2019 年 66 巻 9 号 p. 817-826
2019 年 66 巻 9 号 p. 817-826
Laparoscopic sleeve gastrectomy has been proven effective in treating obesity-associated type 2 diabetes mellitus (T2DM). However, reports of the effect of laparoscopic sleeve gastrectomy on glucose metabolism in Japanese obese patients with T2DM are rare. The aim of this study was to evaluate the effects of laparoscopic sleeve gastrectomy on glucose tolerance in Japanese obese patients with T2DM, and to analyze factors influencing diabetes remission after surgery. This was a retrospective analysis of data for 24 consecutive patients with T2DM who underwent laparoscopic sleeve gastrectomy. We investigated weight loss and its impact on T2DM 1 year postoperatively. We also compared baseline characteristics and postoperative factors between patients who achieved diabetes remission and patients without remission. Mean body weight loss and percent total weight loss were 23.9 kg and 23.3%, respectively. Mean hemoglobin A1c levels dropped from 7.3 ± 0.3% to 6.1 ± 0.2%, and 18 patients (75%) achieved diabetes remission 1 year postoperatively. Patients achieving remission had significantly lower hemoglobin A1c levels (p = 0.026), higher fasting C-peptide values (p < 0.001), shorter diabetes duration (p < 0.001), lower insulin requirement (p = 0.002), and higher area under the insulin response curve (p < 0.001) and insulinogenic index (p < 0.001) during oral glucose tolerance testing. In conclusion, laparoscopic sleeve gastrectomy is an effective treatment for Japanese obese patients with T2DM. Preserving insulin secretion is the major determinant of diabetes remission.
BARIATRIC SURGERY has been proven effective for long-term weight reduction, and significantly reduces obesity-related morbidity and mortality. In particular, bariatric surgery is a powerful treatment for obesity-associated type 2 diabetes mellitus (T2DM) [1-3] with high reported rates of postoperative resolution of T2DM [4, 5]. Randomized controlled studies have shown that bariatric surgery is more effective than intensive medical therapy for glycemic control [3, 6, 7].
Previous reports suggest that resolution of T2DM is more common following predominantly malabsorptive procedures, such as Roux-en-Y gastric bypass (RYGB), compared with purely restrictive operations, with approximately 60% of patients achieving diabetes remission [8]. As a result, RYGB is the most popular bariatric surgical procedure worldwide [9]. In comparison, laparoscopic sleeve gastrectomy (LSG) is a purely-restrictive operation without malabsorptive effects. Weight loss post-LSG is similar to weight loss post-RYGB, and LSG is associated with a high rate of T2DM remission [3, 10, 11]. Moreover, because of the relatively high prevalence of gastric cancer and anatomical difficulties with cancer screening in the remnant stomach using endoscopy, RYGB is not widely accepted in Japan. Because of these issues, LSG is the only approved procedure covered by the national health insurance system in Japan. As a result, 98% of bariatric surgery performed in Japan was LSG according to the 2018 survey of the Japanese Society for Treatment of Obesity.
Because of differences in T2DM pathophysiology between Japanese and non-Asian patients [12], outcomes related to diabetes after bariatric surgery could also differ. Therefore, it is important to determine the effectiveness of LSG in Japanese obese patients with T2DM regarding glycemic control; however, to our knowledge, a few reports have evaluated this question.
The aim of this study was to evaluate the effects of LSG on glucose tolerance in Japanese diabetic patients in detail using oral glucose tolerance testing (OGTT) and to determine the factors influencing postoperative diabetes remission.
This was a retrospective analysis. We included consecutively-selected patients with T2DM who underwent LSG from June 2009 to December 2017 at the Shiga University of Medical Science Hospital and who were followed for at least 1 year. All patients met the criteria for bariatric surgery in Japan [13], which includes body mass index (BMI) ≥ 32 kg/m2 and T2DM. Diagnosis of T2DM was based on the criteria of the Japanese Diabetes Association [14]. We also classified patients with a positive history of T2DM and receiving diabetes medications before surgery as having T2DM. Patients were admitted to the hospital 2–3 weeks before surgery to achieve as much weight reduction as possible before surgery and for preoperative glycemic control, and consumed a diet of 25 kcal/ideal body weight. This dietary regimen was maintained until surgery. The surgical procedure was performed in a supine position with a standard five-port laparoscopic technique, as previously described [15]. On the first day postsurgery, patients received a clear liquid diet, which progressed to a full liquid diet for 2 weeks and a soft diet for 1 week, eventually advancing to a standard diet. Standard follow-up included visits to the outpatient clinic at 1, 2, and 3 months postoperatively, then every 2 months thereafter. OGTT was performed 1 week preoperatively in all patients, and oral hypoglycemic agents, glucagon-like peptide-1 (GLP-1) receptor agonists, or insulin were stopped for that day.
