2020 年 67 巻 1 号 p. 73-80
2020 年 67 巻 1 号 p. 73-80
Those who smoke nicotine-based cigarettes have elevated plasma levels of ghrelin, a hormone secreted from the stomach. Ghrelin has various physiological functions and has recently been shown to be involved in regulating biological rhythms. Therefore, in this study, in order to clarify the significance of the plasma ghrelin increase in smokers, we sought to clarify how nicotine and ghrelin affect the expression dynamics of clock genes using a mouse model. A single dose of nicotine administered intraperitoneally increased plasma ghrelin concentrations transiently, whereas continuous administration of nicotine with an osmotic minipump did not induce any change in the plasma ghrelin concentration. Single administration of nicotine resulted in a transient increase in ghrelin gene expression in the pancreas but not in the stomach, which is the major producer of ghrelin. In addition, in the pancreas, the expression of clock genes was also increased temporarily. Therefore, in order to clarify the interaction between nicotine-induced ghrelin gene expression and clock gene expression in the pancreas, nicotine was administered to ghrelin gene-deficient mice. Administration of nicotine to ghrelin-gene deficient mice increased clock gene expression in the pancreas. However, upon nicotine administration to mice pretreated with octanoate to upregulate ghrelin activity, expression levels of nicotine-inducible clock genes in the pancreas were virtually the same as those in mice not administered nicotine. Thus, our findings indicate that pancreatic ghrelin may suppress nicotine-induced clock gene expression in the pancreas.
GHRELIN is a hormone secreted mainly from the upper gastrointestinal tract such as the stomach and duodenum [1]. The main functions of ghrelin are growth hormone release, hyperphagia, and fat accumulation [1-4], but it is considered to have diverse physiological roles owing to the wide distribution of ghrelin receptors [5]. In fact, ghrelin is also involved in the regulation of reward behavior and of the cardiovascular system [6-8]. Recently, there have been reports suggesting that ghrelin is involved in the regulation of clock gene expression [9], and there is increasing interest in the maintenance of various systems by ghrelin, in particular, the control of biological rhythm and/or sleep.
In turn, ghrelin secretion can be affected by various factors. Ghrelin concentrations in the plasma are high in low-energy states such as fasting [3, 10-12] and low in excessive energy states such as obesity [13]. There are also reports that in patients with Prader-Willi syndrome, ghrelin levels are significantly elevated both before and after meals [14, 15]. In addition, ghrelin secretion is known to be affected by various physiological and pathological factors. For example, epidemiological investigations have revealed that plasma ghrelin secretion is also increased by smoking [16], but its physiological significance in this case remains unknown.
The main component of tobacco is nicotine. Because nicotine can cross the blood-brain barrier [17], when ingested it affects various functions including central functions via nicotinic receptors. There are also reports that electronic cigarette vapor containing nicotine can affect circadian molecular clock gene expression in the mouse lungs [18].
These findings imply that nicotine and ghrelin may be involved in the regulation of biological rhythm via some potential interaction. However, few studies have investigated the combined effects of nicotine and ghrelin. Therefore, in this study, we used experimental mouse models to examine whether nicotine alters ghrelin secretion and, if so, whether it is also involved in the regulation of biological rhythm.
Male C57BL/6J Jcl mice (CLEA, Tokyo, Japan) and ghrelin-gene deficient (ghrl–/–) mice weighing 19–21 g were used in these experiments [19]. Mice were individually housed in a room maintained under controlled temperature (23 ± 1°C, 55 ± 5%) and light conditions (lights on at 0700 h, lights off at 1900 h; therefore, we set 0700 h as zeitgeber time 0 (ZT0)) with ad libitum access to standard rodent chow pellets (CLEA) and water. ZT represents the time of the biological clock in a 12/12 hour light/dark cycle where the start time of the light cycle is ZT0 and the start time of the dark cycle is ZT12. All animal protocols were approved by the Ethical Committee for the Research of Life Science of Kurume University.
Administration of nicotineTo investigate the effect of a single dose of nicotine, mice were given intraperitoneal injections of nicotine (2 mg/kg; FUJIFILM Wako Pure Chemical Co., Osaka, Japan) at ZT6 (1:00 pm). Control groups were intraperitoneally injected with sterile saline.
To investigate the effect of continuous nicotine administration, mice were subcutaneously implanted with ALZET Osmotic Pumps (model 1007D; DURECT Co., Cupertino, CA, USA) containing nicotine. The pumps delivered nicotine at 1.67 ng/0.5 μL/hr/20 g body weight for 7 days. Control groups were implanted with osmotic pumps containing sterile saline.
