the membrane was exposed to BioMax film (Eastman Kodak Co., Rochester, NY, USA).
Xenograft experimentsEight-week-old male KSN/Slc nude mice (about 23–26 g) were subcutaneously inoculated with AtT-20 cells (5 × 106/mouse) in vehicle (200 μL). After 1 week, the mice were randomized either to control (vehicle only) or lapatinib (100 mg/kg per day) groups. Lapatinib, dissolved in vehicle (0.5% methylcellulose, 0.5% Tween 80/PBS; 100 μL), was administered by oral gavage daily for 14 days from 1 week after the cell inoculation. This dose was chosen because it had sufficient long-lasting effects on pituitary prolactin secretion in mice [14].
At the end of the experiment, the mice were euthanized from 1:00 to 3:00 PM and their tumors were carefully removed and weighed. The tumors were subjected to total RNA isolation for quantitative real-time RT-PCR. Trunk blood samples were also collected from the mice for the measurement of their glucose, ACTH and corticosterone levels. Blood glucose levels were measured using Nipro StatStrip XP (Nipro, Osaka, Japan) at the time of the experiment. All animal experiments were performed in accordance with the institutional regulations for animal care of Hirosaki University School of Medicine. The protocol was approved by the Institute for Animal Experiments and Institutional Review Board of Hirosaki University School of Medicine.
ACTH and corticosterone assaysAtT-20 cells were treated with the appropriate concentrations (0.1–10 μM) of lapatinib for 24 h, and medium was collected. After blood centrifugation, medium and plasma ACTH concentrations were measured with an ACTH enzyme-linked immunosorbent assay (ELISA) kit (MD Bioproducts, Zurich, Switzerland).
Plasma corticosterone concentrations were measured by a rodent corticosterone ELISA kit (Endocrine Technologies, Newark, CA).
Statistical analysisData are presented as the mean ± standard error of the mean. Each in vitro experiment was performed three times. Statistical analyses of in vitro studies were performed with ANOVA followed by Fisher’s protected least-significant difference post hoc test. For in vivo studies, analyses were performed with an unpaired Student’s t-test. P < 0.05 was considered statistically significant.
AtT-20 cells were treated with 10 μM lapatinib for the indicated times to examine its effects on Pomc mRNA levels. Lapatinib significantly decreased Pomc mRNA levels (ANOVA; p < 0.05), with the levels decreased to 76% of the basal level 6 h after the addition of lapatinib (Fig. 1A). The effects were dose-dependent (ANOVA; p < 0.005) and significant at 10 μM (Fig. 1B). In addition, lapatinib significantly decreased ACTH levels in the culture medium (Fig. 1C).

Lapatinib decreases Pomc mRNA and ACTH levels in mouse corticotroph tumor cells. (A) Time-dependent effects of lapatinib on Pomc mRNA levels. Cells were treated with 10 μM lapatinib for the indicated times. (B) Dose-dependent effects of lapatinib on Pomc mRNA levels. Cells were treated with increasing concentrations of lapatinib (0.1 to 10 μM) for 6 h. (C) Dose-dependent effects of lapatinib on ACTH levels. Cells were treated with increasing concentrations of lapatinib (0.1 to 10 μM) for 24 h. *p < 0.05 (compared with basal [0] or control [C]). The cells were treated in triplicate, and the average of three independent experiments is shown (n = 3).
We next tested the effects of lapatinib on corticotroph tumor cell proliferation and cell death. Lapatinib dose-dependently decreased cell proliferation (Fig. 2A) but dose-dependently increased the level of cytoplasmic histone-associated DNA fragmentation (Fig. 2B).

Lapatinib decreases the proliferation and induces the death of mouse corticotroph tumor cells. (A) Dose-dependent effects of lapatinib on the proliferation of AtT-20 cells. Cells were treated with increasing concentrations of lapatinib (0.1 to 10 μM) for 48 h, and viable cells were measured using a Cell Counting Kit-8. (B) Dose-dependent effects of lapatinib on cell death in AtT-20 cells. Cells were treated with increasing concentrations of lapatinib (0.1 to 10 μM) for 24 h, and DNA fragmentation was measured using a Cell Death Detection ELISA kit. *p < 0.05 (compared with control [C]). The cells were treated in triplicate, and the average of three independent experiments is shown (n = 3).