We calculated the total area under the curve (AUC) for each measured hormone using the trapezoidal method. The insulinogenic index was obtained by dividing plasma insulin enhancement relative to the fasting value by the corresponding net increase in blood glucose (Δinsulin/Δglucose) at 30 min during the OGTT. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated from the fasting plasma glucose and fasting insulin by the formula: HOMA-IR = fasting insulin (IU/mL) × fasting plasma glucose (mg/dL)/405. The secretory units of islets in transplantation (SUIT) index, which has been shown to be useful for evaluating residual pancreatic β-cell function, was calculated as follows: 1,500 × fasting C peptide/(fasting plasma glucose (mg/mL) – 61.7) [16].
The ABCD diabetes surgery score was proposed by Lee et al. to predict diabetes remission after bariatric surgery [17]. The score system consists of four variables that are independent predictors of T2DM remission: patient age, BMI, C-peptide level, and diabetes duration. Higher values indicate a higher possibility of diabetes remission.
The individualized metabolic surgery (IMS) score was developed more recently by Aminian et al. [18]. This scoring system consists of four variables: preoperative number of diabetes medications, insulin use, diabetes duration, and HbA1c levels, and patients are categorized into three stages of T2DM severity: mild, moderate, and severe. The IMS score has been reported useful to guide selecting the procedure for metabolic surgery for patients with T2DM.
Diabetes remissionDiabetes remission was defined according to the American Diabetes Association criteria [19]. Specifically, diabetes remission was defined as hemoglobin A1c (HbA1c) <6.5% without the use of any diabetes medication.
Ethics statementAll patients gave written informed consent prior to participating in the study. The study protocol was approved by the Ethics Committee of the Shiga University of Medical Science (#18-77) and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Statistical analysisStatistical analyses were performed with SAS (ver. 9.4; SAS Institute Inc., Cary, NC, USA). All data except for baseline characteristics are expressed as the mean ± SE. The distribution of variables was analyzed by assessing histograms and normal plots of the data, and normality was tested using the Kolmogorov–Smirnov and Shapiro–Wilk tests. Baseline patients’ characteristics and differences between patients achieving diabetes remission after surgery (remission group) vs. those not achieving remission (nonremission group) were compared using the t-test for continuous variables and Fisher’s exact test for categorical variables. We used the Mann–Whitney test to compare AUC-glucose and insulin during OGTT between the two groups. We used a receiver operating characteristic (ROC) curve analysis to evaluate the predictive value of factors indicating diabetes remission. Changes in body weight and total weight loss were compared using mixed-model analysis. p-values <0.05 were considered statistically significant.
During the study period, 52 patients underwent bariatric surgery, and of these, 29 patients had diabetes. One patient underwent RYGB, and one patient underwent LSG with duodenal-jejunal bypass (LSG-DJB); 27 patients underwent LSG. Of the 27 patients undergoing LSG, three were excluded because of missing data at the 1-year follow-up. Baseline patients’ characteristics are summarized in Table 1. Patients had a mean age of 46.4 ± 11.3 years, and the mean body weight at their first visit to our hospital was 109.3 ± 18.8 kg (BMI: 41.3 ± 4.1). The mean diabetes duration was 9.1 ± 6.8 years, and the mean HbA1c level was 8.4 ± 1.8%. One patient were using dietary therapy and receiving no medication. Eight patients were taking oral hypoglycemic agents, five patients were taking GLP-1 receptor agonists, and 10 patients were using insulin at baseline.
| Characteristics | Values |
|---|---|
| n | 24 |
| Female (%) | 63 |
| Age (years) | 46.4 ± 11.3 |
| Body Weight (kg) | 109.3 ± 18.8 |
| BMI (kg/m2) | 41.3 ± 4.1 |
| Duration (years) | 9.1 ± 6.8 |
| HbA1c (%) | 8.4 ± 1.8 |
| Fasting CPR (ng/mL) | 2.5 ± 1.2 |
| Urinary CPR (μg/day) | 61.7 ± 40.7 |
| HOMA-IR | 3.7 ± 2.2 |
| Insulinogenic Index | 0.33 ± 0.26 |
| Treatment, n (%) | |
| Diet | 1 (4.2) |
| OHA | 8 (33.3) |
| GLP-1 | 5 (20.8) |
| Insulin | 10 (41.4) |
Values are expressed as mean ± SD for continuous variables.