Administration of octanoic acidIngested medium-chain fatty acids are directly utilized for the acyl modification of ghrelin [20]. We orally administered 0.5% octanoic acid solution via free access to a water bottle to C57BL/6J mice for 1 week. In this experimental system, plasma ghrelin concentration was 17.5 ± 2.1 (fmol/mL) in mice given water only, but 31.5 ± 6.3 (fmol/mL) in mice given octanoic acid. There was no difference in water consumption between the two groups.
Hormone assayTo prepare plasma samples, whole blood was mixed with EDTA-2Na (2 mg/mL) and aprotinin (500 kIU/mL) and centrifuged (1,200 × g, 20 min) at 4°C. Immediately after plasma separation, HCl was added to the sample to a final concentration of 0.1 N. Plasma samples were loaded onto Sep-Pak C18 cartridges (Waters Corp., Milford, MA, USA), and the cartridges were washed in 0.9% NaCl and 10% CH3CN/0.1% TFA. Bound protein was eluted with 60% CH3CN/0.1% TFA. The eluate was lyophilized and subjected to ghrelin-specific ELISA. To quantify plasma ghrelin levels, we used an Active Ghrelin ELISA Kit (SCETI KK, Tokyo, Japan) to assess n-octanoyl-modified ghrelin and a Des-acyl-Ghrelin ELISA Kit (SCETI KK) to measure des-acyl ghrelin according to the manufacturer’s instructions.
Quantitative RT-PCRIn the single-dose nicotine experiment, the hypothalamus, stomach, and pancreas were collected at 0, 30, 60, and 120 min after administration of nicotine. In the continuous nicotine administration experiment, the hypothalamus, stomach, and pancreas were collected 1 week after osmotic minipump implantation. All samples were immediately frozen on dry ice after collection.
Total RNA was isolated using a Sepasol-RNA Super G (Nacalai Tesque, Kyoto, Japan), according to the manufacturer’s instructions. To synthesize cDNA, total RNA (1 μg/animal) derived from each tissue was used. cDNA amplification was performed using Power SYBR Green Master Mix (Thermo Fischer Scientific KK, Tokyo, Japan), as suggested by the manufacturer. Reaction mixtures (QuantiTect Reverse Transcription Kit, Qiagen KK, Tokyo, Japan) were incubated at 37°C for 15 min. Reactions were stopped by incubation at 95°C for 3 min. Real-time PCR was performed using a StepOnePlus Real-Time PCR System (Thermo Fischer Scientific KK). We measured the expression levels of the ghrelin gene in the mouse stomach and pancreas and of the period circadian clock 1 (Per1), period circadian clock 2 (Per2), circadian locomotor output cycles kaput (Clock), cryptochrome 1 (Cry1), brain and muscle Arnt-like protein 1 (Bmal1), and glucose transporter 2 (Glut2) genes in the mouse hypothalamus and pancreas. In the oscillatory system of the vertebrate biological clock, the heterodimer of CLOCK and BMAL1, which are bHLH/PAS type transcription factors, is in the promoter region of the Per or Cry which is the clock gene. Transcription is activated by binding to the existing E-box. The products of these genes, PER and CRY, act directly on the CLOCK-BMAL1 heterodimer to suppress transcriptional activity, thus establishing a negative feedback mechanism that negatively regulates the transcription of their own genes. Since the rhythm of the biological clock is generated by one cycle of this core loop over a period of 24 hours [21], these four genes were targeted for measurement. All samples were amplified in a single MicroAmp Optical 96-well reaction plate (Thermo Fischer Scientific KK). Results reflect duplicate runs of at least two independent experiments. All primers used for quantitative PCR analysis were purchased from Takara Bio, Inc. (Shiga, Japan). PCR cycling conditions were initiated by a 2-min incubation at 50°C to eliminate any deoxyuridine triphosphate-containing PCR products resulting from carryover contamination. After a 15-min period at 95°C to activate HotStarTaq DNA polymerase, PCR fragments were amplified by 40 cycles of 95°C for 15 sec and 60°C for 1 min. Each standard well contained the pGEM-T Easy vector as a standard cDNA fragment. The concentration of the standards covered at least 6 orders of magnitude. We also included negative controls containing no template on each plate. Experimental samples with a threshold cycle value within 2 SD of the mean threshold cycle value for the no-template controls were considered to be below the limits of detection. The relative expression levels were standardized to that of the housekeeping gene ribosomal protein S18 (Rps18) to correct for any bias among the samples caused by RNA isolation, RNA degradation, or the efficiencies of the reverse transcription. After amplification, PCR products were analyzed by melting curve to confirm amplification specificity. Amplicon size and reaction specificity were confirmed by agarose gel electrophoresis.