Because PTTG1 may be involved in the regulation of ACTH production and cell proliferation in corticotroph tumor cells, we treated AtT-20 cells with lapatinib to examine its effects on Pttg1 mRNA levels. Lapatinib (10 μM) significantly decreased Pttg1 mRNA levels (ANOVA; p < 0.01), with the levels decreased to 64% of the basal level 24 h after the addition of lapatinib (Fig. 3A). Pttg1 mRNA levels were dose-dependently decreased by lapatinib (ANOVA; p < 0.05), with 10 μM lapatinib exerting a significant effect (Fig. 3B).

Lapatinib decreases Pttg1 mRNA levels in mouse corticotroph tumor cells. (A) Time-dependent effects of lapatinib on Pttg1 mRNA levels. Cells were treated with 10 μM lapatinib for the indicated times. (B) Dose-dependent effects of lapatinib on Pttg1 mRNA levels. Cells were treated with increasing concentrations of lapatinib (0.1 to 10 μM) for 24 h. *p < 0.05 (compared with basal [0] or control [C]). The cells were treated in triplicate, and the average of three independent experiments is shown (n = 3).
To characterize EGFR protein expression in mouse corticotroph tumor cells, we determined the effects of lapatinib on EGFR protein expression in corticotroph tumor cells using western blot analysis. The protein levels of EGFR were decreased within 24 h of treatment with lapatinib (ANOVA; p < 0.05; Fig. 4).

Lapatinib decreases EGFR protein expression in mouse corticotroph tumor cells. Cells were treated with 10 μM lapatinib for the indicated times, and western blot analysis was performed to examine the protein levels of EGFR. *p < 0.05 (compared with control [0 min]). The cells were treated in triplicate, and the average of three independent experiments is shown (n = 3). A representative blot is shown.
To generate a mouse model of ACTH-dependent Cushing’s syndrome, KSN/Slc nude mice were subcutaneously injected with AtT-20 cells. To determine the effects of lapatinib in vivo, the mice received oral lapatinib or vehicle. Drug treatment was started 1 week after AtT-20 cell injection.