BMI, body mass index; HbA1c, hemoglobin A1c; CPR, C-peptide; HOMA-IR, homeostasis model assessment of insulin resistance; OHA, oral hypoglycemic agents; GLP-1, glucagon-like peptide-1 receptor agonist
Presurgical dietary therapy and 3 weeks of caloric restriction (25 kcal/ideal body weight) during hospitalization reduced patients’ weight below their preadmission values (from 108.9 kg to 99.5 kg). Patients’ body weight decreased significantly from baseline (at operation) compared with 3, 6, and 12 months postoperatively (–17.2 ± 1.0 kg, –22.6 ± 1.6 kg, and –22.9 ± 2.1 kg, respectively), and percent total weight loss (%TWL) was 16.7 ± 0.8%, 22.5 ± 1.3%, and 23.3 ± 1.6%, respectively (Fig. 1a and b).
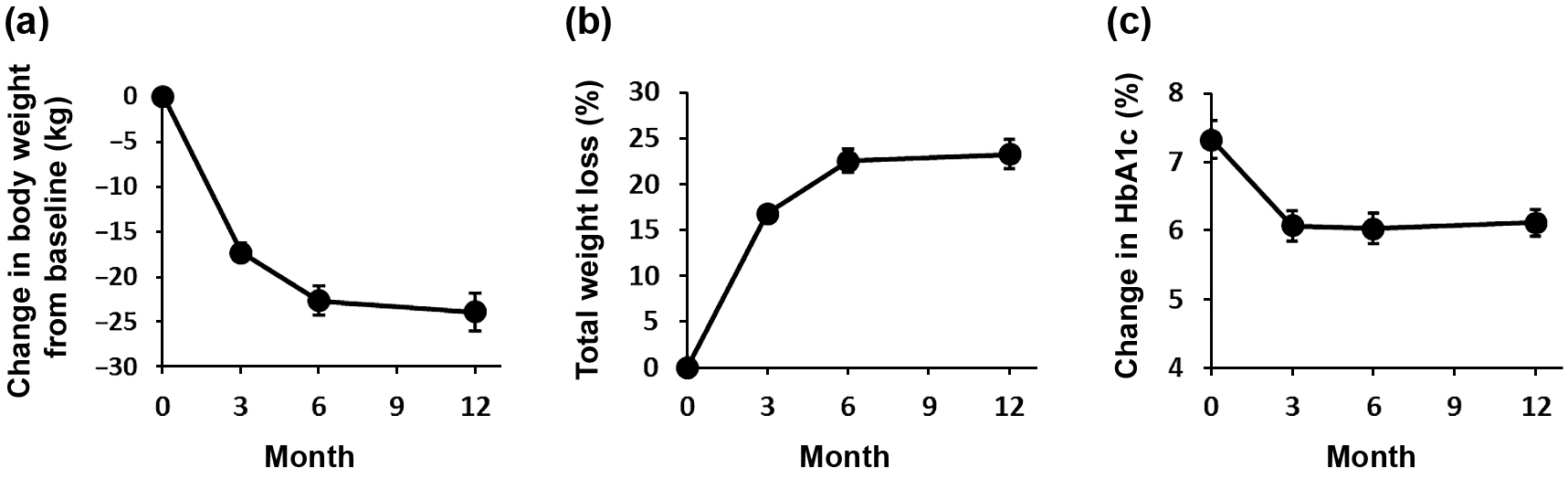
Changes in body weight expressed as the change from baseline (a), %TWL (b), and HbA1c (c). Data represent the mean ± SE.
%TWL, percent total weight loss; HbA1c, hemoglobin A1c
Preoperative weight reduction and intensive glucose control before surgery reduced HbA1c level at baseline from 8.4 ± 1.8% at first visit to our hospital to 7.3 ± 1.3% at surgery. Three patients who were taking oral hypoglycemic agents stopped medication just before surgery, and of three patients who were taking GLP-1 analogues, two were able to discontinue their medication, and one changed to oral hypoglycemic agents. Postoperatively, HbA1c decreased significantly to 6.07 ± 0.24%, 6.03 ± 0.24%, and 6.12 ± 0.22% at 3, 6, and 12 months postoperatively, respectively (Fig. 1c).