Statistical analysesAll continuous variables are presented as the mean ± standard deviation. Results with a p-value <0.05 were considered statistically significant. Statistical analyses were performed using GraphPad Prism 8 (MDF, Tokyo, Japan).
In order to investigate the effect of nicotine on ghrelin secretion in mice, the following two groups were established: a group given a single dose of nicotine intraperitoneally and a group given nicotine subcutaneously for 1 week via an osmotic minipump. In mice given a single dose of nicotine intraperitoneally, plasma concentrations of both ghrelin and des-acyl ghrelin transiently increased 60 min after administration (Fig. 1A). In addition, we investigated the synthesis of ghrelin by assessing changes in ghrelin mRNA levels using quantitative PCR. Little change was observed in the stomach (Fig. 1B); however, in the pancreas, the expression of the ghrelin gene increased over time. At about 60–120 min after nicotine administration, the expression level had increased approximately 6-fold (Fig. 1B). Thus, a single dose of nicotine administered intraperitoneally enhances ghrelin secretion, including ghrelin synthesis and release, at least in the pancreas.
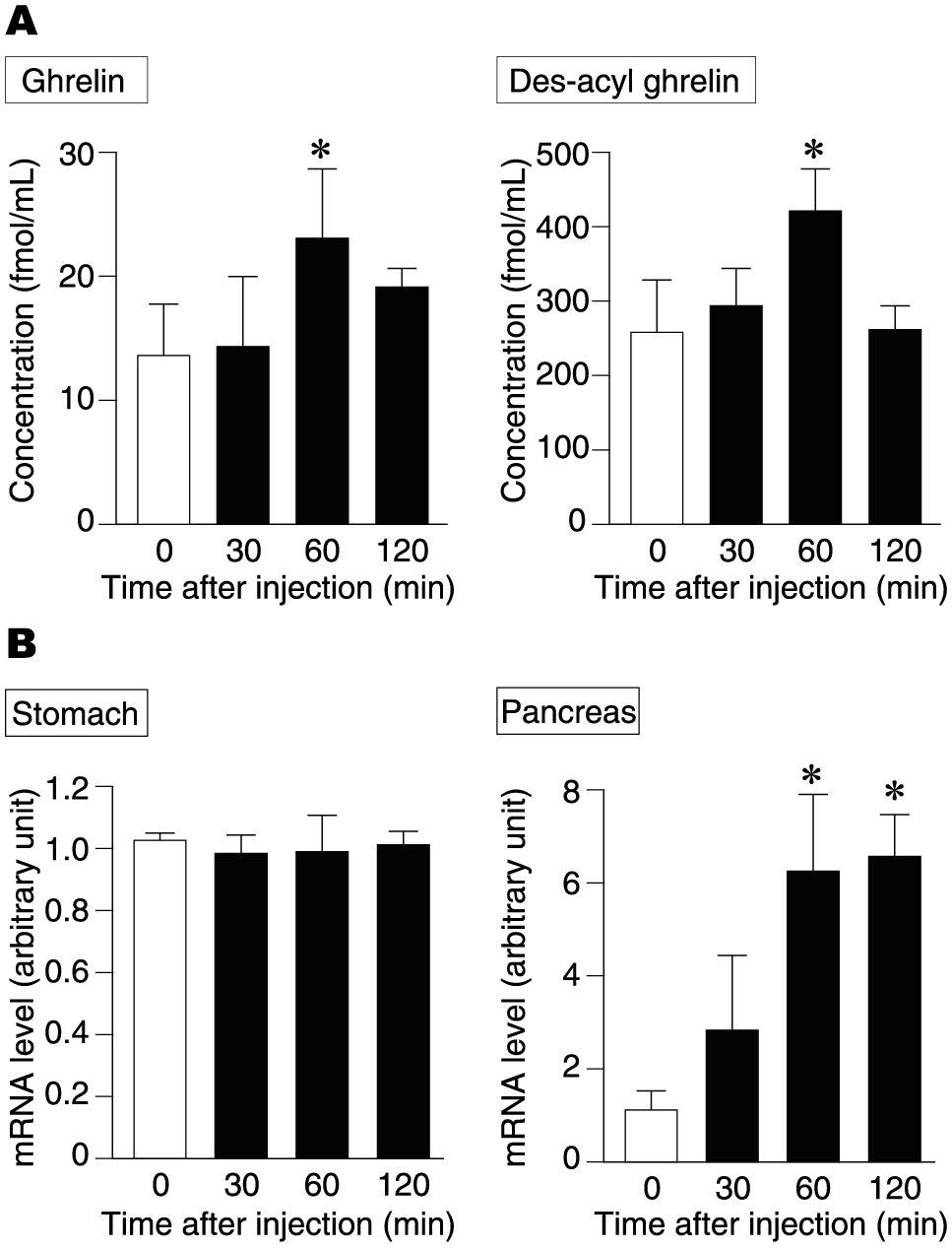
Ghrelin secretion after single administration of nicotine. (A) Plasma ghrelin and des-acyl ghrelin concentrations at 0, 30, 60 and 120 min after administration of nicotine intraperitoneally in mice. (B) Ghrelin gene expression levels in the stomach and pancreas at 0, 30, 60, and 120 min after nicotine was administered intraperitoneally in mice. *p < 0.05 (compared with 0 min after administration).