The increase in body weight was smaller, although not significantly, following treatment with lapatinib (Fig. 5A). Lapatinib also decreased tumor weight compared with control mice (p < 0.001; Fig. 5B). Pomc and Pttg1 mRNA expression levels in the tumor were decreased by lapatinib compared with control (p < 0.005 and p < 0.05; Fig. 5C and 5D, respectively). The elevated plasma ACTH and corticosterone levels in mice allografted with AtT-20 cells were also ameliorated by oral lapatinib treatment (p < 0.005 and p < 0.01; Fig. 5E and 5F, respectively). Blood glucose levels were unaltered in lapatinib-treated mice (Fig. 5G).
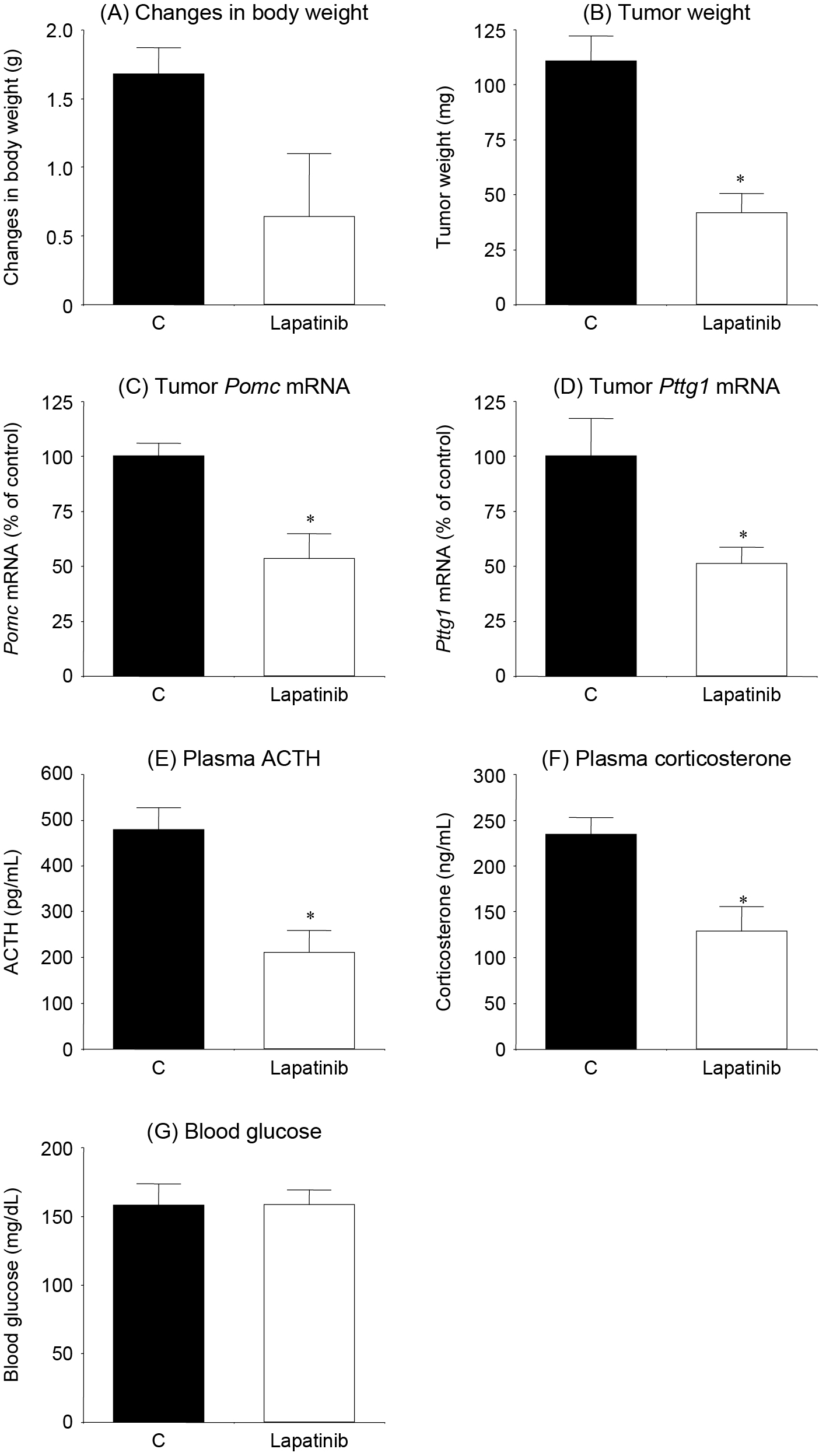
Lapatinib decreases tumor weight and hormone secretion in vivo. For 14 days from 1 week after AtT-20 cell injection, lapatinib (n = 7) or vehicle (n = 7) was administered by oral gavage. *p < 0.05 [compared with control (C)]. (A) Changes in mouse body weight. Body weight in each mouse was determined at the end of the experiment (day 14) and compared with that at day 1. (B) Subcutaneous tumors were resected and weighed on day 14. (C) Tumors were resected, and Pomc mRNA levels were measured by real-time RT-PCR. (D) Tumors were resected, and Pttg1 mRNA levels were measured by real-time RT-PCR. (E) Plasma ACTH levels were measured by ELISA. (F) Plasma corticosterone levels were measured by ELISA. (G) Changes in blood glucose levels. Blood was collected at the end of the experiment and measured.
In this study, we found that the EGFR inhibitor lapatinib decreased Pomc mRNA levels in corticotroph tumor cells and ACTH levels in their medium. Because ACTH is produced EGFR-dependently [10], EGFR inhibitor would suppresses the autonomic ACTH production in AtT-20 cells. Lapatinib also decreased AtT-20 cell proliferation and induced DNA fragmentation in corticotroph tumor cells. These data suggest that the inhibitor would induce cell death in the tumor cells [18]. The ACTH levels in the medium might be less decreased, compared with cell proliferation. The decreased ACTH levels in the medium, therefore, may be caused mainly by decreases in cell proliferation and partially by those in ACTH synthesis [19]. Similar to that of gefitinib, lapatinib also binds to the ATP binding pocket of both EGFR and HER2 protein kinase [11]. Fukuoka’s report [10] shows the effects of gefitinib only on EGFR-overexpressing AtT-20 cells. His study suggests that the effects of these inhibitors depend on EGFR, but not HER2.