Diabetes remissionPostoperatively, 21 patients were able to discontinue medications, but three patients required oral hypoglycemic agents during follow-up. At 1-year postoperatively, 18 patients (75%) had achieved diabetes remission (HbA1c <6.5% without diabetes medication), and 13 patients (54%) had HbA1c levels <6.0% without diabetes medication.
Comparison of baseline characteristics between patients achieving remission and those without remissionWe compared the baseline characteristics of 18 patients constituting the remission group and six patients constituting the nonremission group (Table 2). The remission group had significantly lower body weight (p = 0.019) but not BMI (p = 0.900), lower HbA1c (p = 0.012), higher baseline fasting C-peptide levels (p < 0.001), shorter diabetes duration (p < 0.001), higher SUIT index (p = 0.001), higher ABCD score (p < 0.001), lower IMS score (p < 0.001), and lower insulin requirement (p = 0.002). All patients who had not been using insulin before surgery achieved diabetes remission. In contrast, of the 10 patients who had been using insulin, four patients achieved diabetes remission; the remaining six patients did not achieve remission. In the analysis of preoperative OGTT results, plasma glucose response to oral glucose loading was slightly lower, but not significant, in the remission group compared with that in the nonremission group based on AUC (26,653 ± 6,168 and 33,018 ± 9,474, respectively, p = 0.069; Fig. 2, upper panel). In contrast, plasma insulin response was significantly greater in the remission group vs. the nonremission group based on AUC (7,066 ± 3,034 and 2,184 ± 443, respectively, p < 0.001; Fig. 2, lower panel). The insulinogenic index was higher in the remission group vs. the nonremission group (0.42 ± 0.25 and 0.08 ± 0.06, respectively, p < 0.001) (Table 2).
| Remission | Non-Remission | p-value | |
|---|---|---|---|
| n | 18 | 6 | |
| Female (%) | 10 (56) | 5 (83) | 0.351 |
| Age (years) | 45.0 ± 11.2 | 50.5 ± 11.3 | 0.310 |
| Body Weight (kg) | 113.6 ± 18.9 | 94.7 ± 13.3 | 0.019 |
| BMI (kg/m2) | 41.4 ± 4.2 | 41.7 ± 7.0 | 0.900 |
| Duration (years) | 6.4 ± 5.2 | 17.3 ± 4.2 | <0.001 |
| HbA1c (%) | 7.8 ± 1.5 | 9.9 ± 1.6 | 0.012 |
| Fasting CPR (ng/mL) | 3.0 ± 1.0 | 1.0 ± 0.5 | <0.001 |
| Urinary CPR (μg/day) | 68.5 ± 43.8 | 41.2 ± 20.8 | 0.160 |
| HOMA-IR | 4.1 ± 2.4 | 2.6 ± 1.3 | 0.191 |
| Insulinogenic Index | 0.42 ± 0.25 | 0.08 ± 0.06 | <0.001 |
| SUIT Index | 137.6 ± 106.4 | 38.0 ± 13.6 | 0.001 |
| ABCD score | 5.2 ± 1.5 | 2.5 ± 0.5 | <0.001 |
| IMS score, n mild/moderate/severe | 3/13/2 | 0/0/6 | <0.001 |
| Insulin user, n (%) | 4 (22) | 6 (100) | 0.002 |
Value are expressed as mean ± SD. P-values represent comparisons between patients who achieved remission (remission group) vs. those who did not achieve remission (nonremission group).