In contrast, in mice implanted with osmotic minipumps for 1 week and administered nicotine subcutaneously, there was no change in the plasma concentrations of ghrelin or des-acyl ghrelin (Fig. 2A). Furthermore, no difference was found in the expression level of the ghrelin gene in implanted mice, either in the stomach or the pancreas (Fig. 2B). Thus, ghrelin secretion in the mouse pancreas was affected only by the single dose of nicotine.

Ghrelin secretion after continuous administration of nicotine. (A) Plasma ghrelin and des-acyl ghrelin concentrations after 1 week of continuous subcutaneous administration of nicotine (Ni) or saline (Sal) by osmotic minipump in mice. (B) Ghrelin gene expression levels in the stomach and pancreas after 1 week of continuous subcutaneous administration by osmotic minipump in mice.
Next, we investigated whether nicotine regulates the expression of clock genes. Following nicotine administration, we did not observe any change in the expression levels of clock genes in the brain samples containing the suprachiasmatic nucleus (Fig. 3A). However, we observed increased expression of all clock genes except Bmal1 in the pancreas at 30 min after intraperitoneal administration of nicotine (Fig. 3B). All clock genes whose expression levels were altered by nicotine returned to pre-administration levels by 60 min after nicotine administration (Fig. 3B). Therefore, administration of nicotine influences not only pancreatic ghrelin gene expression but also pancreatic clock gene expression.