PTTG1, an oncogene cloned from a rat pituitary tumor [20], triggers the development of pituitary tumors [21, 22]. PTTG1 would activate cell proliferation [23]. A positive correlation has been suggested between PTTG1 and histone deacetylases or heat shock protein 90 [24]. In our previous studies [25, 26], histone deacetylase and heat shock protein 90 inhibitors suppress Pttg1 mRNA levels in AtT-20 cells. PTTG1 expression levels contribute to the proliferation of corticotroph tumor cells [25]. In present study, lapatinib gradually decreased Pttg1 mRNA levels in corticotroph tumor cells. Thus, PTTG1 might also participate in the EGFR-induced corticotroph tumor cell proliferation.
EGFR signaling contributes to ACTH production and tumor growth in corticotroph tumors [10]. Cleavage of USP8 induces increased deubiquitination of EGFR, impairing its downregulation and boosting intracellular EGFR signaling [7], although the mechanism has not been determined in AtT-20 cells. Janus kinase/signal transducer and activator of transcription (Jak/STAT) is located downstream of EGFR signaling [27] and is essential for EGFR-driven migration and invasion [28]. In our previous study, SD-1029, a potent Jak2 and STAT3 inhibitor, also decreased ACTH synthesis and secretion in corticotroph tumor cells [17].
While AtT-20 cells did not express endogenous EGFR in Fukuoka’s report [10], our study shows that EGFR protein is expressed in AtT-20 cells. This discrepancy is unclear, but AtT-20 has some subtypes. Each AtT-20 subtype might have a different character. Furthermore, lapatinib decreased the EGFR protein levels in our study. Nuclear EGFR expression is detected in both human and canine Cushing’s tumors [10]. Nuclear EGFR acts as a transcription factor and selectively induces ACTH synthesis and secretion [10]. Lapatinib may reduce autonomous EGFR signaling via these changes in EGFR expression. Together, this receptor and its downstream effectors would be a promising target for the therapy of Cushing’s disease.
In our in vivo study, corticotroph tumor weight was decreased by lapatinib treatment versus the control. Fukuoka’s report [10] shows effects of gefitinib only on EGFR-overexpressing AtT-20 allografts. Because EGFR is endogenously expressed in our AtT-20 cells, this discrepancy may be caused. Additionally, some Cushing’s complications, such as obesity and high blood glucose levels, were improved in his study, but not in our experiment. These results suggest that the effects of an EGFR inhibitor might be enhanced in EGFR-overexpressing AtT-20 allografts. Lapatinib also decreased tumor Pttg1 mRNA levels. In our previous study, tumor Pttg1 mRNA levels were decreased by SOM230, a multiligand somatostatin analog [18]. Pttg1 knockdown inhibits cell proliferation [25] and PTTG1 might thus be associated with inhibition of cell proliferation in corticotroph tumor cells. ACTH-dependent hypercortisolemia is a characteristic of Cushing’s disease. We found extremely high plasma ACTH and corticosterone levels in mice with AtT-20 allografts. The levels of both components were suppressed by treatment with lapatinib. Lapatinib also decreased tumor POMC levels. Although most Cushing’s diseases have microadenoma, lapatinib could suppress plasma ACTH levels via inhibition of synthesis and/or suppression of tumor proliferation. At this point, tumor POMC expression was suppressed by gefitinib in EGFR-transgenic mice [29]. It is possible that inhibition of EGFR signaling contributes to direct inhibition of ACTH production.
In conclusion, lapatinib decreases the production and cell proliferation of corticotroph tumor cells. In mice with AtT-20 xenografts, tumor weight is decreased by treatment with lapatinib. Lapatinib also decreases plasma ACTH and corticosterone levels. Inhibition of EGFR signaling contributes to inhibition of ACTH production and cell proliferation in corticotroph tumor cells. Therefore, an EGFR-targeting therapy might be clinically important in Cushing’s disease.
This research did not receive any specific grants from any funding agencies in the public, commercial, or not-for-profit sector.
Disclosure potential conflicts of interestNone of the authors has any potential conflicts of interest associated with this research.
Human participants were not involved in this research.
All authors were involved in the treatments, drafted the manuscript, and approved the final manuscript.