LSG, laparoscopic sleeve gastrectomy; BMI, body mass index; HbA1c, hemoglobin A1c; CPR, C-peptide; HOMA-IR, homeostasis model assessment of insulin resistance; SUIT, The secretory units of islets in transplantation index; ABCD score, ABCD diabetes surgery score; IMS score, individualized metabolic surgery score
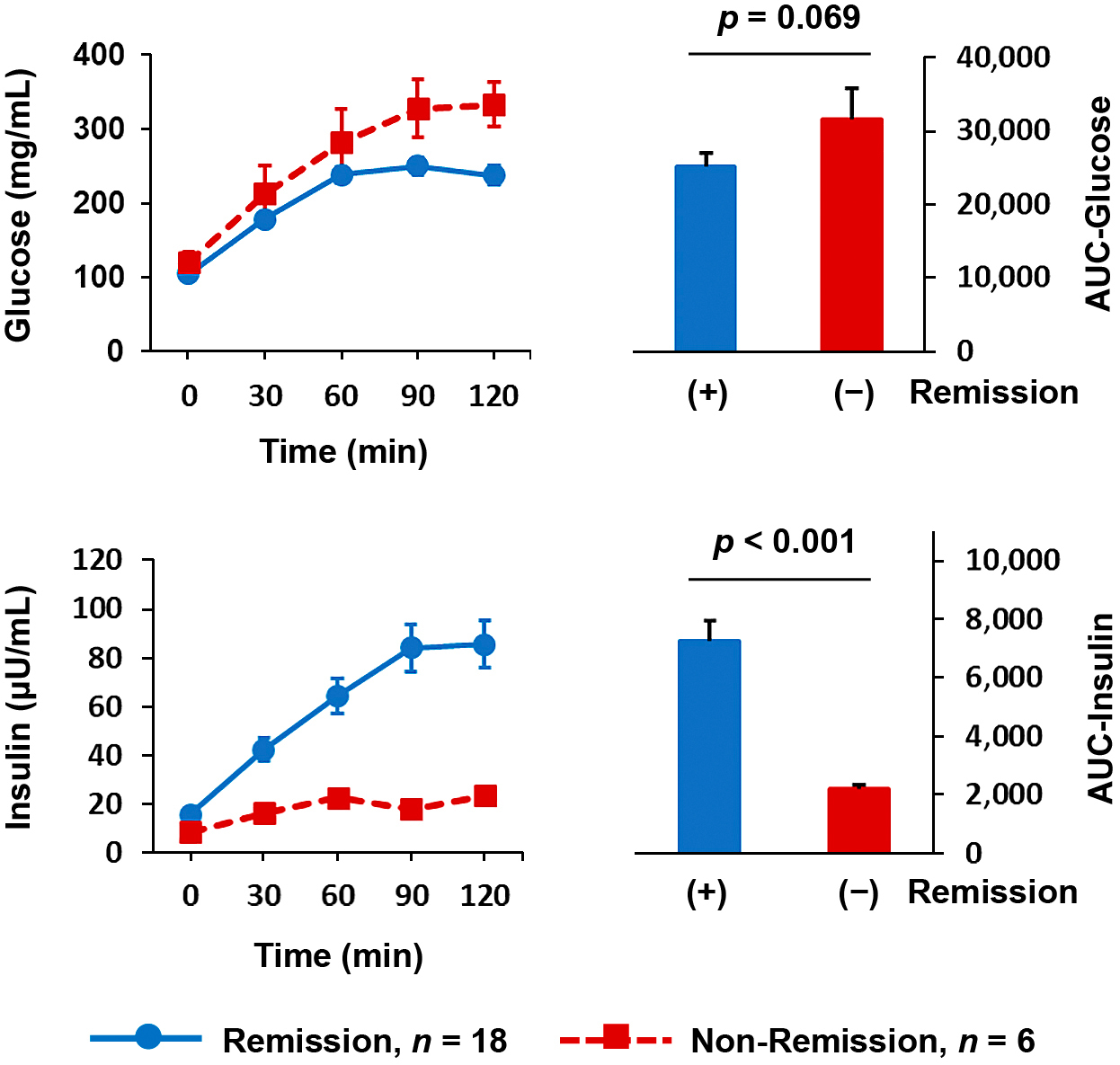
Comparison of plasma glucose concentration (upper panel) and insulin levels (lower panel) during OGTT before LSG between patients who achieved diabetes remission (blue line, n = 18) and those without remission (red line, n = 6) 1 year after LSG.
Right panel: The AUC for indicated measurements appears as arbitrary units (blue bars, remission group; red bars, nonremission group).
Data represent the mean ± SE. P-values are for comparisons between the remission and nonremission groups and were calculated using the Mann–Whitney test.
OGTT, oral glucose tolerance test; LSG, laparoscopic sleeve gastrectomy
We compared the distribution of fasting C-peptide level, SUIT index, insulinogenic index, and ABCD sores between the remission group and the nonremisison group (Fig. 3). To evaluate these measurements’ predictive values for diabetes remission, we performed a ROC curve analysis for each index and score; AUCs were 0.981, 0.898, 0.935, and 0.958, for C-peptide level, SUIT index, insulinogenic index, and ABCD score, respectively. These results suggest that all four measurements are good predictors for diabetes remission 1 year post-LSG. Cut-off values calculated from the ROC curve analysis were 1.82, 63.1, 0.17, and 3.5, for C-peptide level, SUIT index, insulinogenic index, and ABCD score, respectively (Fig. 3).