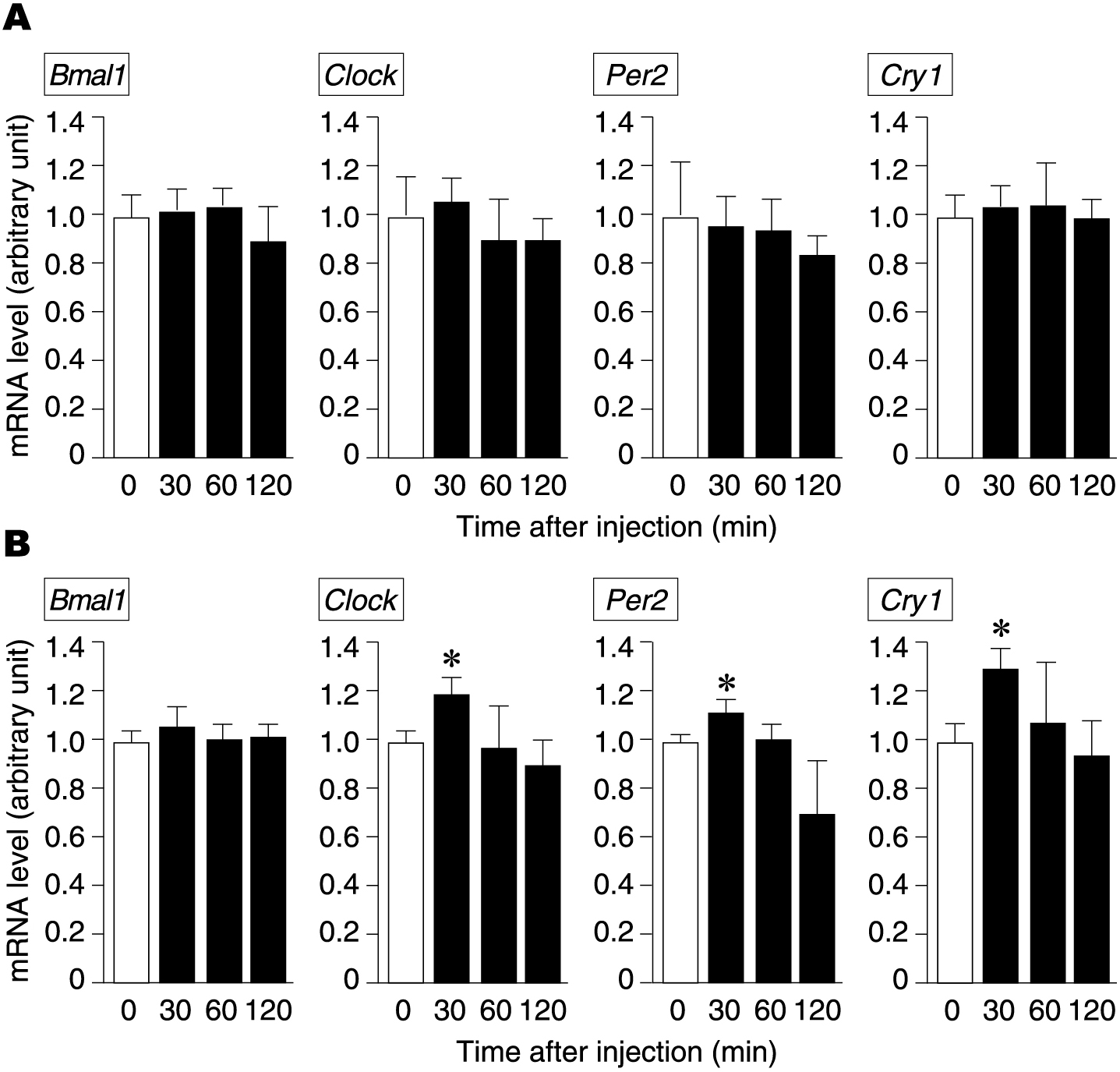
Changes in clock gene expression levels in the hypothalamus and pancreas after a single dose of nicotine. Changes in the expression levels of clock genes (Bmal1, Clock, Per2, Cry1) in the hypothalamus and pancreas at 0, 30, 60, and 120 min after a single dose of intraperitoneal nicotine in mice. *p < 0.05 (compared with 0 min after administration).
To further investigate the relationship between ghrelin secretion and clock gene expression after nicotine administration in the pancreas, we studied clock gene expression in ghrelin gene-knockout (ghrl–/–) mice using quantitative RT-PCR. The expression levels of all clock genes except Bmal1 were higher in ghrl–/– mice than in nicotine-treated wild-type mice, indicating that ghrelin suppresses nicotine-induced clock gene expression (Fig. 4).
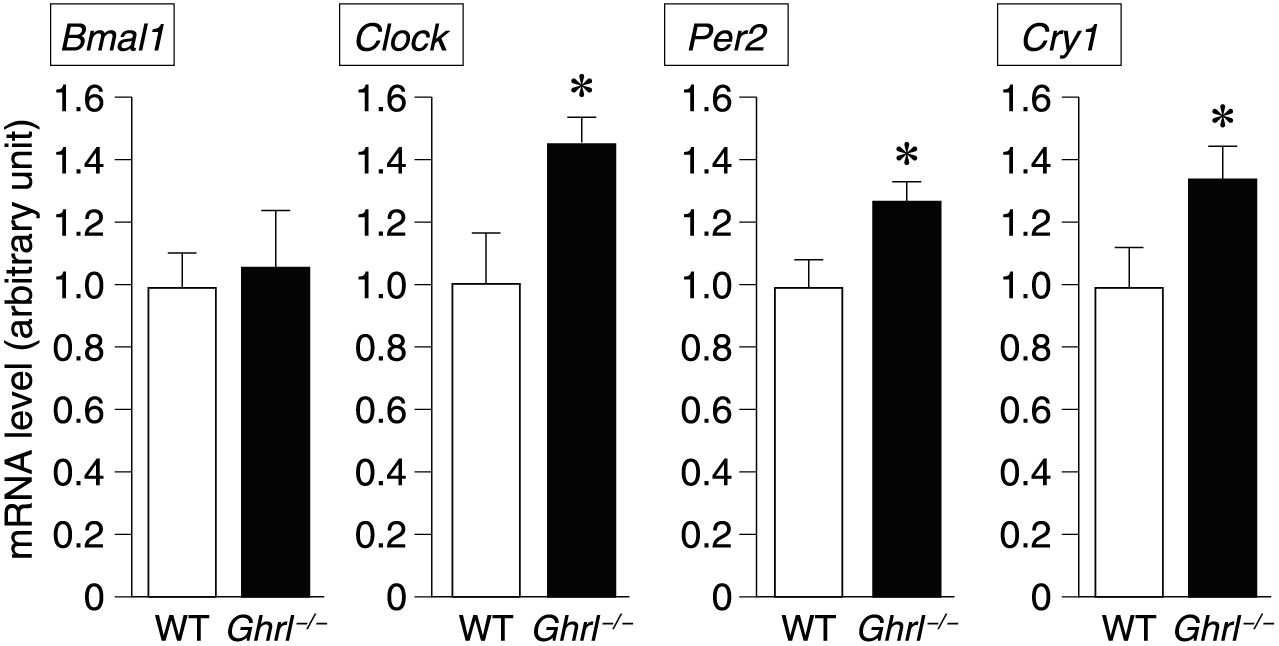
Expression levels of clock genes in the pancreas after administration of nicotine in ghrl–/– mice. Expression level of clock genes (Bmal1, Clock, Per2, Cry1) 30 min after nicotine was administered intraperitoneally in mice. WT: wild-type mice, Ghrl–/–: ghrelin gene-deficient mice. *p < 0.05.