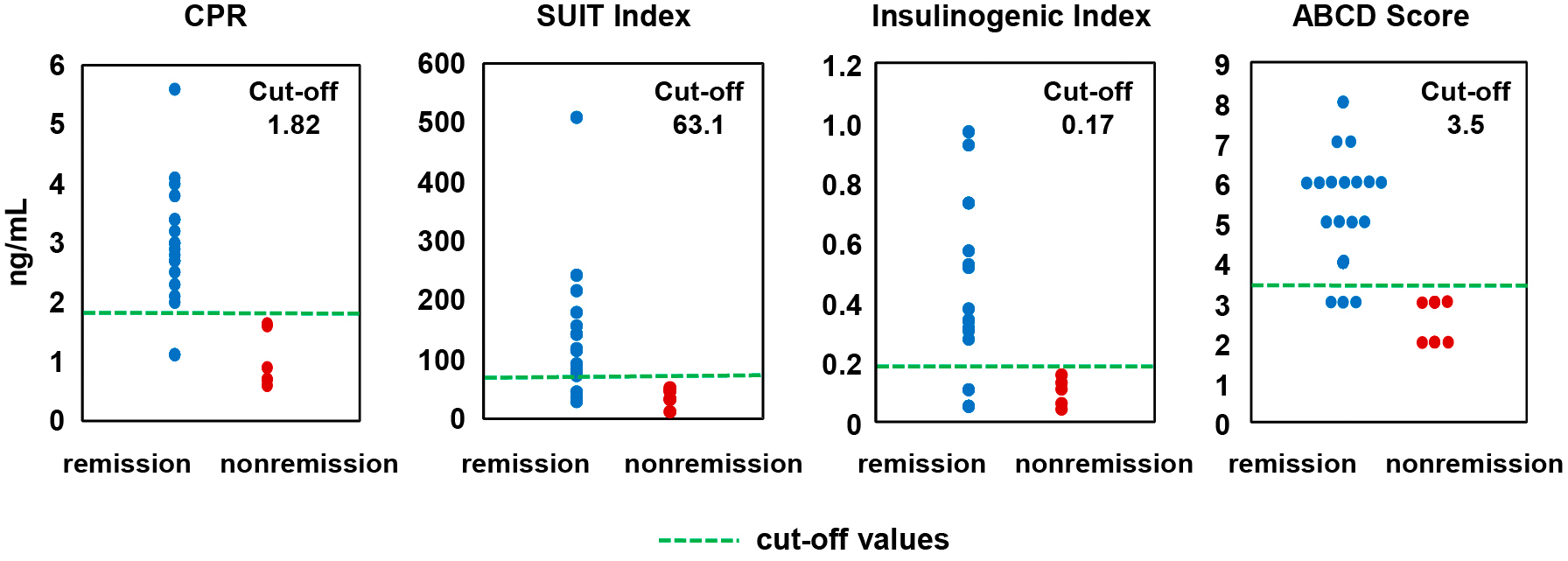
Distributions for the four variables in the remission group (blue dot, n = 18) and nonremission group (red dot, n = 6). The green dashed lines indicate cut-off values for diabetes remission calculated from the ROC curve analysis.
CPR, C-peptide; SUIT, the secretory units of islets in transplantation index; ABCD score, ABCD diabetes surgery score
Body weight reduction was comparable at 3 months between the groups, with a wider difference at 12 months (remission group: 25.9 ± 2.6 kg and nonremission group: 18.0 ± 2.5 kg); however, the difference was not significant (Fig. 4a). Similarly, when body weight reduction was assessed by %TWL, the difference was not significant at 1 year (remission group: 24.5 ± 1.8% and nonremission group: 19.5 ± 2.5%; Fig. 4b).

Changes in body weight expressed as the change from baseline (a), %TWL (b), and HbA1c (c) in the remission group (blue line, n = 18) and nonremission group (red line, n = 6).
Data represent the mean ± SE. P-values are for comparisons between the remission group and nonremission group and were calculated using mixed-model analysis.
%TWL, percent total weight loss; HbA1c, hemoglobin A1c
HbA1c at surgery was higher in the nonremission group (8.9 ± 0.8%) vs. the remission group (6.8 ± 1.05) (p = 0.026; Fig. 4c). HbA1c in the remission group decreased to 5.6% at 3 months and remained stable for 1 year (5.6% at 1 year). In contrast, HbA1c in the nonremission group decreased to 7.5% at 3 months but did not decrease further (7.6% at 1 year) (Fig. 4c).
Changes in diabetes medication in patients without remissionAll nonremission patients had been using insulin before surgery, and the mean total daily insulin dose at baseline was 48.7 ± 17.6 units/day. Three patients changed to oral hypoglycemic agents, and one changed to a glucagon-like peptide-1 receptor agonist after surgery. Two patients continued insulin, and their insulin dose changed from baseline to 1 year as follows: 69 to 10 units/day and 48 to 10 units/day (patient 1 and patient 2, respectively).
This study has two major findings. First, LSG is effective in weight reduction and glycemic control for 1 year in Japanese obese-T2DM patients; a high rate of diabetes remission was seen 1 year postoperatively. Second, preserving insulin secretion is the major determinant of diabetes remission after LSG.
Bariatric surgery is a recognized powerful strategy for treating morbid obesity. The most recent survey by the International Federation for the Surgery of Obesity and Metabolic Disorders showed that approximately 580,000 bariatric surgeries were performed in 2014 [9]. In contrast, the number of bariatric surgeries in Japan remains low; 831 patients underwent bariatric procedures from 2005–2015 [20]. However, the number of bariatric surgeries has increased since LSG was approved and covered by Japan’s national health insurance system in 2014; 471 patients underwent bariatric procedures in 2017 [21].
In this study, we confirmed that LSG is effective for weight reduction in Japanese obese patients; we saw significant weight reduction in our patients. The mean weight loss and %TWL were 23.9 kg and 23.3%, respectively, 1 year after surgery. These results are comparable with results for non-Asian patients. Schauer et al. reported that, in a randomized controlled trial comparing bariatric surgery and medical therapy for T2DM, weight reduction among 50 American patients 1 year after LSG was 25.1 kg (%TWL: 24.7%) [22]. Importantly, in addition to weight reduction, patients also showed dramatically-improved glucose tolerance after LSG. A narrative review of published observational studies showed a high rate of resolution of T2DM after bariatric surgery [4, 5], and several randomized controlled trials showed that bariatric surgery is superior to intensive medical treatment regarding glycemic control [3, 6, 7]. Based on these results, bariatric surgery has been defined as a powerful treatment for obesity-associated T2DM in clinical guidelines in several countries including Japan [23-25]. However, most of these results were derived from data for patients undergoing RYGB, one of the malabsorptive procedures, and T2DM resolution may be more common following predominantly malabsorptive procedures compared with purely restrictive techniques [23-25]. LSG is a purely-restrictive procedure and has proven effective for both weight reduction and resolution of diabetes [10, 11]. Adding to its relative simplicity and safety, the most commonly-performed procedure in the world is now LSG based on the 2014 International Federation for the Surgery of Obesity and Metabolic Disorders survey [26].
Insulin resistance and β-cell failure are two major features of T2DM [27]. According to current understanding, the pathophysiology of T2DM is different in East Asian patients, including Japanese patients, compared with non-Asian patients, in that Japanese patients develop diabetes easily when they cannot compensate for insulin resistance with increased insulin secretion. [12, 28]. Thus, we cannot necessarily expect similar effects on glycemic control following bariatric surgery in Japanese patients with T2DM because decreased insulin resistance secondary to weight reduction might be a major cause of diabetes remission if insulin secretion is restored, as with non-Asian patients. Therefore, it is important to evaluate the effectiveness of LSG for glycemic control in Japanese obese diabetic patients. Our results showed that LSG dramatically improved glycemic control in Japanese obese diabetic patients, with a diabetes remission rate at 1 year of 75%, similar to results in non-Asian patients [10].
Factors influencing diabetes resolutionIdentifying preoperative predictors of diabetes resolution is critical for determining which diabetic patients will obtain the greatest benefit from surgery. We showed that diabetes duration, C-peptide level, AUC for insulin and insulinogenic index during OGTT, and insulin use were correlated with diabetes resolution. Previous reports following RYGB showed that factors correlating with diabetes resolution included T2DM duration, elevated HbA1c, insulin use [29], younger age [30], higher BMI, greater weight loss, higher C-peptide level, higher insulin resistance, and higher ABCD score [30, 31]. Most of these factors are related to β-cell function. Our results are consistent with previous reports and clearly confirmed that preserving β-cell function is the key factor determining whether remission can be expected.
Body weight at baseline was higher in our remission group compared with the nonremission group. However, we saw no difference in BMI between the groups because the number of female patients was smaller in the remission group, and therefore, the average height was higher in the remission group compared with the nonremission group (167.1 cm vs. 155.7 cm, respectively).