Next, we examined clock gene expression after nicotine administration using mice in which endogenous ghrelin activity had been previously increased by prior oral administration of 0.5% octanoic acid for 1 week. Clock gene expression levels were higher in the nicotine administration group than in the control group (Fig. 5). However, upon nicotine administration to mice pretreated with octanoic acid, no change in clock gene expression was observed, with expression levels similar to those in the control group.

Expression levels of clock genes in the pancreas after single-dose administration of intraperitoneal nicotine to octanoate-pretreated mice. A single dose of nicotine was intraperitoneally administered to mice in which endogenous ghrelin activity was enhanced by pretreatment with oral octanoic acid, and clock gene (Bmal1, Clock, Per2, Cry1) expression levels in the pancreas were examined after 30 min. Mice were divided into three groups: an untreated group (Int), a nicotine administration group (Ni), and a nicotine administration group after octanoic acid pretreatment (OC + Ni). *p < 0.05 (group Ni compared to groups Int and OC + Ni).
We investigated how pancreatic function is affected by clock genes regulated by nicotine and ghrelin. In the pancreas, it is known that there is a circadian rhythm in gene expression of Glut2, Ins1, Ins2, and Rab37 involved in insulin secretion [22]. A single dose of nicotine to C57BL/6J mice decreased the rhythmicity of Glut2 mRNA peaking at ZT18 observed with a single dose of physiological saline (Fig. 6A). Furthermore, to clarify the relationship between nicotine intake and ghrelin, we administered nicotine once to WT mice and ghrl–/– mice. Nicotine administration to ghrl–/– mice almost canceled the rhythm of Glut2 mRNA expression in the pancreas (Fig. 6B).
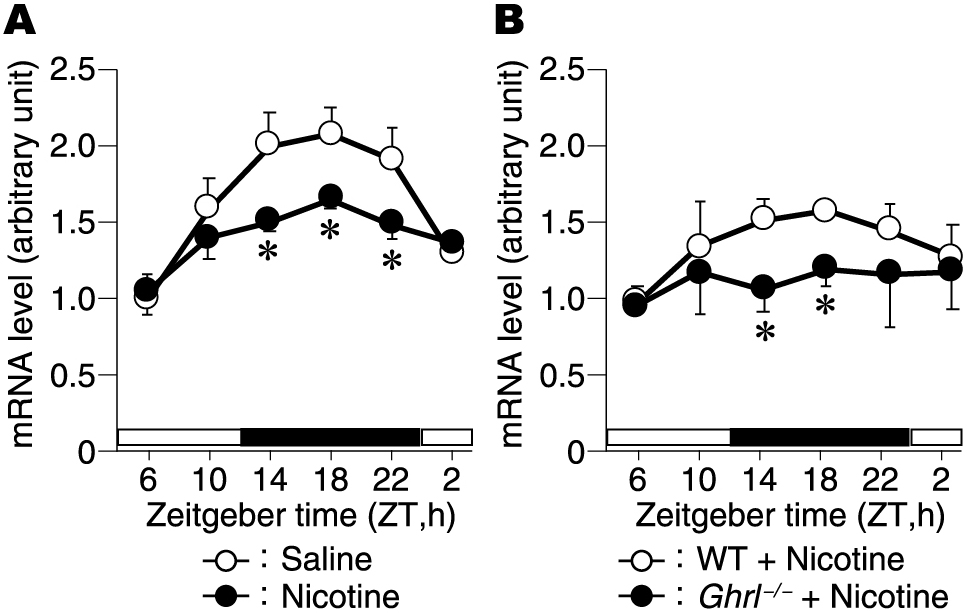
Changes in Glut2 expression levels in the pancreas after a single dose of nicotine. Changes in the expression levels of Glut2 in the pancreas at ZT6, 10, 14, 18, 22, 2 after a single dose of intraperitoneal nicotine in mice. (A) C57BL/6J mice, and (B) ghrl–/– mice. The open square represents the light period, and the closed square represents the dark period. *p < 0.05 (compared with Saline group (A) and with WT mice (B)).