Preoperative weight loss can significantly improve glucose metabolism, reduce the surgical risk, and affect postoperative clinical outcomes. Watanabe et al. reported that the extent of preoperative weight loss statistically affected postoperative weight loss and might have minor advantages in reducing the risk of early postoperative complications in patients who undergoing LSG [32]. In our study, five patients were able to discontinue their diabetes medication before surgery even though their OGTT results still met the criteria for diabetes. However, further weight reduction using surgery was necessary to achieve diabetes remission.
We saw no difference in weight reduction 1 year postoperatively compared with baseline between the remission and nonremission groups. Similarly, there was no difference in %TWL, which was not affected by initial body weight. It is still a matter of debate how much weight loss contributes to diabetes resolution following bariatric surgery. It is known that improved glucose metabolism becomes apparent within days of surgery, before significant weight loss occurs [29, 33]. Thus, total body weight loss, per se, is unlikely to play a significant role in mediating early glycemic improvements. However, results are conflicting regarding the association between preoperative BMI, weight loss, and the magnitude of diabetes improvement over longer periods [34-36]. Further study is needed to elucidate these issues in Japanese patients.
Although diabetes remission was not achieved in 25% of our patients, their glycemic control improved. Average HbA1c in nonremission patients dropped from 8.9% at surgery to 7.6% 1 year postoperatively; 4/6 patients were able to discontinue insulin, and two patients were able to drastically reduce their insulin dose. Although diabetes remission is the ideal outcome, bariatric surgery is still beneficial in patients less likely to achieve diabetes remission because improved glycemic control may prevent diabetic complications.
There are concerns about the long-term effects of LSG. Golomb et al. reported that LSG was associated with significant weight regain and decreased diabetic remission rates in their 5-year follow-up [37]. We evaluated the longer follow-up data in our study and found that, of 18 patients in the remission group at 1 year, 16 patients were followed at 2 years. Of the 18 patients, one patient had restarted oral hypoglycemic agents 17 months after surgery. HbA1c levels among the 16 patients followed at 2 years were 5.85 ± 0.14%, suggesting that LSG provides effective glycemic control for longer periods (at least up to 2 years).
It has been reported that patients with severe diabetes were unlikely to achieve diabetes remission after LSG [17]. Thus, there is an opinion that malabsorptive procedures should be recommended in patients with severe diabetes [17, 38]. LSG-DJB is a combination of LSG and proximal intestinal bypass through duodenal exclusion, in which exploration of the remnant stomach can be performed endoscopically through a physiological route [39]. LSG-DJB is not covered by the national health insurance system in Japan but has been approved and performed at limited facilities, and evidence supporting the superiority of LSG-DJB over LSG for glycemic control, especially in severe diabetes, has been accumulating in Japan [38, 40-43].
Elucidating the mechanisms underlying diabetes improvement following bariatric surgery is an important issue to clarify. It is widely accepted that postprandial plasma GLP-1 levels are markedly increased after bariatric surgery including after RYGB and LSG, which is believed to be the one of the major mechanisms in diabetes improvement [4, 5]. Consistent with this finding, we reported significantly enhanced GLP-1 secretion during OGTT along with enhanced insulin secretion after LSG [15]. However, extensive research has revealed that the underlying mechanisms for diabetes improvement following bariatric surgery might be more complex. Several different mechanisms, namely, bile acid metabolism, nutrient sensing, glucose utilization in the gut, a possible anti-incretin (decretin), and the microbiome, are reported to be involved [33]. Further study is needed to clarify and understand these complex factors.
There are several limitations in this study, and the most important is the small number of patients. A simple predictor for diabetes remission would be useful. Several predictors have been proposed [17, 18, 31], and it is important to determine which predictor is better for Japanese patients. We calculated the cut-off values for four parameters describing diabetes remission in this study; however, verifying our cut-off values requires larger studies with higher numbers of patients. Our study is also limited by its relatively short duration. Long-term glycemic control is the aim of diabetes therapy; therefore, studies with more patients and a longer follow-up are required for the precise evaluation of the effect of LSG in Japanese obese T2DM patients. Another limitation is that we did not quantitatively evaluate patients’ quality of life (QOL) after surgery, in this study. There are controversies regarding the impact of bariatric surgery on QOL [44-46]. Preoperative predictors for QOL after surgery and a method for postoperative monitoring are important issues requiring clarification.
In conclusion, this study showed that LSG is effective in weight reduction and glycemic control in Japanese obese T2DM patients. Preserving insulin secretion is the major determinant of diabetes remission.
We thank Jane Charbonneau, DVM, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
MT received research support from Sunny Health Co., Ltd.
None of the other authors have any potential conflicts of interest associated with this research.