These facts indicated that ghrelin suppresses nicotine-induced clock gene expression to maintain pancreatic function (Fig. 7). Notably, under octanoic acid administration, there was no change in body weight or food intake in mice, although ghrelin’s main function is to promote GH secretion and to enhance feeding.
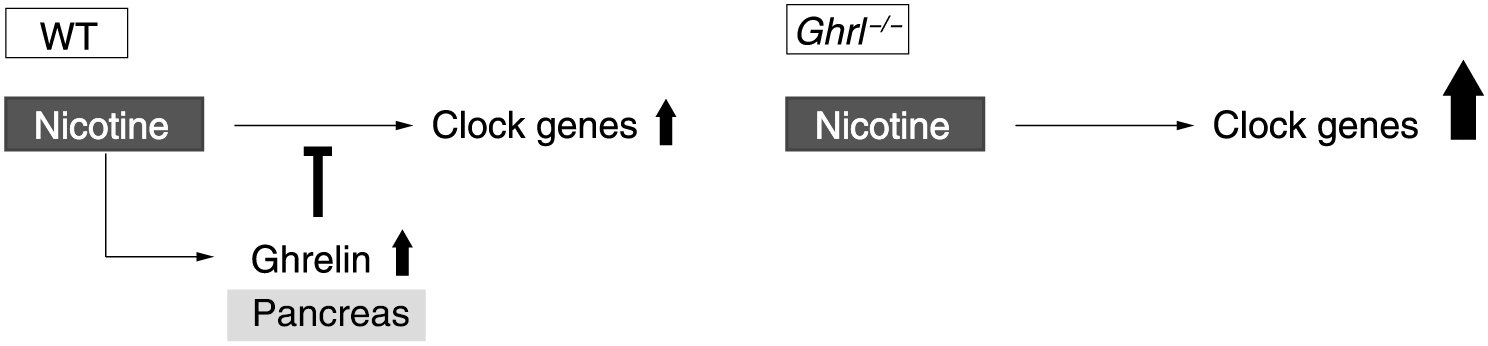
Schematic representation of ghrelin regulation of nicotine-induced clock gene expression in the pancreas. Ghrelin suppresses transient increases in nicotine-induced clock gene expression in the wild-type mouse pancreas. In contrast, in the ghrelin gene-deficient mouse pancreas, expression of clock genes is upregulated compared to that in wild-type mice because ghrelin has no suppressive effect.
In this study, we demonstrated that nicotine transiently increased the expression of the ghrelin gene in the pancreas. Nicotine upregulated the pancreatic expression of clock genes. Furthermore, nicotine-induced clock gene expression in the pancreas was greater in ghrl–/– mice than in WT mice. In contrast, in mice with increased endogenous ghrelin activity, there was almost no change in clock gene expression, even when nicotine was administered. This suggests that transient nicotine ingestion may affect the rhythm of the pancreas. Notably, sustained nicotine administration had no effect on the expression of ghrelin or clock genes.
As plasma concentrations of ghrelin and des-acyl ghrelin gradually increased following administration of a single dose of nicotine, the expression of the ghrelin gene was examined in ghrelin-producing tissues such as the stomach and pancreas. Expression of the ghrelin gene increased only in the pancreas, with no significant change found in other tissues such as the stomach. This indicates that ghrelin is only transiently released in the stomach upon nicotine administration, whereas ghrelin secretion, including both its synthesis and release, is accelerated in the pancreas. However, plasma concentrations of ghrelin and des-acyl ghrelin are known to largely reflect secretion from the upper gastrointestinal tract such as the stomach and duodenum, with very little ghrelin biosynthesized in the pancreas [1, 23, 24]. Thus, the influence of the pancreas on the plasma ghrelin concentration is considered to be small. Therefore, it is thought that the ghrelin secreted in the pancreas upon nicotine administration functions in an autocrine and/or paracrine manner in the pancreas, as revealed by morphological observations [25]. One such function is thought to be the control of clock gene expression in the pancreas, which is supported by our finding that nicotine temporarily increases the expression of pancreatic clock genes. Moreover, this increase in expression was completely inhibited by the upregulation of ghrelin activity via oral administration of octanoic acid, a medium-chain fatty acid. Nicotine enhances lipolysis and increases the amount of free fatty acids in the circulation [26]. However, orally administered fatty acids can directly bind to the inactive form, des-acyl ghrelin, to produce the active form of ghrelin [20]. Therefore, in normal mice, intake of nicotine increases free fatty acids in circulation, and thus the amount of active ghrelin is considered to increase. Thus, in normal mice treated with nicotine, ghrelin from two sources may be present in the pancreas. As demonstrated in this study, ghrelin secreted from the pancreas by nicotine treatment suppresses nicotine-induced clock gene expression in the pancreas. On the other hand, there may be an active form of ghrelin obtained by the binding of free fatty acids to des-acyl ghrelin although its function is unknown. It cannot be denied that such ghrelin may also act to protect the pancreas from abnormalities in clock gene expression induced by a single administration of nicotine in the pancreas.
Mammalian circadian clocks are organized into a hierarchical structure [27]. The central clock in the suprachiasmatic nucleus of the hypothalamus controls rhythms such as behavior and blood hormone concentrations. However, the peripheral clock, located in peripheral tissues such as the liver and kidney, controls functions unique to each organ, such as the regulation of metabolism and ion concentrations. Interestingly, in life-threatening situations, the peripheral clock can escape central clock control and perform its own time adjustments [28]. The precise effect of nicotine in the pancreas is unclear. However, since the rapid upregulation of the Per1 gene resets the biological clock in Drosophila [29], this study suggests that nicotine may reset the pancreatic clock and affect downstream pancreatic functions. Ghrelin secretion has been shown to be negatively correlated with obesity. When ghrelin levels are downregulated due to obesity or other physiological factors, the pancreas is even more affected by nicotine intake. As dysregulation of clock genes is a trigger for various diseases including cancer, additional research on nicotine ingestion and control of the expression of pancreatic clock genes is required.
In the pancreas, both the secretion of ghrelin during fasting and the secretion of insulin after eating are required to maintain homeostasis. Because these hormones are antagonistic, secretion of one is low when secretion of the other is high. Nicotine also acts antagonistically on these hormones, with ghrelin secretion accelerated by nicotine and glucose-induced insulin secretion suppressed by high concentrations of nicotine [30]. We have shown in this study that pancreatic clock genes exhibit abnormal expression patterns in the absence of ghrelin, and there are also reports that administration of an insulin inhibitor in mice delays the circadian clock of the liver [31]. Thus, when secretion of one of the antagonistic hormones ghrelin or insulin is reduced via some external or internal factor, the peripheral biological clock is dysregulated. Therefore, in the pancreas, both ghrelin and insulin are necessary for maintaining peripheral clock homeostasis, and more detailed studies investigating both of these endocrine factors are needed in the future.
We investigated what abnormalities in the rhythm of actual pancreatic function are caused by the suppressive effect of ghrelin on nicotine-induced clock gene expression. Among the genes expressed in the pancreas, genes such as Glut2, Ins1, Ins2, and Rab37 are known to exhibit circadian rhythm [22]. Of these, it was found that the expression rhythm of Glut2 mRNA was attenuated by the increased expression of clock genes by nicotine. Since nocturnal mice begin feeding before and after the dark phase, pancreatic Glut2 mRNA expression usually increases towards ZT12, the starting point of feeding time, with a maximum around ZT18 [22]. However, this study shows that nicotine administration inhibits this rhythm. Furthermore, we found that this inhibitory effect becomes more prominent in the absence of ghrelin. Since GLUT2 present in pancreatic β cells has a function of monitoring blood glucose concentration, nicotine intake is thought to attenuate this monitoring function. In addition, the results obtained from ghrl–/– mice imply that ghrelin has a role in maintaining the normal rhythm of GLUT2 and complementing its function.
This study showed that nicotine enhances ghrelin secretion and induces clock gene expression in the pancreas. Furthermore, we revealed that ghrelin suppresses nicotine-induced clock gene expression. While nicotine administration induces various responses throughout the body, the results of this study show that in the pancreas, the abnormal clock gene expression induced by nicotine can be suppressed by the presence or activation of ghrelin. These results are expected to lead to a new endocrinological or pathophysiological understanding of conditions such as obesity that involve reduced ghrelin secretion.
We thank Shigetoshi Koga (Kurume University) and Masayo Yamane (Kurume University) for laboratory animal care. This work was supported in part by MEXT-Supported Program for the Strategic Research Foundation at Private Universities, JSPS KAKENHI Grant Number JP22790889 (Grant-in-Aid for Scientific Research (C)) and the Smoking Research Foundation of Japan. We thank Editage (www.editage.jp) for English language editing.
None of the authors have any potential conflicts of interest associated with this research.